Abstract
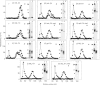
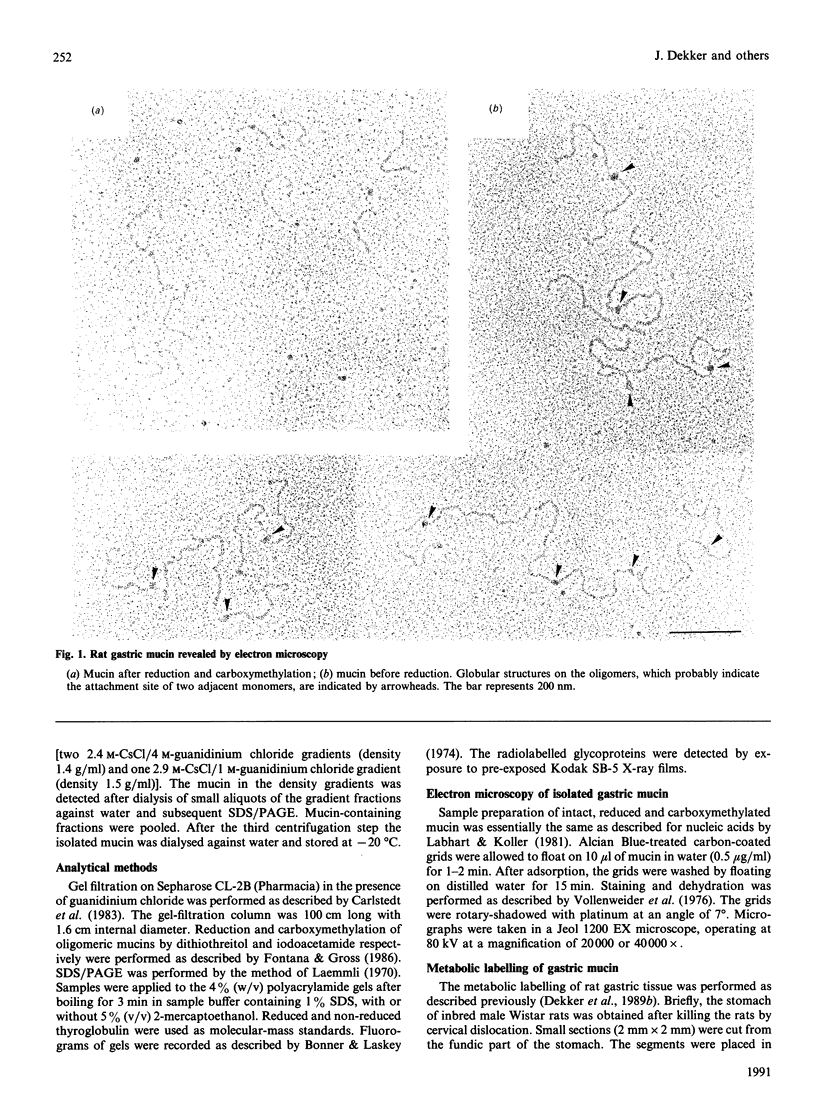
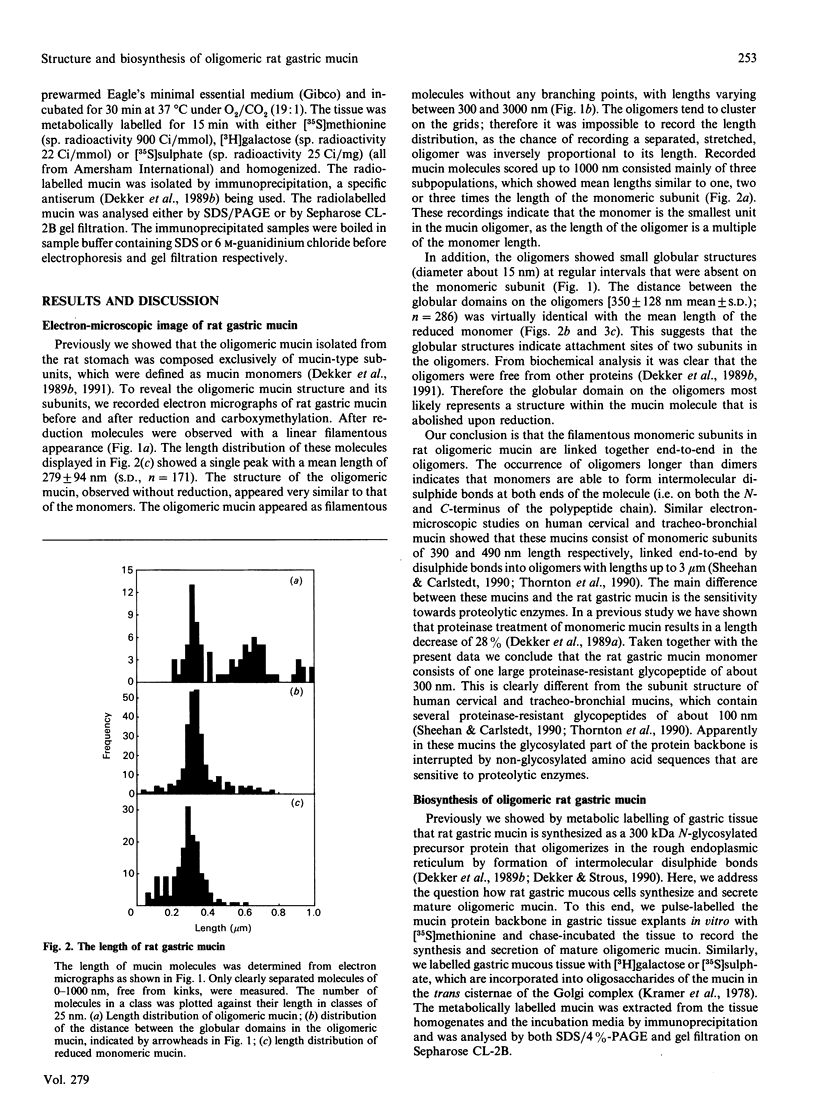
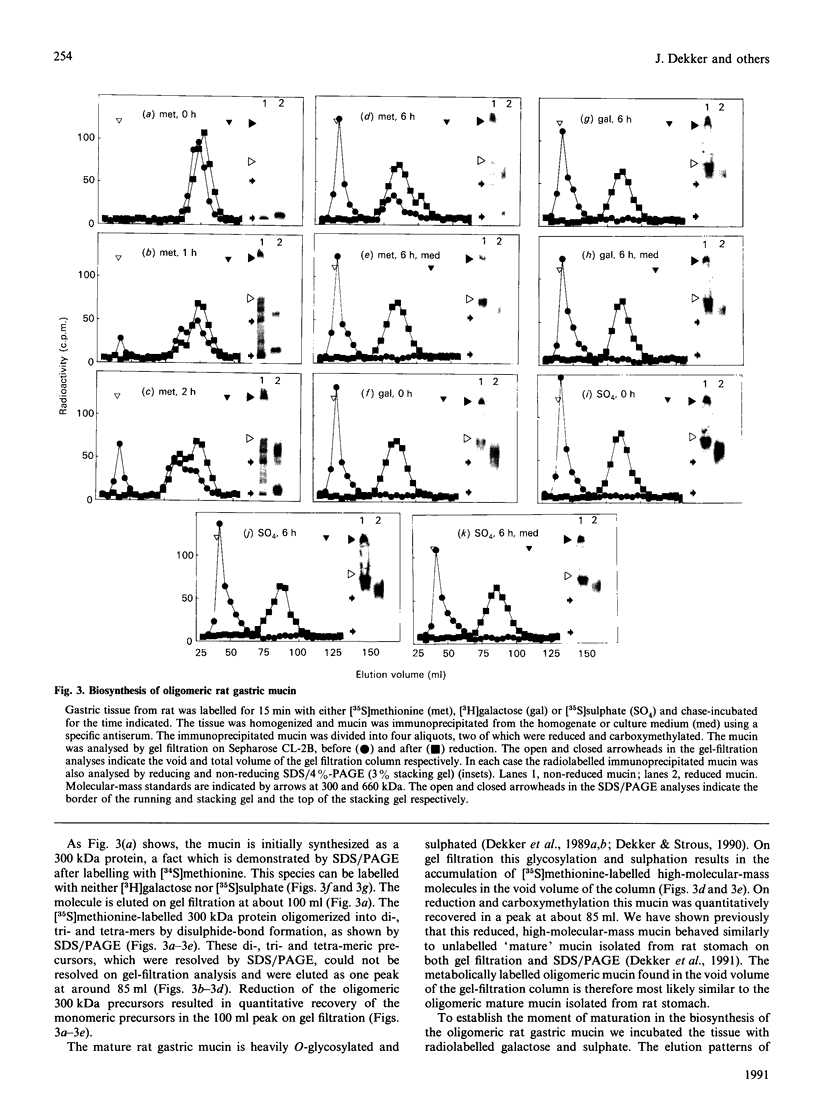
Oligomeric gastric mucin was isolated from the fundic part of the rat stomach. Previously we have shown by biochemical analysis that this oligomeric mucin consists of disulphide-linked homo-oligomers, which contain no other covalently attached proteins [Dekker, Aelmans & Strous (1991) Biochem. J. 277, 423-427]. Electron-microscopic images of the oligomeric mucin revealed a heterogenous population of long filamentous molecules of 300-3000 nm length. After reduction and carboxymethylation the monomeric mucins displayed a length distribution with a single peak at about 279 nm. Length-distribution analysis of oligomeric molecules with length up to 1000 nm revealed three subpopulations with one, two or three times the length of the monomeric mucin. The oligomers displayed small globular domains of about 15 nm, which were equally spaced along the molecule's length. As the distance between these globular domains was similar to the monomer length, these domains most likely indicate attachment sites of the monomers. These results show that the mucin monomers attached end-to-end in the oligomer. Biosynthesis of the mucin oligomers was studied by labelling of stomach explants in vitro with [35S]methionine, [3H]galactose or [35S]sulphate and subsequent immunoprecipitation of the mucin with a specific antiserum. Analysis by electrophoresis and gel filtration revealed that the oligomerization takes place by formation of disulphide bonds between the 300 kDa mucin precursors. The mucin was exclusively synthesized and secreted as fully glycosylated oligomers, as neither precursor proteins nor monomeric mucin were detected in the culture medium. A model for the biosynthesis of rat gastric mucin is proposed in which the filamentous mucin monomers are linked end-to-end by disulphide bonds.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Bell A., Mantle M., Pearson J. P. The structure and physiology of gastrointestinal mucus. Adv Exp Med Biol. 1982;144:115–133. doi: 10.1007/978-1-4615-9254-9_15. [DOI] [PubMed] [Google Scholar]
