Abstract
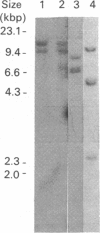
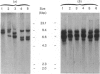
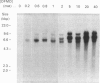
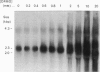
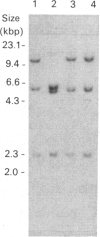
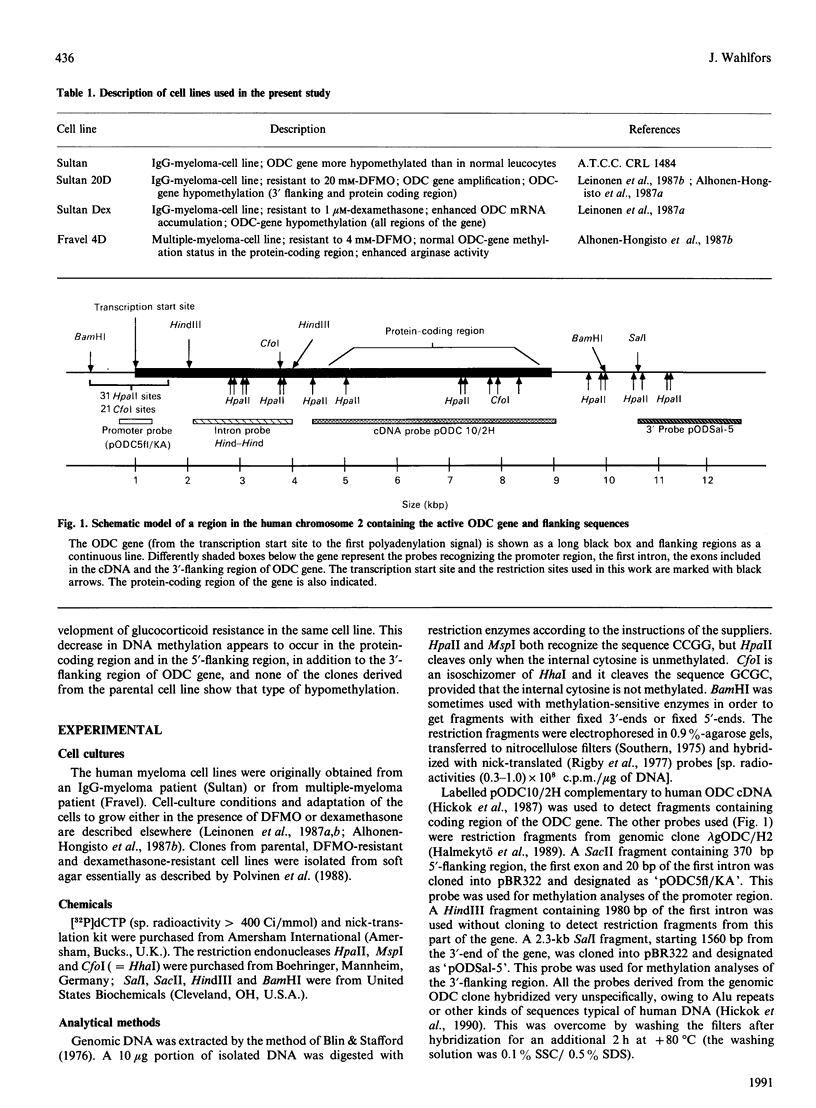
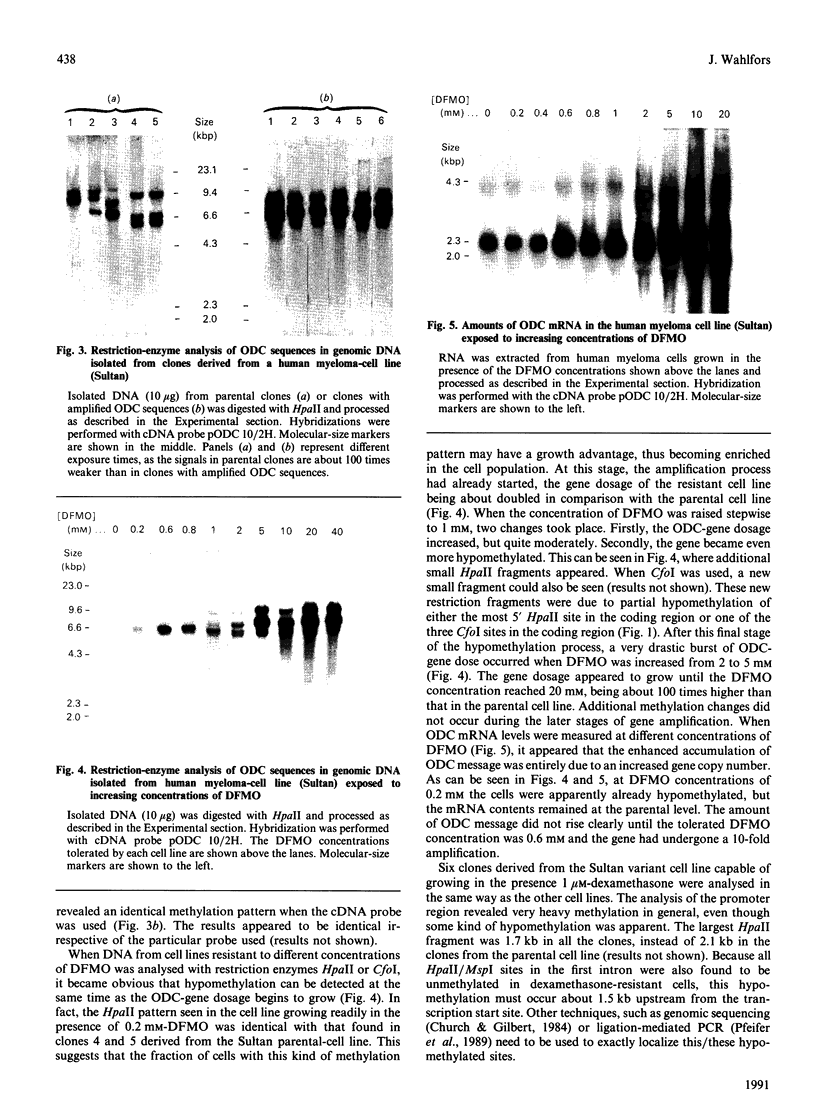
The ornithine decarboxylase (ODC; EC 4.1.1.17) gene in parental, dexamethasone-resistant and 2-difluoromethylornithine (DFMO)-resistant human IgG-myeloma-cell lines was studied with the aid of methylation-sensitive restriction endonucleases and probes recognizing different parts of the gene. In all cell lines the promoter region of the ODC gene appeared to be heavily methylated, whereas the first long intron was unmethylated. Methylation analyses of several clones from the parental cell line revealed that these cells are heterogeneous with respect to the methylation status of the ODC gene, whereas all clones from DFMO-resistant cell lines displayed the same methylation pattern. Two of the parental clones represented a hypomethylated type very close to that exclusively found among the DFMO-resistant clones with ODC gene amplification. This typical methylation pattern was due to decreased methylation of a few CCGG sequences in the 3'-flanking region of the gene. It is possible that this kind of hypomethylation favours the initiation of the gene-amplification process in certain individual cells. This hypothesis was supported by the finding that no hypomethylation was present in the ODC gene of another human myeloma cell line that had acquired resistance to DFMO without gene amplification. In a dexamethasone-resistant cell line that overproduced ODC mRNA at normal gene dosage there were some minor differences between the methylation pattern of the ODC gene of different clones, but no such hypomethylation could be found in clones from the parental cell line. In dexamethasone-resistant cells the ODC gene was hypomethylated around the two HpaII sites and three CfoI sites in the coding region and also, as well as in cells with amplified ODC sequences, in the 3'-flanking region of the gene. Some hypomethylation in the distant 5'-flanking region was also observed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Toffenetti J., Zhu Z., Chader G. J., Noonan D. M. Hypomethylation of the interphotoreceptor retinoid-binding protein (IRBP) promotor and first exon is linked to expression of the gene. Nucleic Acids Res. 1990 Sep 11;18(17):5181–5187. doi: 10.1093/nar/18.17.