Abstract
Background
Endometriosis is a complex disorder with genetic, immune, inflammatory, and multifactorial etiologies. Zinc, an essential trace element, plays a crucial role in various physiological processes. Dysregulation or deficiency of zinc can lead to aberrations in human physiology. However, the association between dietary zinc and endometriosis remains ambiguous. This study aimed to investigate the link between dietary zinc intake and endometriosis.
Methods
Utilizing cross-sectional data from the National Health and Nutrition Examination Survey, we analyzed information from American women aged 20–54 years between 1999 and 2006. After adjusting for relevant covariates, multivariable logistic regression analysis was employed to assess correlations.
Results
A total of 4315 women were included in the study. The multivariable logistic regression model revealed a positive correlation between dietary zinc intake and the risk of endometriosis, even after controlling for confounding variables. Relative to individuals with lower zinc consumption (≤ 8 mg/day), the adjusted odds ratio (OR) values for dietary zinc intake and endometriosis in the 8–14 mg/day and > 14 mg/day groups were 1.19 (95% CI: 0.92–1.54, p = 0.189) and 1.60 (95% CI: 1.12–2.27, p = 0.009), respectively.
Conclusions
Our findings suggest a positive correlation between dietary zinc intake and the prevalence of endometriosis. However, further investigations are necessary to better understand this association and explore the potential role of dietary zinc in endometriosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-20433-9.
Keywords: Endometriosis, Zinc, NHANES, Cross-sectional study
Introduction
Endometriosis, a prevalent inflammatory condition, is characterized by the presence of tissue resembling endometrial tissue outside the uterus on organs and tissues within the pelvic region. Globally affecting 176 million women, 5–10% of fertile women experience pelvic pain and infertility due to this condition [1]. Endometriosis is 40–60% more common in women with dysmenorrhea, 21–47% more common in women with subfertility, and 71–87% more common in women with pelvic pain [2]. Notably, women with endometriosis incur healthcare costs more than twice as high as those without the condition [3]. I Its widespread prevalence has led to its classification as a public health issue [4]. Despite the significant impact of endometriosis on healthcare costs and quality of life [5], little is known about modifiable risk factors preventing its occurrence.
Zinc, an essential trace element vital for normal bodily functions, plays a crucial role in reproductive potential. Any malfunction or deficiency in zinc can lead to abnormalities in the human body [6]. Since the human body cannot store zinc, maintaining adequate levels through dietary intake is essential, especially for individuals of reproductive age. Based on dietary surveys conducted in nine countries within the European Union (EU) [7], the average daily intake of zinc varied between 4.6 and 6.2 mg for children under three years old, 5.5 to 9.3 mg for children aged three to under ten years old, 6.8 to 14.5 mg for adolescents aged ten to under eighteen years old, and 8.0 to 14.0 mg for adults. Meat and meat products, grains and goods made from them, and milk and dairy products were the main sources of zinc intake. A broad range of dietary phytate intakes was suggested by the scant data on phytate intake in the EU. A zinc shortage can lead to changes in immune cell counts and function, which raises the risk of cancer, autoimmune illnesses, and infectious diseases [8, 9]. Excessive zinc consumption may exert an immunosuppressive effect, promoting pathogen multiplication [10].
Given zinc’s essential role in overall health, its specific involvement in reproductive function and potential impact on conditions such as endometriosis deserve further exploration. Zinc supports key reproductive processes by facilitating DNA synthesis, cell division, and gene expression [11]. Zinc deficiency can lead to hormonal imbalances, impacting gonadal development and ovarian function [12]. In endometriosis, zinc modulates immune responses and reduces oxidative stress, both key factors in the disease's progression [12]. Additionally, zinc regulates matrix metalloproteinases (MMPs), enzymes involved in tissue remodeling, which may affect the invasiveness of endometriotic lesions [13].
Unlike the well-studied effects of zinc on the male reproductive system, limited investigations have been conducted on its effects on the female reproductive system [14, 15]. While most studies have focused on zinc's role and supplementation during pregnancy and fetal development [16, 17], only a few clinical studies suggest decreased serum zinc levels in women with endometriosis [18]. However, these studies, with their low sample numbers, have limitations, and the impact of factors such as smoking, obesity, and race/ethnicity remains unknown. This cross-sectional study aims to explore this connection among American women aged 20 to 54, utilizing a substantial sample size (4315 participants) to address these information gaps. Specifically, we hypothesize that higher dietary zinc intake is associated with a lower risk of endometriosis, given zinc’s role in modulating immune responses, reducing oxidative stress, and regulating matrix metalloproteinases (MMPs), which are involved in tissue remodeling. Our findings aim to provide new insights into endometriosis prevention strategies in the United States.
Methods
Data source
The National Center for Health Statistics (NCHS) and the National Health and Nutrition Examination Survey (NHANES) played crucial roles in data collection for this study. Data were gathered from four consecutive 2-year NHANES cycles conducted between 1999 and 2006, utilizing a nationally representative stratified sample through interviews and physical exams. Ethical approval was obtained from the NCHS Ethics Review Committee, and participants provided written informed consent.
Participants and definition
Endometriosis was assessed based on participants' responses to a specific question in the reproductive health questionnaire: "Has a doctor or other health professional ever told you that you had endometriosis?" Those responding affirmatively were classified as patients. Dietary zinc consumption was evaluated in the NHANES dietary survey, part of the "What We Eat in America" survey, conducted at the Mobile Examination Center (MEC) using a 24-h recall completed by professional interviewers. The NHANES computer-assisted dietary interview (CADI) system documented participants' food and beverage intake 24 h before the interview.
Participants were randomized to data collection sessions in the morning or afternoon/evening, following the study protocol. The US Department of Agriculture Survey Nutrients Database and the University of Texas Food Intake Analysis System determined dietary zinc and nutrient consumption. Nutritional calculations excluded pharmaceuticals or dietary supplements. Two 24-h dietary recall interviews were conducted, and a second interview was completed 3–10 days later over the phone. The first interview, conducted in person at the MEC, was selected for analysis, considering that 24-h recall is the most commonly used method in large-scale surveys [19].