- Allen A., Hutton D. A., Leonard A. J., Pearson J. P., Sellers L. A. The role of mucus in the protection of the gastroduodenal mucosa. Scand J Gastroenterol Suppl. 1986;125:71–78. doi: 10.3109/00365528609093820. [DOI] [PubMed] [Google Scholar]
- Allen A., Snary D. The structure and function of gastric mucus. Gut. 1972 Aug;13(8):666–672. doi: 10.1136/gut.13.8.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. Structure of gastrointestinal mucus glycoproteins and the viscous and gel-forming properties of mucus. Br Med Bull. 1978 Jan;34(1):28–33. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K., Ulmsten U., Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. Biochem J. 1983 Apr 1;211(1):13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K. Macromolecular properties and polymeric structure of mucus glycoproteins. Ciba Found Symp. 1984;109:157–172. doi: 10.1002/9780470720905.ch11. [DOI] [PubMed] [Google Scholar]
- Dekker J., Strous G. J. Covalent oligomerization of rat gastric mucin occurs in the rough endoplasmic reticulum, is N-glycosylation-dependent, and precedes initial O-glycosylation. J Biol Chem. 1990 Oct 25;265(30):18116–18122. [PubMed] [Google Scholar]
- Dekker J., Van Beurden-Lamers W. M., Oprins A., Strous G. J. Isolation and structural analysis of rat gastric mucus glycoprotein suggests a homogeneous protein backbone. Biochem J. 1989 Jun 15;260(3):717–723. doi: 10.1042/bj2600717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J., Van Beurden-Lamers W. M., Strous G. J. Biosynthesis of gastric mucus glycoprotein of the rat. J Biol Chem. 1989 Jun 25;264(18):10431–10437. [PubMed] [Google Scholar]