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhonen-Hongisto L., Leinonen P., Laine R., Jänne J. Human myeloma cells acquire resistance to difluoromethylornithine without overproducing ornithine decarboxylase. Biochem Biophys Res Commun. 1987 Apr 14;144(1):132–137. doi: 10.1016/s0006-291x(87)80485-0. [DOI] [PubMed] [Google Scholar]
- Alhonen-Hongisto L., Leinonen P., Sinervirta R., Laine R., Winqvist R., Alitalo K., Jänne O. A., Jänne J. Mouse and human ornithine decarboxylase genes. Methylation polymorphism and amplification. Biochem J. 1987 Feb 15;242(1):205–210. doi: 10.1042/bj2420205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antequera F., Boyes J., Bird A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell. 1990 Aug 10;62(3):503–514. doi: 10.1016/0092-8674(90)90015-7. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Di Giambattista M., Thielen A. P., Maassen J. A., Möller W., Cocito C. Localization of virginiamycin S binding site on bacterial ribosome by fluorescence energy transfer. Biochemistry. 1986 Jun 17;25(12):3540–3547. doi: 10.1021/bi00360a011. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Toth M., Kochanek S., Achten S., Freisem-Rabien U., Behn-Krappa A., Orend G. Eukaryotic DNA methylation: facts and problems. FEBS Lett. 1990 Aug 1;268(2):329–333. doi: 10.1016/0014-5793(90)81280-2. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Gehrke C. W., Kuo K. C., Ehrlich M. Reduced genomic 5-methylcytosine content in human colonic neoplasia. Cancer Res. 1988 Mar 1;48(5):1159–1161. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983 Jan 6;301(5895):89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. Hypomethylation of ras oncogenes in primary human cancers. Biochem Biophys Res Commun. 1983 Feb 28;111(1):47–54. doi: 10.1016/s0006-291x(83)80115-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. C., Flanagan M. A. Characterization and sequence analysis of the human ornithine decarboxylase gene. DNA. 1989 Nov;8(9):623–634. doi: 10.1089/dna.1.1989.8.623. [DOI] [PubMed] [Google Scholar]