Measurements
Covariates considered in our study encompassed a wide range of variables from the literature [20–22], including age, marital status, race/ethnicity, education level, family income, smoking status, physical activity, BMI, calorie intake, and use of dietary supplements. Racial and ethnic categories included non-Hispanic white, non-Hispanic black, Mexican American, and other races. Marital status categories were living with a partner or living alone. Educational achievement was stratified into fewer than nine years, nine to twelve years, and more than twelve years. Family income, assessed using the Poverty Income Ratio (PIR), categorized income into low, medium, and high based on ranges from 1.3 to 3.5, according to the US government's Agriculture report [23]. Smoking status was dichotomized into smokers and never smokers (those who smoked fewer than 100 cigarettes each). Physical activity levels were segmented into three categories: unable to perform physical activity, moderate (defined as at least 10 min of movement within the previous 30 days resulting in only light perspiration or a mild to moderate increase in respiration or heart rate), and vigorous (at least 10 min of activity within the last 30 days resulting in profuse sweating or an increased heart rate).
Prior to the MEC interview, participants underwent a dietary recall interview to obtain 24-h nutritional data, including calorie intake and macronutrients. Information on medications, including dietary supplements, taken in the previous month was collected.
Statistical analyses
This study, a secondary analysis of freely available datasets, used descriptive statistics to characterize continuous variables (mean/SD or median/IQR) and proportions (%) for categorical variables. Group differences were assessed using Kruskal–Wallis tests and one-way analyses of variance. Logistic regression models explored the relationship between dietary zinc intake and endometriosis in three models. Model 1 was a crude model, Model 2 adjusted for age, race/ethnicity, education level, smoking status, and dietary supplements taken, and Model 3 adjusted for Model 2 plus vigorous activity, moderate activity, marital status, family income, and BMI. These models aimed to comprehensively account for potential confounding factors and refine the understanding of the relationship between Dietary Zinc Intake and endometriosis.
Moreover, potential modifiers of the association between dietary zinc intake and endometriosis were investigated, incorporating variables such as family income (low vs. medium or high), marital status (living with a partner vs. living alone), smoking status, and dietary supplements taken. Multivariate logistic regression assessed heterogeneity among subgroups, and interactions between subgroups and dietary zinc intake were scrutinized through likelihood ratio testing. To ensure the robustness of our findings, sensitivity analyses were conducted by excluding participants with extreme energy intake, defined as those consuming less than 500 or more than 5000 kcal per day. This meticulous approach aimed to evaluate the consistency and reliability of our results under different conditions, enhancing the validity of the study outcomes.
Sample size determination relied on available data, and no a priori statistical power assessments were carried out. Statistical analyses were performed using R 3.3.2, a statistical software program developed by The R Foundation, Shanghai, China (accessed on 10 January 2023), along with Free Statistics Software 1.5 [24]. A descriptive study was conducted on all individuals, characterizing the dataset comprehensively. For hypothesis testing, a two-tailed analysis was employed, and a significance level of 0.05 was considered for determining statistical significance. This widely accepted threshold is indicative of a standard level of confidence in interpreting the results and drawing meaningful conclusions from the conducted analyses.
Results
Study population
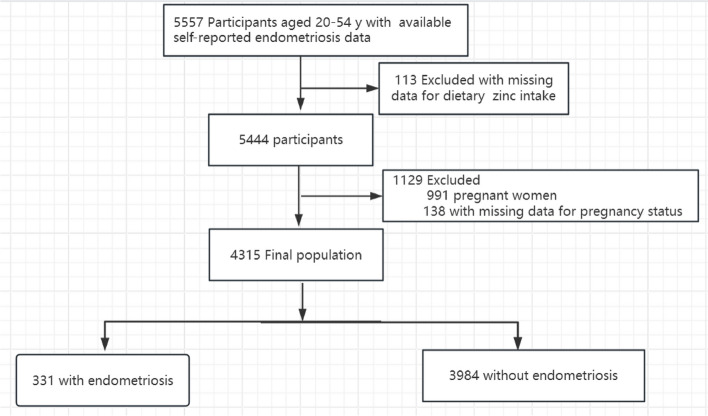
The original dataset comprised 5557 female participants. Following the exclusion of individuals with missing endometriosis-related information and those falling outside the age range of 20 to 54 years, our final study population consisted of 4315 women. Among them, 331 were diagnosed with endometriosis, while 3984 were not. Exclusions were applied to 991 pregnant women, 138 with missing pregnancy status data, and 113 lacking information on Dietary Zinc Intake. Figure 1 provides a detailed illustration of the inclusion and exclusion process.
Fig. 1.
The study’s flow diagram
Baseline characteristics
Table 1 outlines the fundamental characteristics of the 4315 participants in the study. Among the sample, 331 individuals (7.7%) received a diagnosis of endometriosis, and 2742 (64%) were categorized as overweight. A total of 1699 participants (39.4%) reported being smokers, while 2030 (47.1%) acknowledged the use of dietary supplements. Regarding age distribution, 2298 participants (53.3%) were below 40 years old, 1403 (32.5%) were between 40 and 50 years old, and 614 (14.2%) were above 50 years old. Notably, dietary zinc intake seemed to be higher in individuals who used dietary supplements, engaged in moderate exercise, and lived with a partner or had a higher income. These baseline characteristics offer an overview of the diversity within the study population and establish the foundation for subsequent analyses exploring the association between these factors and endometriosis.
Table 1.