- Harding S. E., Rowe A. J., Creeth J. M. Further evidence for a flexible and highly expanded spheroidal model for mucus glycoproteins in solution. Biochem J. 1983 Mar 1;209(3):893–896. doi: 10.1042/bj2090893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerss S., Allen A., Garner A. A simple method for measuring thickness of the mucus gel layer adherent to rat, frog and human gastric mucosa: influence of feeding, prostaglandin, N-acetylcysteine and other agents. Clin Sci (Lond) 1982 Aug;63(2):187–195. doi: 10.1042/cs0630187. [DOI] [PubMed] [Google Scholar]
- Kramer M. F., Geuze J. J., Strous G. J. Site of synthesis, intracellular transport and secretion of glycoprotein in exocrine cells. Ciba Found Symp. 1978;(54):25–51. doi: 10.1002/9780470720356.ch3. [DOI] [PubMed] [Google Scholar]
- Labhart P., Koller T. Electron microscope specimen preparation of rat liver chromatin by a modified Miller spreading technique. Eur J Cell Biol. 1981 Jun;24(2):309–316. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McQueen S., Hutton D., Allen A., Garner A. Gastric and duodenal surface mucus gel thickness in rat: effects of prostaglandins and damaging agents. Am J Physiol. 1983 Sep;245(3):G388–G393. doi: 10.1152/ajpgi.1983.245.3.G388. [DOI] [PubMed] [Google Scholar]
- Mian N., Pope A. J., Anderson C. E., Kent P. W. Factors influencing the viscous properties of chicken tracheal mucins. Biochim Biophys Acta. 1982 Jul 16;717(1):41–48. doi: 10.1016/0304-4165(82)90377-4. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Mantle M., Fahim R. E., Specian R. D., Bennick A., Kawagishi S., Sherman P., Forstner J. F. The putative 'link' glycopeptide associated with mucus glycoproteins. Composition and properties of preparations from the gastrointestinal tracts of several mammals. Biochem J. 1989 Jul 15;261(2):637–647. doi: 10.1042/bj2610637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. C., Voter W. A., Sage H., Brown C. F., Kaufman B. Effects of deglycosylation on the architecture of ovine submaxillary mucin glycoprotein. J Biol Chem. 1984 Mar 10;259(5):3167–3172. [PubMed] [Google Scholar]
