Abstract
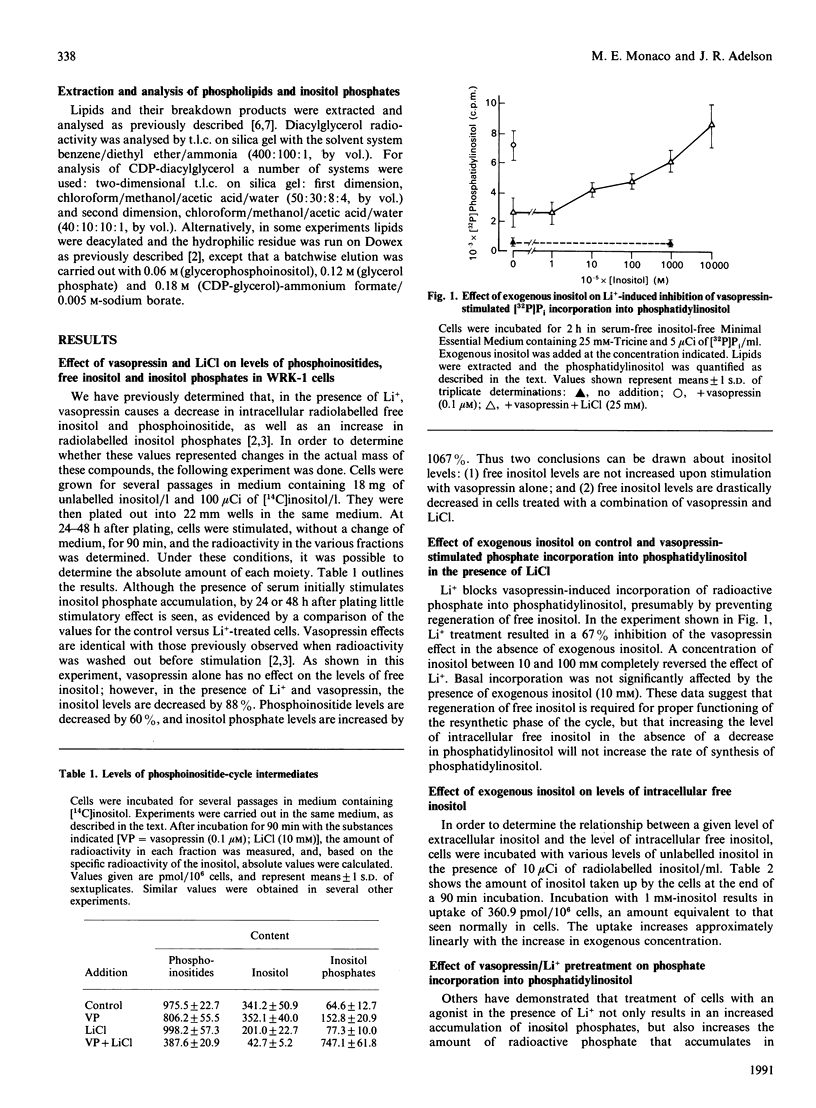
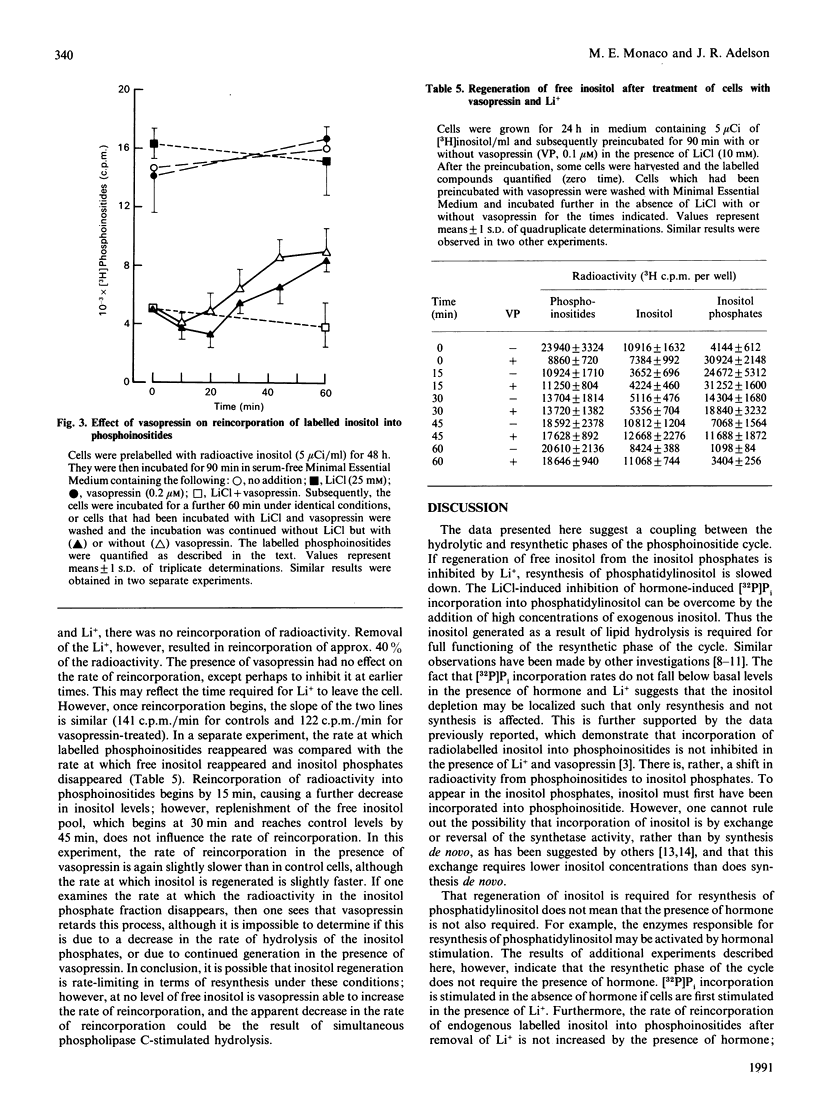
Previous data suggest that agonist-induced hydrolysis of phosphatidylinositol bisphosphate is accompanied by resynthesis through phosphatidylinositol such that these metabolic events function in a cyclic manner. However, it is not known whether resynthesis depends on the presence of agonist or is a direct result of agonist-induced breakdown. In the present study we demonstrate that: (1) increasing the intracellular free inositol concentration will not stimulate phosphatidylinositol synthesis, as measured by assessing the amount of [32P]Pi incorporation; (2) regeneration of free inositol is required for resynthesis; however, addition of exogenous inositol can sustain resynthesis under conditions which inhibit the regeneration of endogenous inositol; (3) resynthesis can take place in the absence of agonist provided that cells have been previously incubated under conditions which prevent resynthesis; and (4) the presence of agonist does not increase the rate of resynthesis. Thus the resynthetic phase of the phosphoinositide cycle is a compensatory event triggered either by the decrease in the level of phosphatidylinositol or by an increase in precursor substrates. The agonist itself appears to have no direct effect on the resynthesis process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balla T., Enyedi P., Hunyady L., Spät A. Effects of lithium on angiotensin-stimulated phosphatidylinositol turnover and aldosterone production in adrenal glomerulosa cells: a possible causal relationship. FEBS Lett. 1984 Jun 11;171(2):179–182. doi: 10.1016/0014-5793(84)80483-4. [DOI] [PubMed] [Google Scholar]
- Cubitt A. B., Zhang B., Gershengorn M. C. Analysis by base exchange of thyrotropin-releasing hormone responsive and unresponsive inositol lipid pools in rat pituitary tumor cells. J Biol Chem. 1990 Jun 15;265(17):9707–9714. [PubMed] [Google Scholar]
- Downes C. P., Stone M. A. Lithium-induced reduction in intracellular inositol supply in cholinergically stimulated parotid gland. Biochem J. 1986 Feb 15;234(1):199–204. doi: 10.1042/bj2340199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. H., Raeburn C. A. The interaction of lithium with thyrotropin-releasing hormone-stimulated lipid metabolism in GH3 pituitary tumour cells. Enhancement of stimulated 1,2-diacylglycerol formation. Biochem J. 1984 Nov 15;224(1):129–136. doi: 