Population characteristics by categories of dietary zinc intake
| Variables | Zinc Intake, mg/d | ||||
|---|---|---|---|---|---|
| Total | < 8 | 8–14 | > 14 | p | |
| 4315 | 1864 | 1652 | 799 | ||
| Age(year), n (%) | 0.022 | ||||
| < 40 | 2298 (53.3) | 942 (50.5) | 907 (54.9) | 449 (56.2) | |
| 40–50 | 1403 (32.5) | 630 (33.8) | 524 (31.7) | 249 (31.2) | |
| > 50 | 614 (14.2) | 292 (15.7) | 221 (13.4) | 101 (12.6) | |
| BMI(Kg/m2), n (%) | 0.004 | ||||
| < 25 | 1544 (36.0) | 625 (33.9) | 633 (38.5) | 286 (36) | |
| 25–30 | 1153 (26.9) | 545 (29.5) | 415 (25.2) | 193 (24.3) | |
| > 30 | 1589 (37.1) | 676 (36.6) | 597 (36.3) | 316 (39.7) | |
| Race/Ethnicity, n (%) | < 0.001 | ||||
| Mexican American | 973 (22.5) | 410 (22) | 396 (24) | 167 (20.9) | |
| Other Race | 381 ( 8.8) | 173 (9.3) | 153 (9.3) | 55 (6.9) | |
| Non-Hispanic White | 2001 (46.4) | 812 (43.6) | 793 (48) | 396 (49.6) | |
| Non-Hispanic Black | 960 (22.2) | 469 (25.2) | 310 (18.8) | 181 (22.7) | |
| Family Income, n (%) | 0.008 | ||||
| Low | 1134 (28.1) | 530 (30.5) | 406 (26.1) | 198 (26.4) | |
| Medium | 1475 (36.5) | 637 (36.7) | 577 (37.1) | 261 (34.8) | |
| High | 1433 (35.5) | 570 (32.8) | 572 (36.8) | 291 (38.8) | |
| Education Level(year), n (%) | 0.002 | ||||
| < 9 | 369 ( 8.6) | 179 (9.6) | 141 (8.5) | 49 (6.1) | |
| 9–12 | 1647 (38.2) | 748 (40.2) | 601 (36.4) | 298 (37.3) | |
| > 12 | 2295 (53.2) | 935 (50.2) | 909 (55.1) | 451 (56.5) | |
| Marital Status, n (%) | 0.031 | ||||
| Living with a partner | 2531 (60.2) | 1046 (58) | 992 (61.4) | 493 (62.8) | |
| Living alone | 1675 (39.8) | 759 (42) | 624 (38.6) | 292 (37.2) | |
| Smoking status, n (%) | 0.958 | ||||
| Yes | 1699 (39.4) | 736 (39.5) | 648 (39.2) | 315 (39.4) | |
| No | 2613 (60.6) | 1127 (60.5) | 1003 (60.7) | 483 (60.5) | |
| Vigorous activity, n (%) | 0.018 | ||||
| Yes | 1470 (34.1) | 597 (32) | 582 (35.2) | 291 (36.4) | |
| No | 2761 (64.0) | 1222 (65.6) | 1049 (63.5) | 490 (61.3) | |
| Unable to do activity | 82 ( 1.9) | 44 (2.4) | 20 (1.2) | 18 (2.3) | |
| Moderate activity, n (%) | < 0.001 | ||||
| Yes | 2257 (52.3) | 919 (49.3) | 872 (52.8) | 466 (58.3) | |
| No | 1995 (46.2) | 908 (48.7) | 767 (46.4) | 320 (40.1) | |
| Unable to do activity | 61 (1.4) | 36 (1.9) | 12 (0.7) | 13 (1.6) | |
| Dietary supplements taken, n (%) | < 0.001 | ||||
| Yes | 2030 (47.1) | 803 (43.1) | 795 (48.1) | 432 (54.1) | |
| No | 2281 (52.9) | 1059 (56.8) | 855 (51.8) | 367 (45.9) | |
Relationship between Dietary Zinc Intake and endometriosis
Table 2 presents the relationships between dietary zinc intake and endometriosis. The univariate analysis revealed significant associations, indicating that dietary zinc intake, age, race, family income, education level, smoking status, and the use of dietary supplements were correlated with the presence of endometriosis. These findings underscore a complex interplay between dietary factors, demographic variables, and lifestyle choices influencing the occurrence of endometriosis. Subsequent multivariate analyses will further dissect these associations to elucidate the independent contributions of each factor to the risk of endometriosis.
Table 2.
Association of covariates and endometriosis risk
| Variable | OR_95CI | P_value | Variable | OR_95CI | P_value |
|---|---|---|---|---|---|
| zinc | 1.03 (1.01 ~ 1.05) | 0.008 | BMI(Kg/m2), n (%) | ||
| Age(year), n (%) | < 25 | 1 (reference) | |||
| < 40 | 1 (reference) | 25–30 | 0.97 (0.73 ~ 1.28) | 0.828 | |
| 40–50 | 0.55 (0.43 ~ 0.7) | < 0.001 | > 30 | 1.08 (0.83 ~ 1.41) | 0.57 |
| > 50 | 0.53 (0.39 ~ 0.73) | < 0.001 | Smoking status, n (%) | ||
| Race/Ethnicity, n (%) | Yes | 1 (reference) | |||
| Mexican American | 1 (reference) | No | 1.61 (1.28 ~ 2.01) | < 0.001 | |
| Other Race | 0.61 (0.33 ~ 1.13) | 0.119 | Vigorous activity, n (%) | ||
| Non-Hispanic White | 0.23 (0.15 ~ 0.34) | < 0.001 | Yes | 1 (reference) | |
| Non-Hispanic Black | 0.41 (0.26 ~ 0.66) | < 0.001 | No | 0.96 (0.76 ~ 1.22) | 0.758 |
| Education Level(year), n (%) | Unable to do activity | 0.58 (0.29 ~ 1.15) | 0.118 | ||
| < 9 | 1 (reference) | Moderate activity, n (%) | |||
| 9–12 | 0.19 (0.08 ~ 0.44) | < 0.001 | Yes | 1 (reference) | |
| > 12 | 0.18 (0.08 ~ 0.4) | < 0.001 | No | 1.16 (0.92 ~ 1.46) | 0.198 |
| Marital Status, n (%) | Unable to do activity | 0.51 (0.25 ~ 1.04) | 0.066 | ||
| Living with a partner | 1 (reference) | Dietary supplements taken, n (%) | |||
| Living alone | 1.25 (0.98 ~ 1.58) | 0.067 | Yes | 1 (reference) | |
| Family Income, n (%) | No | 1.57 (1.25 ~ 1.97) | < 0.001 | ||
| Low | 1 (reference) | ||||
| Medium | 0.77 (0.56 ~ 1.06) | 0.108 | |||
| High | 0.5 (0.37 ~ 0.68) | < 0.001 | |||
In comparison to individuals with lower zinc consumption (≤ 8 mg/day), and with adjustments for age, race, education level, smoking status, dietary supplements taken, vigorous activity, moderate activity, marital status, family income, and BMI, the adjusted odds ratio (OR) values for dietary zinc intake and endometriosis in the 8–14 mg/day and > 14 mg/day groups were 1.19 (95% CI: 0.92–1.54, p = 0.189) and 1.60 (95% CI: 1.12–2.27, p = 0.009), respectively (Table 3).
