Abstract
The kinetic and regulatory properties of cytosolic pyruvate kinase (PKc) isolated from endosperm of germinating castor oil seeds (Ricinus communis L.) have been studied. Optimal efficiency in substrate utilization (in terms of Vmax/Km for phosphoenolpyruvate or ADP) occurred between pH 6.7 and 7.4. Enzyme activity was absolutely dependent on the presence of a bivalent and a univalent metal cation, with Mg2+ and K+ fulfilling this requirement. Mg2+ binding showed positive and negative co-operativity at pH 6.5 (h = 1.6) and pH 7.2 (h = 0.69) respectively. Hyperbolic saturation kinetics were observed with phosphoenolpyruvate (PEP) and K+, whereas ADP acted as a mixed-type inhibitor over 1 mM. Glycerol (10%, v/v) increased the S0.5(ADP) 2.3-fold and altered the pattern of nucleotide binding from hyperbolic (h = 1.0) to sigmoidal (h = 1.79) without modifying PEP saturation kinetics. No activators were identified. ATP, AMP, isocitrate, 2-oxoglutarate, malate, 2-phosphoglycerate, 2,3-bisphosphoglycerate, 3-phosphoglycerate, glycerol 3-phosphate and phosphoglycolate were the most effective inhibitors. These metabolites yielded additive inhibition when tested in pairs. ATP and 3-phosphoglycerate were mixed-type inhibitors with respect to PEP, whereas competitive inhibition was observed for other inhibitors. Inhibition by malate, 2-oxoglutarate, phosphorylated triose sugars or phosphoglycolate was far more pronounced at pH 7.2 than at pH 6.5. Although 32P-labelling studies revealed that extensive phosphorylation in vivo of soluble endosperm proteins occurred between days 3 and 5 of seed germination, no alteration in the 32P-labelling pattern of 5-day-germinated endosperm was observed after 30 min of anaerobiosis. Moreover, no evidence was obtained that PKc was a phosphoprotein in aerobic or anoxic endosperms. It is proposed that endosperm PKc activity of germinating castor seeds is enhanced after anaerobiosis through concerted decreases in ATP levels, cytosolic pH and concentrations of several key inhibitors.
Full text
PDF


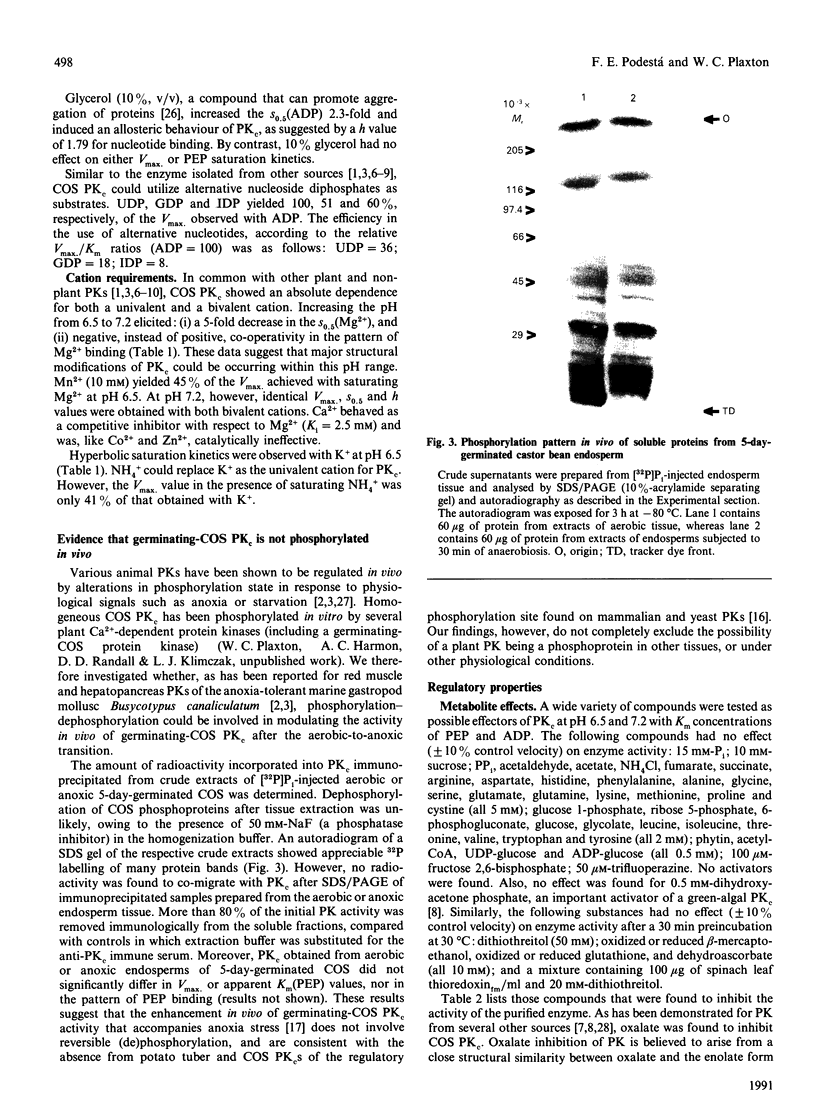

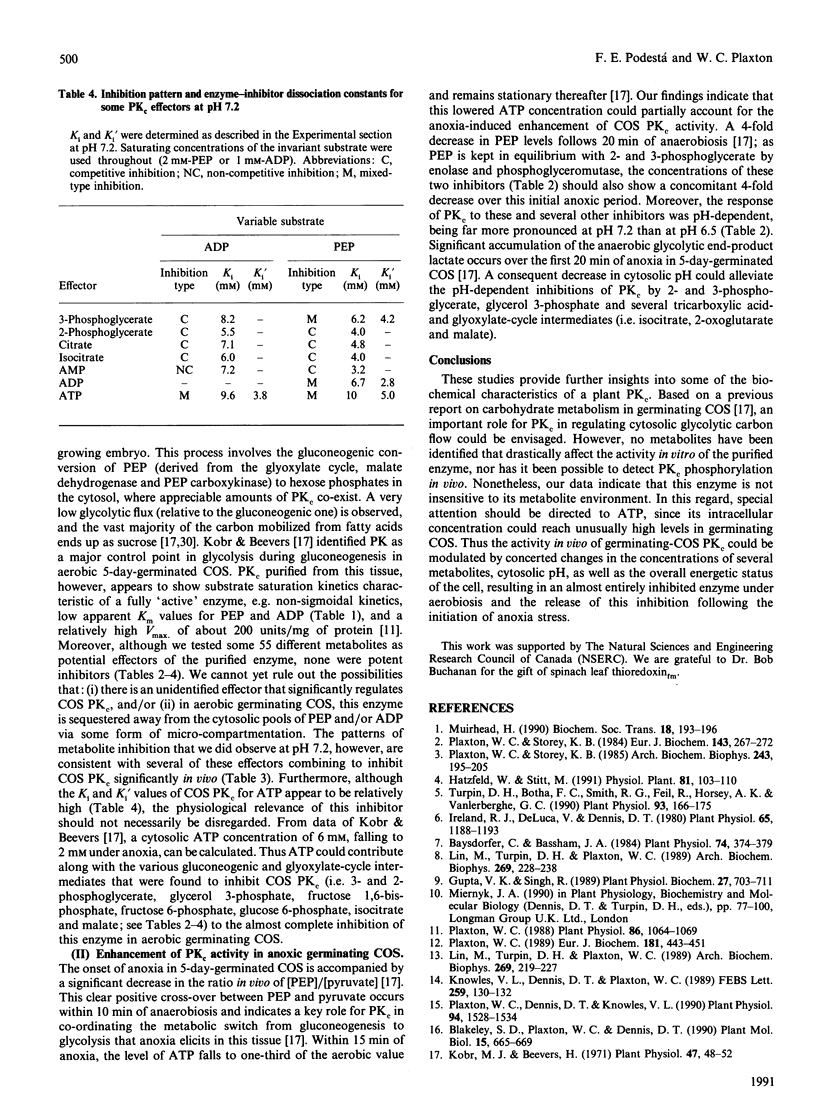

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimacopoulos-Jeannet F., Jeanrenaud B. Insulin activates 6-phosphofructo-2-kinase and pyruvate kinase in the liver. Indirect evidence for an action via a phosphatase. J Biol Chem. 1990 May 5;265(13):7202–7206. doi: 10.1016/0261-5614(90)90109-6. [DOI] [PubMed] [Google Scholar]