- Fukunaga R., Matsuyama M., Okamura H., Nagata K., Nagata S., Sokawa Y. Undermethylation of interferon-gamma gene in human T cell lines and normal T lymphocytes. Nucleic Acids Res. 1986 Jun 11;14(11):4421–4436. doi: 10.1093/nar/14.11.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama-Sosa M. A., Slagel V. A., Trewyn R. W., Oxenhandler R., Kuo K. C., Gehrke C. W., Ehrlich M. The 5-methylcytosine content of DNA from human tumors. Nucleic Acids Res. 1983 Oct 11;11(19):6883–6894. doi: 10.1093/nar/11.19.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz S. E., Vogelstein B., Hamilton S. R., Feinberg A. P. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science. 1985 Apr 12;228(4696):187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- Halmekytö M., Hirvonen A., Wahlfors J., Alhonen L., Jänne J. Methylation of human ornithine decarboxylase gene before transfection abolishes its transient expression in Chinese hamster ovary cells. Biochem Biophys Res Commun. 1989 Jul 14;162(1):528–534. doi: 10.1016/0006-291x(89)92029-9. [DOI] [PubMed] [Google Scholar]
- Hickok N. J., Seppänen P. J., Gunsalus G. L., Jänne O. A. Complete amino acid sequence of human ornithine decarboxylase deduced from complementary DNA. DNA. 1987 Jun;6(3):179–187. doi: 10.1089/dna.1987.6.179. [DOI] [PubMed] [Google Scholar]
- Hickok N. J., Wahlfors J., Crozat A., Halmekytö M., Alhonen L., Jänne J., Jänne O. A. Human ornithine decarboxylase-encoding loci: nucleotide sequence of the expressed gene and characterization of a pseudogene. Gene. 1990 Sep 14;93(2):257–263. doi: 10.1016/0378-1119(90)90233-h. [DOI] [PubMed] [Google Scholar]
- Hyttinen I. M., Halmekytö M., Alhonen L., Jänne J. Levels of ornithine decarboxylase genomic sequences, heterogeneous nuclear RNA and mRNA in human myeloma cells resistant to alpha-difluoromethylornithine. Biochem J. 1991 Sep 15;278(Pt 3):871–874. doi: 10.1042/bj2780871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Wolkowicz M. J., Rideout W. M., 3rd, Gonzales F. A., Marziasz C. M., Coetzee G. A., Tapscott S. J. De novo methylation of the MyoD1 CpG island during the establishment of immortal cell lines. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6117–6121. doi: 10.1073/pnas.87.16.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen P., Alhonen-Hongisto L., Laine R., Jänne O. A., Jänne J. Chronic exposure to dexamethasone induces hypomethylation of ornithine decarboxylase genes in a human myeloma cell line. FEBS Lett. 1987 May 4;215(1):68–72. doi: 10.1016/0014-5793(87)80115-1. [DOI] [PubMed] [Google Scholar]