Table 3.
Association between dietary zinc intake and endometriosis
| Variable | OR (95%CI) | ||||||
|---|---|---|---|---|---|---|---|
| Dietary zinc intake | total | Model1 | P_value | Model2 | P_value | Model3 | P_value |
| (mg/day) | |||||||
| < 8 | 1864 | 1(Ref) | 1(Ref) | 1(Ref) | |||
| 8–14 | 1652 | 1.08 (0.85 ~ 1.38) | 0.537 | 1.1 (0.86 ~ 1.41) | 0.455 | 1.19 (0.92 ~ 1.54) | 0.189 |
| > 14 | 799 | 1.42 (1.02 ~ 1.98) | 0.041 | 1.51 (1.07 ~ 2.12) | 0.019 | 1.6 (1.12 ~ 2.27) | 0.009 |
| Trend test | 0.053 | 0.025 | 0.008 |
Model 1 was a crude model. Model 2 was adjusted for age, race/ethnicity, education level, smoking status, dietary supplements taken. Model 3 was adjusted for model 2 plus vigorous activity, moderate activity, marital status, family income and BMI
OR Odds ratio, CI Confidence interval, Ref Reference, BMI Body Mass Index
Stratified analyses based on additional variables
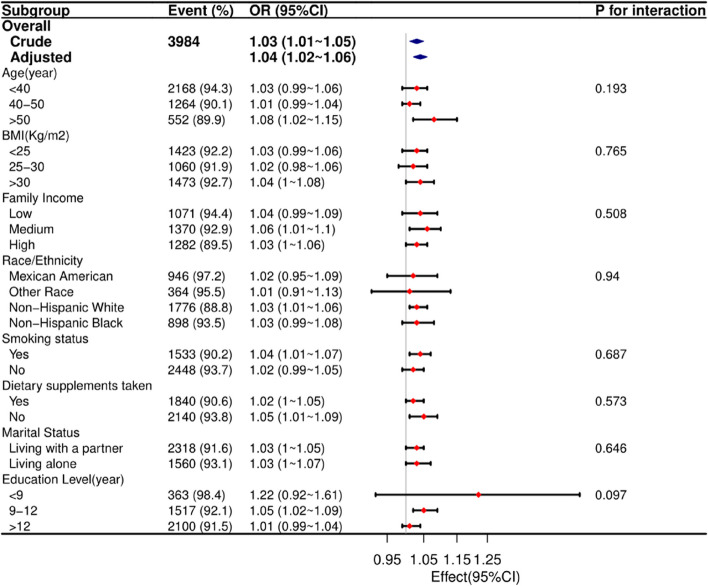
In a meticulous examination of various subgroups, stratified analyses were conducted to assess potential effect modifications on the relationship between dietary zinc intake and endometriosis (refer to Fig. 2). Notably, no significant interactions were identified in any subgroups, whether stratified by age, race, BMI, marital status, family income, smoking status, education level, or dietary supplements taken. These results suggest that the observed association between dietary zinc intake and endometriosis holds consistently across diverse demographic and lifestyle factors, reinforcing the robustness of the findings.
Fig. 2.
The relationship between dietary zinc intake and endometriosis according to basic features. Except for the stratification component itself, each stratification factor was adjusted for all other variables (age, race/ethnicity, education level, smoking status, dietary supplements taken, vigorous activity, moderate activity, marital status, family income and BMI)
Sensitivity analysis
Furthermore, upon excluding individuals with extreme energy intake, the dataset comprised 4237 individuals, and the association between dietary zinc intake and endometriosis remained robust. In comparison to individuals with lower zinc consumption (≤ 8 mg/day), and after adjustments for age, race, education level, smoking status, dietary supplements taken, vigorous activity, moderate activity, marital status, family income, and BMI, the adjusted odds ratio (OR) values for dietary zinc intake and endometriosis in the 8–14 mg/day and > 14 mg/day groups were 1.22 (95% CI: 0.94–1.59, p = 0.128) and 1.61 (95% CI: 1.13–2.29, p = 0.009), respectively. This reinforces the reliability of the observed relationship, demonstrating its resilience to variations in energy intake (Supplementary Table S1).
Discussion
In this extensive cross-sectional study involving American adults, a noteworthy and previously unexplored positive correlation between endometriosis and dietary zinc intake was identified. Endometriosis, characterized by inflammation and altered immune regulation, has immune dysregulation as a central element in its development. Previous studies have delineated the existence of a robust immunosuppressive microenvironment in endometriosis [25]. While zinc's roles as an antioxidant, anti-inflammatory agent, and immune regulator suggest it may have a potential influence on endometriosis, further studies are required to confirm this relationship. Specifically, zinc has been reported to exert a pronounced impact on the immune system, primarily through its influence on T lymphocytes. Even minor fluctuations in serum zinc concentration have been shown to correspondingly affect T cell levels [26]. This novel finding sheds light on a potential avenue for understanding and managing endometriosis, emphasizing the intricate interplay between dietary factors, immune responses, and the pathogenesis of this complex inflammatory condition.