- Baysdorfer C., Bassham J. A. Spinach pyruvate kinase isoforms : partial purification and regulatory properties. Plant Physiol. 1984 Feb;74(2):374–379. doi: 10.1104/pp.74.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley S. D., Plaxton W. C., Dennis D. T. Cloning and characterization of a cDNA for the cytosolic isozyme of plant pyruvate kinase: the relationship between the plant and non-plant enzyme. Plant Mol Biol. 1990 Oct;15(4):665–669. doi: 10.1007/BF00017842. [DOI] [PubMed] [Google Scholar]
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Gekko K., Timasheff S. N. Mechanism of protein stabilization by glycerol: preferential hydration in glycerol-water mixtures. Biochemistry. 1981 Aug 4;20(16):4667–4676. doi: 10.1021/bi00519a023. [DOI] [PubMed] [Google Scholar]
- Ireland R. J., De Luca V., Dennis D. T. Characterization and kinetics of isoenzymes of pyruvate kinase from developing castor bean endosperm. Plant Physiol. 1980 Jun;65(6):1188–1193. doi: 10.1104/pp.65.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Cochet C., Dhien A., Chambaz E. M. A rapid method for screening inhibitor effects: determination of I50 and its standard deviation. Anal Biochem. 1978 Jan;84(1):68–77. doi: 10.1016/0003-2697(78)90484-0. [DOI] [PubMed] [Google Scholar]
- Kobr M. J., Beevers H. Gluconeogenesis in the castor bean endosperm: I. Changes in glycolytic intermediates. Plant Physiol. 1971 Jan;47(1):48–52. doi: 10.1104/pp.47.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K., Ashihara H. Identification of non-equilibrium glycolytic reactions in suspension-cultured plant cells. Biochim Biophys Acta. 1990 Nov 9;1036(2):138–142. doi: 10.1016/0304-4165(90)90025-r. [DOI] [PubMed] [Google Scholar]
- Lin M., Turpin D. H., Plaxton W. C. Pyruvate kinase isozymes from the green alga, Selenastrum minutum. I. Purification and physical and immunological characterization. Arch Biochem Biophys. 1989 Feb 15;269(1):219–227. doi: 10.1016/0003-9861(89)90103-3. [DOI] [PubMed] [Google Scholar]
- Lin M., Turpin D. H., Plaxton W. C. Pyruvate kinase isozymes from the green alga, Selenastrum minutum. II. Kinetic and regulatory properties. Arch Biochem Biophys. 1989 Feb 15;269(1):228–238. doi: 10.1016/0003-9861(89)90104-5. [DOI] [PubMed] [Google Scholar]