- Leinonen P., Alhonen-Hongisto L., Laine R., Jänne O. A., Jänne J. Human myeloma cells acquire resistance to difluoromethylornithine by amplification of ornithine decarboxylase gene. Biochem J. 1987 Feb 15;242(1):199–203. doi: 10.1042/bj2420199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsanen V., Leinonen P., Alhonen L., Jänne J. Hypomethylation of ornithine decarboxylase gene and erb-A1 oncogene in human chronic lymphatic leukemia. Blood. 1988 Dec;72(6):2042–2044. [PubMed] [Google Scholar]
- Moshier J. A., Gilbert J. D., Skunca M., Dosescu J., Almodovar K. M., Luk G. D. Isolation and expression of a human ornithine decarboxylase gene. J Biol Chem. 1990 Mar 25;265(9):4884–4892. [PubMed] [Google Scholar]
- Pfeifer G. P., Steigerwald S. D., Mueller P. R., Wold B., Riggs A. D. Genomic sequencing and methylation analysis by ligation mediated PCR. Science. 1989 Nov 10;246(4931):810–813. doi: 10.1126/science.2814502. [DOI] [PubMed] [Google Scholar]
- Pilz R. B., Steglich C., Scheffler I. E. Molecular and genetic characterization of an ornithine decarboxylase-deficient Chinese hamster cell line. J Biol Chem. 1990 May 25;265(15):8880–8886. [PubMed] [Google Scholar]
- Polvinen K., Sinervirta R., Alhonen L., Jänne J. Overproduction of ornithine decarboxylase confers an apparent growth advantage to mouse tumor cells. Biochem Biophys Res Commun. 1988 Aug 30;155(1):373–378. doi: 10.1016/s0006-291x(88)81095-7. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wegner M., Schwender S., Dinkl E., Grummt F. An amplification-promoting sequence from mouse genomic DNA: interaction with a trans-acting factor that also affects gene expression. DNA Cell Biol. 1990 Jun;9(5):311–321. doi: 10.1089/dna.1990.9.311. [DOI] [PubMed] [Google Scholar]
- de Bustros A., Nelkin B. D., Silverman A., Ehrlich G., Poiesz B., Baylin S. B. The short arm of chromosome 11 is a "hot spot" for hypermethylation in human neoplasia. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5693–5697. doi: 10.1073/pnas.85.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steeg H., van Oostrom C. T., Martens J. W., van Kreyl C., Schepens J., Wieringa B. Nucleotide sequence of the human ornithine decarboxylase gene. Nucleic Acids Res. 1989 Nov 11;17(21):8855–8856. doi: 10.1093/nar/17.21.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]