To date, a limited number of studies have explored the association between zinc and endometriosis, with varying results. Messalli et al. [27] reported significantly lower serum zinc levels in women with endometriosis compared to controls (1,010 ± 59.24 µg/L vs. 1,294 ± 62.22 µg/L). The authors suggested that zinc deficiency may contribute to endometriosis by impairing immune regulation and increasing oxidative stress. This highlights zinc’s critical role in immune function and antioxidative defense in relation to the disease. Another cross-sectional study [28] involving 190 Asian women found an inverse association between zinc blood levels and endometriosis, suggesting that lower zinc concentrations may contribute to the disease's pathophysiology. This aligns with prior research indicating that zinc plays a crucial role in reducing oxidative stress and regulating immune responses, both of which are critical in endometriosis development [27, 29]. Moreover, a study by Mier-Cabrera et al. highlighted the role of antioxidants, including zinc, in improving oxidative stress markers in women with endometriosis [29]. The positive impact of dietary antioxidants in managing oxidative stress adds weight to the hypothesis that zinc deficiency may exacerbate endometriosis. The study by Messalli et al. [27] further supports this by exploring zinc’s involvement in modulating immune function and oxidative stress in endometriosis. Furthermore, Singh et al. [30] found that women with endometriosis had lower zinc levels in their follicular fluid compared to those with tubal infertility, linking zinc deficiency to poorer oocyte quality. Interestingly, after successful IVF, zinc levels increased in the follicular fluid of endometriosis patients, suggesting a potential role of zinc in embryo implantation. Meanwhile, Pollack et al. [31] found no significant link between urinary zinc levels and endometriosis, but emphasized the need for further research on zinc's role in reproductive health.
In contrast to these prior findings, our present study drew a distinct conclusion, establishing a positive correlation between dietary zinc intake and the occurrence of endometriosis. Differences in the absorption and metabolism of zinc between blood and urine may be the cause of this discrepancy. A Type 2 statistical mistake that may have resulted from small sample numbers in earlier demographic cohorts is another reasonable explanation. The present study, being a nationwide cohort in the United States, offers a unique opportunity to assess the dose–response relationship of dietary zinc intake and endometriosis. The comprehensive adjustments for numerous covariables, coupled with a range of subgroup and sensitivity analyses, contribute to the strength and reliability of our findings.
While the precise mechanisms underlying the inverse association between zinc intake and endometriosis remain subjects of ongoing investigation, our findings are biologically plausible, supported by existing evidence. Zinc, an abundant element in enzymes, plays a pivotal role at every stage of the cell cycle. Zinc is essential for a variety of processes, including replication and repair, as it functions as a cofactor for enzymes including thymidine kinase, reverse transcriptase, and DNA and RNA polymerase. Zinc also plays a key part in the apoptotic process of programmed cell death because it is a cofactor for the superoxide dismutase (SOD) enzyme, which is essential for regulating oxidative stress in cells. Additionally, zinc contributes to maintaining the appropriate neurodegenerative equilibrium of the extracellular matrix, and the functionality of matrix metalloproteinases (MMPs) is dependent on it.
Increased proliferation, invasion, internal bleeding, and fibrosis are symptomatic features of endometriosis, a hormone-influenced disorder that impacts the stroma and glands outside the uterus. The progression from early to advanced stages involves intricate processes such as estrogen regulation, matrix remodeling, inflammation, and oxidative stress [32]. MMPs, particularly MMP-2 and MMP-9, are deemed crucial for the advancement of invasion, and zinc, serving as an MMP inhibitor, emerges as a significant factor in endometriosis. Studies have indicated that women diagnosed with endometriosis exhibit elevated levels of MMP-2 [33]and MMP-9 [34] compared to controls, pointing to heightened MMP activity in this invasive condition. Investigations further reveal increased MMP levels, notably MMP2 and MMP9, in ectopic tissue, peritoneal fluid, or sera of individuals with endometriosis [35, 36]. A study by Borghese et al. (2008) compared the gene expression profiles of eutopic and ectopic endometrium, identifying overexpression of extracellular matrix genes, including MMP23 and MMP26, with notable expression variations [37]. Recent evidence [38] underscores the essential role of MMPs in the processes underlying the development and management of endometriosis. By breaking down the non-extracellular matrix (ECM), MMPs contribute to the release of several components, creating a vicious cycle that accelerates endometriosis development. MMPs may become integral components in these processes by altering immune cells or autoimmune factors, leading to an imbalance in local microenvironmental immunity that triggers endometriosis onset. Collectively, MMPs play a pivotal role in the progression of endometriosis. While no differences were observed between women with and without endometriosis in a cross-sectional study, women with stage III/IV endometriosis exhibited higher MMP2 expression in their sera than those with stage I/II endometriosis [39], This finding could be attributed to the small sample size (n = 30) of the study. Additionally, Wu et al. identified elevated MMP9 expression in individuals with recurrent ovarian endometriosis [40]. These observations further emphasize the intricate and multifaceted role of MMPs in the progression and severity of endometriosis, warranting continued exploration in larger cohorts for more conclusive insights.
In the context of endometriosis, fibroblastic cells originating from the endometrium have been shown to increase the production of MMPs in response to elevated levels of pro-inflammatory cytokines [41]. Significantly, these cytokines contribute to the modulation of zinc metabolism [42]. Numerous studies have reported heightened ion consumption in the presence of cytokines such as IL-6 and TNF-α [43]. Consequently, the ions become less predisposed to binding and inactivating MMPs. This scenario leads to a substantial activation of these enzymes, facilitating the progression of endometriotic lesions. MMPs play a crucial role by metabolizing the extracellular matrix and facilitating the penetration of endometrial cells [44]. Moreover, the impact of chronic inflammation on the homeostasis of metallothionein, a protein that binds zinc after dietary absorption, is noteworthy [45]. Metallothionein levels tend to increase with age and in the presence of chronic inflammation, leading to continuous zinc sequestration at the intracellular level. This, in turn, results in a subsequent decrease in the availability of zinc to fulfill its enzymatic functions, where it serves as a cofactor [46, 47]. The intricate interplay between inflammatory cytokines, zinc metabolism, and MMP activation underscores the multifaceted mechanisms involved in the progression of endometriosis. These findings further support the biological plausibility of our observed positive correlation between dietary zinc intake and the occurrence of endometriosis.
Conversely, while Zn2 + ions exhibit immunomodulatory properties under normal circumstances [48, 49], there exists a dichotomy where, in certain situations, they can paradoxically promote the proliferation of pathogens. Excessive zinc levels pose potential dangers due to their immunosuppressive effects. In elevated concentrations, zinc may exert immunosuppression by impeding lymphocyte function and interferon-gamma (IFN-γ) production [50–52]. Moreover, it is crucial to acknowledge that the harmful effects of zinc in high doses may be accentuated by the fact that endometriosis is an estrogen-dependent disease. Compelling evidence, derived from both animal and human studies, suggests that metal ions can activate estrogen receptors. These metal ions, classified as a subgroup of xenoestrogens, are referred to as metalloestrogens [53, 54]. The potential role of metalloestrogens as endocrine disruptors adds another layer of complexity to the intricate relationship between zinc, estrogen, and the pathogenesis of endometriosis. This multifaceted interplay warrants further investigation to elucidate the nuanced mechanisms at play in the context of endometriosis development.