10.1042/bj2240129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey P. P. Potentiation by lithium of CMP-phosphatidate formation in carbachol-stimulated rat cerebral-cortical slices and its reversal by myo-inositol. Biochem J. 1989 Mar 1;258(2):621–624. doi: 10.1042/bj2580621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Regulation by phosphatidylinositol of rat pituitary plasma membrane and endoplasmic reticulum phosphatidylinositol synthase activities. A mechanism for activation of phosphoinositide resynthesis during cell stimulation. J Biol Chem. 1987 May 15;262(14):6457–6459. [PubMed] [Google Scholar]
- Kidwell W. R., Monaco M. E., Wicha M. S., Smith G. S. Unsaturated fatty acid requirements for growth and survival of a rat mammary tumor cell line. Cancer Res. 1978 Nov;38(11 Pt 2):4091–4100. [PubMed] [Google Scholar]
- Koréh K., Monaco M. E. The relationship of hormone-sensitive and hormone-insensitive phosphatidylinositol to phosphatidylinositol 4,5-bisphosphate in the WRK-1 cell. J Biol Chem. 1986 Jan 5;261(1):88–91. [PubMed] [Google Scholar]
- Maccallum S. H., Barker C. J., Hunt P. A., Wong N. S., Kirk C. J., Michell R. H. The use of cells doubly labelled with [14C]inositol and [3H]inositol to search for a hormone-sensitive inositol lipid pool with atypically rapid metabolic turnover. J Endocrinol. 1989 Jul;122(1):379–389. doi: 10.1677/joe.0.1220379. [DOI] [PubMed] [Google Scholar]
- McPhee F., Lowe G., Vaziri C., Downes C. P. Phosphatidylinositol synthase and phosphatidylinositol/inositol exchange reactions in turkey erythrocyte membranes. Biochem J. 1991 Apr 1;275(Pt 1):187–192. doi: 10.1042/bj2750187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco M. E. Calcium and the phosphoinositide cycle in WRK-1 cells. Effects of A23187 on metabolism of specific phosphatidylinositol pools. J Biol Chem. 1987 Jan 5;262(1):147–151. [PubMed] [Google Scholar]
- Monaco M. E. Calcium and the phosphoinositide cycle in WRK-1 cells. Effects of A23187 on metabolism of specific phosphatidylinositol pools. J Biol Chem. 1987 Jan 5;262(1):147–151. [PubMed] [Google Scholar]
- Monaco M. E. Inositol metabolism in WRK-1 cells. Relationship of hormone-sensitive to -insensitive pools of phosphoinositides. J Biol Chem. 1987 Sep 25;262(27):13001–13006. [PubMed] [Google Scholar]
- Monaco M. E., Woods D. Characterization of the hormone-sensitive phosphatidylinositol pool in WRK-1 cells. J Biol Chem. 1983 Dec 25;258(24):15125–15129. [PubMed] [Google Scholar]