- Roussel P., Lamblin G., Lhermitte M., Houdret N., Lafitte J. J., Perini J. M., Klein A., Scharfman A. The complexity of mucins. Biochimie. 1988 Nov;70(11):1471–1482. doi: 10.1016/0300-9084(88)90284-2. [DOI] [PubMed] [Google Scholar]
- Sellers L. A., Allen A., Morris E. R., Ross-Murphy S. B. Mucus glycoprotein gels. Role of glycoprotein polymeric structure and carbohydrate side-chains in gel-formation. Carbohydr Res. 1988 Jul 15;178:93–110. doi: 10.1016/0008-6215(88)80104-6. [DOI] [PubMed] [Google Scholar]
- Sheehan J. K., Carlstedt I. Electron microscopy of cervical-mucus glycoproteins and fragments therefrom. The use of colloidal gold to make visible 'naked' protein regions. Biochem J. 1990 Jan 1;265(1):169–177. doi: 10.1042/bj2650169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan J. K., Oates K., Carlstedt I. Electron microscopy of cervical, gastric and bronchial mucus glycoproteins. Biochem J. 1986 Oct 1;239(1):147–153. doi: 10.1042/bj2390147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayter H. S., Lamblin G., Le Treut A., Galabert C., Houdret N., Degand P., Roussel P. Complex structure of human bronchial mucus glycoprotein. Eur J Biochem. 1984 Jul 16;142(2):209–218. doi: 10.1111/j.1432-1033.1984.tb08273.x. [DOI] [PubMed] [Google Scholar]
- Thornton D. J., Davies J. R., Kraayenbrink M., Richardson P. S., Sheehan J. K., Carlstedt I. Mucus glycoproteins from 'normal' human tracheobronchial secretion. Biochem J. 1990 Jan 1;265(1):179–186. doi: 10.1042/bj2650179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdugo P., Tam P. Y., Butler J. Conformational structure of respiratory mucus studied by laser correlation spectroscopy. Biorheology. 1983;20(2):223–230. doi: 10.3233/bir-1983-20212. [DOI] [PubMed] [Google Scholar]
- Vollenweider H. J., Koller T. Physical mapping of Qbeta replicase binding sites on Qbeta RNA. J Mol Biol. 1976 Mar 5;101(3):367–377. doi: 10.1016/0022-2836(76)90153-4. [DOI] [PubMed] [Google Scholar]