Recent studies have highlighted potential molecular mechanisms that could mediate the relationship between dietary zinc intake and endometriosis. One notable pathway involves the kisspeptin system, which is crucial in regulating the hypothalamic–pituitary–gonadal (HPG) axis and influencing inflammatory and metabolic processes. Dysregulation of the kisspeptin system has been linked to reproductive disorders like endometriosis, where altered expression of KISS1 and its receptor (KISS1R) has been observed in both eutopic and ectopic endometrial tissues. Kisspeptin is believed to modulate inflammation and pain responses, key factors in the pathophysiology of endometriosis [55]. Additionally, the system’s involvement in immune responses and metabolic regulation further underscores its potential role as a mediator between zinc intake and endometriosis development.
While our results suggest a positive correlation between higher dietary zinc intake and lower endometriosis risk, some findings did not reach statistical significance. For instance, the association between zinc intake and endometriosis risk in the middle intake group (8–14 mg/day) was not significant (p = 0.189). This indicates that the protective effect of zinc may not be consistent across all intake levels, suggesting the need for further research on potential threshold effects.
Additionally, variables such as smoking status and physical activity did not significantly interact with zinc intake in influencing endometriosis risk. These non-significant findings highlight the complexity of endometriosis etiology and suggest that zinc, while important, may interact with other factors. Including these results provides a more complete view of the study's outcomes and underscores the importance of investigating other modifiable factors in future research.
Although our study offers insightful information, there are a few important limitations to be aware of. Firstly, we cannot conclusively demonstrate causation or directionality due to the cross-sectional design. Despite meticulous adjustments in the logistic regression model for potential confounders, the presence of unmeasured variables may still introduce confounding factors. Notably, the inclusion of certain dietary factors in our model aimed to mitigate potential confounding effects. Secondly, the challenge of accurately measuring total body zinc status persists. Our reliance on dietary interviews and 24-h recall for zinc intake introduces inherent limitations. Self-reported dietary data, susceptible to recall bias, may not offer precise measurements of total zinc status. Future studies incorporating more sophisticated methodologies for evaluating zinc status could enhance the accuracy of these assessments. Thirdly, the study's exclusive focus on US residents necessitates caution when extrapolating findings to other populations. The unique demographic and lifestyle factors inherent to the US may limit the generalizability of our results. Consequently, conducting well-designed multicenter controlled trials across diverse populations is imperative to validate and extend the implications of our findings. Finally, our study also lacks data on the subtypes of endometriosis and any associated comorbidities, which is a notable limitation. Recent studies have shown that women with different endometriosis subtypes and comorbid conditions may have unique genetic susceptibilities that influence their disease presentation and progression [56]. Understanding these variations is crucial for future research. In conclusion, while our study sheds light on the intricate relationship between dietary zinc intake and endometriosis, the identified limitations underscore the need for further research to deepen our understanding of this association and its broader implications.
Conclusions
In summary, our findings unveil a positive association between dietary zinc intake and the prevalence of endometriosis among adults in the United States. This study highlights a potential association between dietary zinc intake and endometriosis, but further research is necessary to confirm whether dietary zinc plays a modifiable role in endometriosis prevalence. To elucidate the underlying processes and explore potential ramifications for women's health preventive measures and therapies, further research is imperative.
Supplementary Information
Acknowledgements
Acknowledgments The authors express their gratitude to the "Clinical Scientist" team from the public platform for their valuable assistance in the course of this research. The collaborative efforts and insights provided by the team have significantly contributed to the refinement and enhancement of this study.
Institutional review board statement
Since the secondary analysis did not require extra institutional review board approval, ethical review and approval were waived for this investigation.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- EU
European Union
- NCHS
National Center for Health Statistics
- MEC
Mobile Examination Center
- CADI
Computer-assisted dietary interview
- PIR
Poverty Income Ratio
- SOD
Superoxide dismutase
- MMPs
Matrix metalloproteinases
- ECM
Extracellular matrix
Authors’ contributions
Conceptualization, Y.H. and B.X.; Data curation, Y.H.; Formal analysis, Y.W.; Methodology, Y.W., Y.H., J.H. and X.L.; Writing—original draft, Y.H.,Y.W. and F.L.; Writing—review and editing, B.X., Y.H., J.H, X.L. and F.L. All authors have read and agreed to the published version of the manuscript.
Funding
None.
Data availability
The study's publicly available datasets can be found online. Online at http://www.cdc.gov/nchs/nhanes.htm, you can find the name of the repository or repositories as well as their accession numbers (accessed on October 8, 2023).
Declarations
Ethics approval and consent to participate
All participants completed written informed consent forms before to participation in the NHANES, which was approved by the National Center for Health Statistics (NCHS) Ethics Review Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yingmei Huang, and Yumei Wei contributed equally to this work.
References
- 1.Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis Nat Rev Dis Primers. 2018;4(1):9. [DOI] [PubMed] [Google Scholar]
- 2.Giampaolino P, Della Corte L, Foreste V, Barra F, Ferrero S, Bifulco G. Dioxin and endometriosis: a new possible relation based on epigenetic theory. Gynecol Endocrinol. 2020;36(4):279–84. [DOI] [PubMed] [Google Scholar]
- 3.Soliman AM, Surrey ES, Bonafede M, Nelson JK, Vora JB, Agarwal SK: Health Care Utilization and Costs Associated with Endometriosis Among Women with Medicaid Insurance. (2376–1032 (Electronic)). [DOI] [PMC free article] [PubMed]
- 4.Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, Singh SS, Taylor HS: Clinical diagnosis of endometriosis: a call to action. Am J Obstet Gynecol 2019, 220(4):354 e351–354 e312. [DOI] [PubMed]
- 5.Simoens S, Hummelshoj L, Dunselman G, Brandes I, Dirksen C, D’Hooghe T, EndoCost C. Endometriosis cost assessment (the EndoCost study): a cost-of-illness study protocol. Gynecol Obstet Invest. 2011;71(3):170–6. [DOI] [PubMed] [Google Scholar]
- 6.Fallah A, Mohammad-Hasani A, Colagar AH: Zinc is an Essential Element for Male Fertility: A Review of Zn Roles in Men's Health, Germination, Sperm Quality, and Fertilization. (2228–5482 (Print)). [PMC free article] [PubMed]
- 7.Scientific Opinion on Dietary Reference Values for zinc. EFSA Journal 2014, 12(10).