- Lodato D. T., Reed G. H. Structure of the oxalate-ATP complex with pyruvate kinase: ATP as a bridging ligand for the two divalent cations. Biochemistry. 1987 Apr 21;26(8):2243–2250. doi: 10.1021/bi00382a026. [DOI] [PubMed] [Google Scholar]
- Muirhead H. Isoenzymes of pyruvate kinase. Biochem Soc Trans. 1990 Apr;18(2):193–196. doi: 10.1042/bst0180193. [DOI] [PubMed] [Google Scholar]
- Plaxton W. C., Dennis D. T., Knowles V. L. Purification of leucoplast pyruvate kinase from developing castor bean endosperm. Plant Physiol. 1990 Dec;94(4):1528–1534. doi: 10.1104/pp.94.4.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W. C. Molecular and immunological characterization of plastid and cytosolic pyruvate kinase isozymes from castor-oil-plant endosperm and leaf. Eur J Biochem. 1989 May 1;181(2):443–451. doi: 10.1111/j.1432-1033.1989.tb14745.x. [DOI] [PubMed] [Google Scholar]
- Plaxton W. C. Purification of pyruvate kinase from germinating castor bean endosperm. Plant Physiol. 1988 Apr;86(4):1064–1069. doi: 10.1104/pp.86.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W. C., Storey K. B. Phosphorylation in vivo of red-muscle pyruvate kinase from the channelled whelk, Busycotypus canaliculatum, in response to anoxic stress. Eur J Biochem. 1984 Sep 3;143(2):267–272. doi: 10.1111/j.1432-1033.1984.tb08368.x. [DOI] [PubMed] [Google Scholar]
- Plaxton W. C., Storey K. B. Purification and properties of aerobic and anoxic forms of pyruvate kinase from the hepatopancreas of the channelled whelk, Busycotypus canaliculatum. Arch Biochem Biophys. 1985 Nov 15;243(1):195–205. doi: 10.1016/0003-9861(85)90788-x. [DOI] [PubMed] [Google Scholar]
- Stitt J. T. Differential sensitivity in the sites of fever production by prostaglandin E1 within the hypothalamus of the rat. J Physiol. 1991 Jan;432:99–110. doi: 10.1113/jphysiol.1991.sp018378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin D. H., Botha F. C., Smith R. G., Feil R., Horsey A. K., Vanlerberghe G. C. Regulation of Carbon Partitioning to Respiration during Dark Ammonium Assimilation by the Green Alga Selenastrum minutum. Plant Physiol. 1990 May;93(1):166–175. doi: 10.1104/pp.93.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]