- 8.Wessels I, Rink L. Micronutrients in autoimmune diseases: possible therapeutic benefits of zinc and vitamin D. J Nutr Biochem. 2020;77: 108240. [DOI] [PubMed] [Google Scholar]
- 9.Haase H, Schomburg L. You’d Better Zinc-Trace Element Homeostasis in Infection and Inflammation. Nutrients. 2019;11(9):2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skrajnowska D, Bobrowska-Korczak B: Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11(10). [DOI] [PMC free article] [PubMed]
- 11.Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr. 2013;4(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. 2013;4(2):176–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vickram S, Rohini K, Srinivasan S, Nancy Veenakumari D, Archana K, Anbarasu K, Jeyanthi P, Thanigaivel S, Gulothungan G, Rajendiran N et al: Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth. Int J Mol Sci 2021, 22(4). [DOI] [PMC free article] [PubMed]
- 14.Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update. 2007;13(2):163–74. [DOI] [PubMed] [Google Scholar]
- 15.Cummings JE, Kovacic JP. The ubiquitous role of zinc in health and disease. J Vet Emerg Crit Care (San Antonio). 2009;19(3):215–40. [DOI] [PubMed] [Google Scholar]
- 16.Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, Bhutta ZA: Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev 2015, 2015(2):CD000230. [DOI] [PMC free article] [PubMed]
- 17.Wilson RL, Grieger JA, Bianco-Miotto T, Roberts CT: Association between Maternal Zinc Status, Dietary Zinc Intake and Pregnancy Complications: A Systematic Review. In: Nutrients. vol. 8; 2016. [DOI] [PMC free article] [PubMed]
- 18.Messalli Em Fau - Schettino MT, Schettino Mt Fau - Mainini G, Mainini G Fau - Ercolano S, Ercolano S Fau - Fuschillo G, Fuschillo G Fau - Falcone F, Falcone F Fau - Esposito E, Esposito E Fau - Di Donna MC, Di Donna Mc Fau - De Franciscis P, De Franciscis P Fau - Torella M, Torella M: The possible role of zinc in the etiopathogenesis of endometriosis. (0390–6663 (Print)). [PubMed]
- 19.Foster E, Lee C, Imamura F, Hollidge SE, Westgate KL, Venables MC, Poliakov I, Rowland MK, Osadchiy T, Bradley JC, et al. Validity and reliability of an online self-report 24-h dietary recall method (Intake24): a doubly labelled water study and repeated-measures analysis. J Nutr Sci. 2019;8: e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu HA-O, Wang L, Chen C, Dong ZA-O, Yu S: Association between Dietary Niacin Intake and Migraine among American Adults: National Health and Nutrition Examination Survey. LID - 10.3390/nu14153052 LID - 3052. (2072–6643 (Electronic)). [DOI] [PMC free article] [PubMed]
- 21.Xiao Q, Cai B, Yin A, Huo H, Lan K, Zhou G, Shen L, He B. L-shaped association of serum 25-hydroxyvitamin D concentrations with cardiovascular and all-cause mortality in individuals with osteoarthritis: results from the NHANES database prospective cohort study. BMC Med. 2022;20(1):308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YY, Qiu HB, Tian JW: Association Between Vitamin D and Hyperuricemia Among Adults in the United States. (2296–861X (Print)). [DOI] [PMC free article] [PubMed]
- 23.What We Eat in America: Data Tables [https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/]
- 24.Yang Q, Zheng J, Chen W, Chen X, Wen D, Chen W, Xiong X, Zhang Z. Association Between Preadmission Metformin Use and Outcomes in Intensive Care Unit Patients With Sepsis and Type 2 Diabetes: A Cohort Study. Front Med (Lausanne). 2021;8: 640785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Wang K, Xu Y, Guo P, Hong B, Cao Y, Wei Z, Xue R, Wang C, Jiang H. Alteration of Myeloid-Derived Suppressor Cells, Chronic Inflammatory Cytokines, and Exosomal miRNA Contribute to the Peritoneal Immune Disorder of Patients With Endometriosis. Reprod Sci. 2019;26(8):1130–8. [DOI] [PubMed] [Google Scholar]
- 26.Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14(5–6):353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torella M, Franciscis PD, Donna MCD, Esposito E, Falcone F, Fuschillo G, Ercolano S, Mainini G, Schettino MT, Messalli EM. The possible role of zinc in the etiopathogenesis of endometriosis. Clin Exp Obstet Gynecol. 2014;41(5):541–6. [PubMed] [Google Scholar]
- 28.Lai GL, Yeh CC, Yeh CY, Chen RY, Fu CL, Chen CH, Tzeng CR. Decreased zinc and increased lead blood levels are associated with endometriosis in Asian Women. Reprod Toxicol. 2017;74:77–84. [DOI] [PubMed] [Google Scholar]
- 29.Mier-Cabrera J, Aburto-Soto T, Burrola-Mendez S, Jimenez-Zamudio L, Tolentino MC, Casanueva E, Hernandez-Guerrero C. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod Biol Endocrinol. 2009;7(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh AK, Chattopadhyay R, Chakravarty B, Chaudhury K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod Toxicol. 2013;42:116–24. [DOI] [PubMed] [Google Scholar]
- 31.Pollack AZ, Louis GM, Chen Z, Peterson CM, Sundaram R, Croughan MS, Sun L, Hediger ML, Stanford JB, Varner MW, et al. Trace elements and endometriosis: the ENDO study. Reprod Toxicol. 2013;42:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P: Endometriosis. (2056–676X (Electronic)). [DOI] [PubMed]
- 33.Huang HF, Hong Lh Fau - Tan Y, Tan Y Fau - Sheng J-Z, Sheng JZ: Matrix metalloproteinase 2 is associated with changes in steroid hormones in the sera and peritoneal fluid of patients with endometriosis. (0015–0282 (Print)). [DOI] [PubMed]
- 34.Singh AK, Chattopadhyay R Fau - Chakravarty B, Chakravarty B Fau - Chaudhury K, Chaudhury K: Altered circulating levels of matrix metalloproteinases 2 and 9 and their inhibitors and effect of progesterone supplementation in women with endometriosis undergoing in vitro fertilization. (1556–5653 (Electronic)). [DOI] [PubMed]
- 35.Sui X, Li Y, Sun Y, Li C, Li X, Zhang G. Expression and significance of autophagy genes LC3, Beclin1 and MMP-2 in endometriosis. Exp Ther Med. 2018;16(3):1958–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kodarahmian M, Amidi F, Moini A, Kashani L, Shabani Nashtaei M, Pazhohan A, Bahramrezai M, Berenjian S, Sobhani A. The modulating effects of Resveratrol on the expression of MMP-2 and MMP-9 in endometriosis women: a randomized exploratory trial. Gynecol Endocrinol. 2019;35(8):719–26. [DOI] [PubMed] [Google Scholar]
- 37.Borghese B, Mondon F Fau - Noël J-C, Noël Jc Fau - Fayt I, Fayt I Fau - Mignot T-M, Mignot Tm Fau - Vaiman D, Vaiman D Fau - Chapron C, Chapron C: Gene expression profile for ectopic versus eutopic endometrium provides new insights into endometriosis oncogenic potential. (0888–8809 (Print)). [DOI] [PubMed]
- 38.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malvezzi H, Aguiar VG, Paz CC, Tanus-Santos JE, Penna IA, Navarro PA. Increased circulating MMP-2 levels in infertile patients with moderate and severe pelvic endometriosis. Reprod Sci. 2013;20(5):557–62. [DOI] [PubMed] [Google Scholar]
- 40.Wu T, Zhang R, Jiang Q, Li Z, Wu R. Expression of cellular adherent and invasive molecules in recurrent ovarian endometriosis. J Int Med Res. 2020;48(11):300060520971993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen JL, Lin QH, Fang XL, Tao GS, Huang FY. Effect of progesterone on the secretion of matrix metalloproteinase-2 and matrix metalloproteinase-9 in human ectopic endometrial stromal cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2005;30(3):307–11. [PubMed] [Google Scholar]
- 42.Hudelist G, Singer Cf Fau - Keckstein J, Keckstein J: [Matrix metalloproteinases and their role in menstruation and endometriosis]. (0044–4197 (Print)). [DOI] [PubMed]
- 43.Kyama CM, Overbergh L, Debrock S, Valckx D, Vander Perre S, Meuleman C, Mihalyi A, Mwenda JM, Mathieu C, D’Hooghe TM. Increased peritoneal and endometrial gene expression of biologically relevant cytokines and growth factors during the menstrual phase in women with endometriosis. Fertil Steril. 2006;85(6):1667–75. [DOI] [PubMed] [Google Scholar]
- 44.Li T, Li YG, Pu DM. Matrix metalloproteinase-2 and -9 expression correlated with angiogenesis in human adenomyosis. Gynecol Obstet Invest. 2006;62(4):229–35. [DOI] [PubMed] [Google Scholar]
- 45.Ria R, Loverro G, Vacca A, Ribatti D, Cormio G, Roccaro AM, Selvaggi L. Angiogenesis extent and expression of matrix metalloproteinase-2 and -9 agree with progression of ovarian endometriomas. Eur J Clin Invest. 2002;32(3):199–206. [DOI] [PubMed] [Google Scholar]
- 46.Manicone AM, McGuire JK: Matrix metalloproteinases as modulators of inflammation. (1084–9521 (Print)). [DOI] [PMC free article] [PubMed]
- 47.Braundmeier AG, Fazleabas AT, Lessey BA, Guo H, Toole BP, Nowak RA. Extracellular matrix metalloproteinase inducer regulates metalloproteinases in human uterine endometrium. J Clin Endocrinol Metab. 2006;91(6):2358–65. [DOI] [PubMed] [Google Scholar]
- 48.<IAI.00779–17.pdf>.
- 49.Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, Cormack BP, Chazin WJ, Kehl-Fie TE, Culotta VC: Role of Calprotectin in Withholding Zinc and Copper from Candida albicans. LID - 10.1128/IAI.00779-17 LID - e00779–17. (1098–5522 (Electronic)). [DOI] [PMC free article] [PubMed]
- 50.<4w05t0s1407.pdf>.
- 51.Rink L, Kirchner H. Zinc-altered immune function and cytokine production. J Nutr. 2000;130(5S Suppl):1407S–1411S. [DOI] [PubMed] [Google Scholar]
- 52.Wellinghausen N: Immunobiology of gestational zinc deficiency. Br J Nutr 2001, 85 Suppl 2(S2):S81–86. [PubMed]
- 53.Johnson MD, Kenney N, Stoica A, Hilakivi-Clarke L, Singh B, Chepko G, Clarke R, Sholler PF, Lirio AA, Foss C, et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med. 2003;9(8):1081–4. [DOI] [PubMed] [Google Scholar]
- 54.Stoica A, Katzenellenbogen BS, Martin MB. Activation of estrogen receptor-alpha by the heavy metal cadmium. Mol Endocrinol. 2000;14(4):545–53. [DOI] [PubMed] [Google Scholar]
- 55.Salmeri NA-O, Viganò PA-O, Cavoretto PA-OX, Marci RA-O, Candiani MA-O: The kisspeptin system in and beyond reproduction: exploring intricate pathways and potential links between endometriosis and polycystic ovary syndrome. (1573–2606 (Electronic)). [DOI] [PubMed]
- 56.Rahmioglu N, Mortlock S, Ghiasi M, Moller PL, Stefansdottir L, Galarneau G, Turman C, Danning R, Law MH, Sapkota Y, et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nat Genet. 2023;55(3):423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study's publicly available datasets can be found online. Online at http://www.cdc.gov/nchs/nhanes.htm, you can find the name of the repository or repositories as well as their accession numbers (accessed on October 8, 2023).