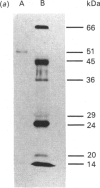
Abstract
The enzyme indol-3-ylacetylglucose synthase (UDP-glucose:indol-3-ylacetate beta-D-glucosyltransferase) catalyses the reaction: [formula: see text] This is the first step in the series of reactions leading to the indol-3-ylacetic acid conjugates found in maize. Previous attempts to purify this enzyme from the liquid endosperm of kernels of Zea mays (sweet corn) were not entirely successful owing to the lability of partially purified preparations during column chromatography. Thus this enzyme has not previously been purified to homogeneity. During the present study it was found that retention of enzyme activity required the combined presence of glycerol and dithiothreitol. Adding these requirements permitted purification of the enzyme to homogeneity with retention of catalytic activity. These purified preparations were used for preparation of rabbit polyclonal antibodies to the enzyme. Antibodies to the Zea mays endosperm enzyme cross-react with the enzyme from Zea mays vegetative tissues and with an enzyme from the liquid endosperm of oak acorns (Quercus sp). In this paper we report a simplified purification procedure adaptable to the preparation of milligram amounts of the enzyme.
Full text
PDF

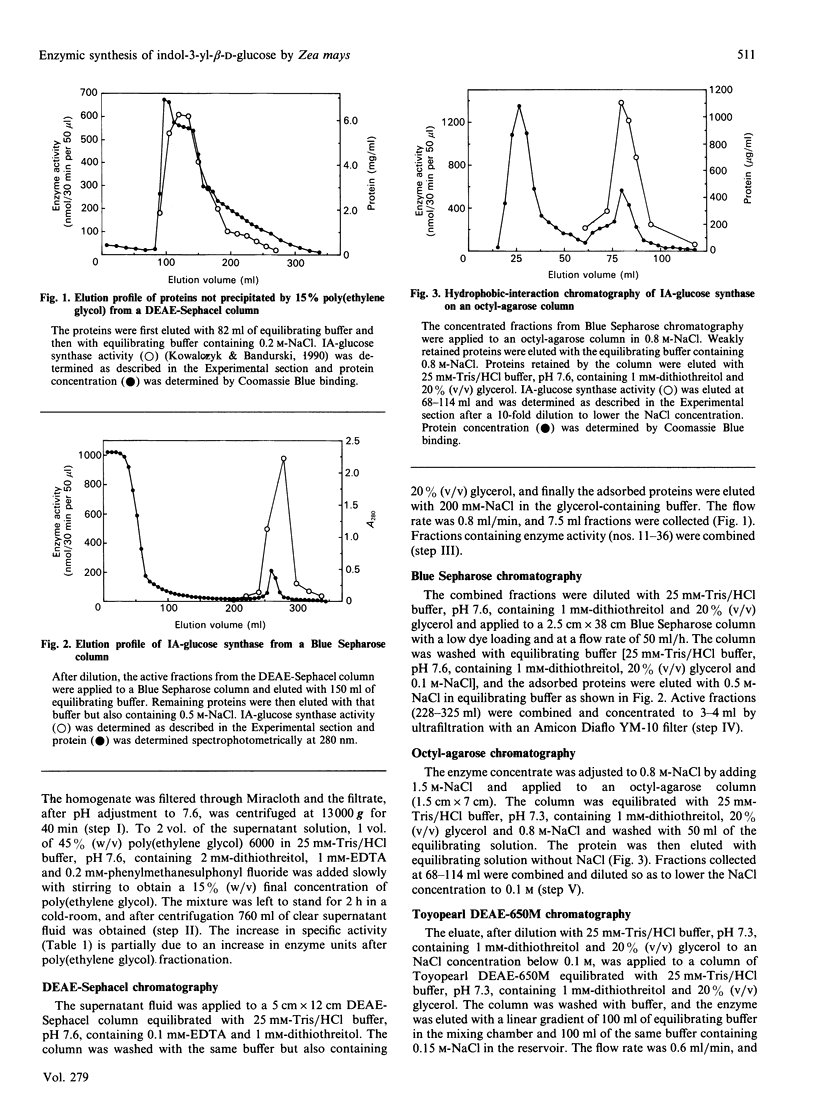
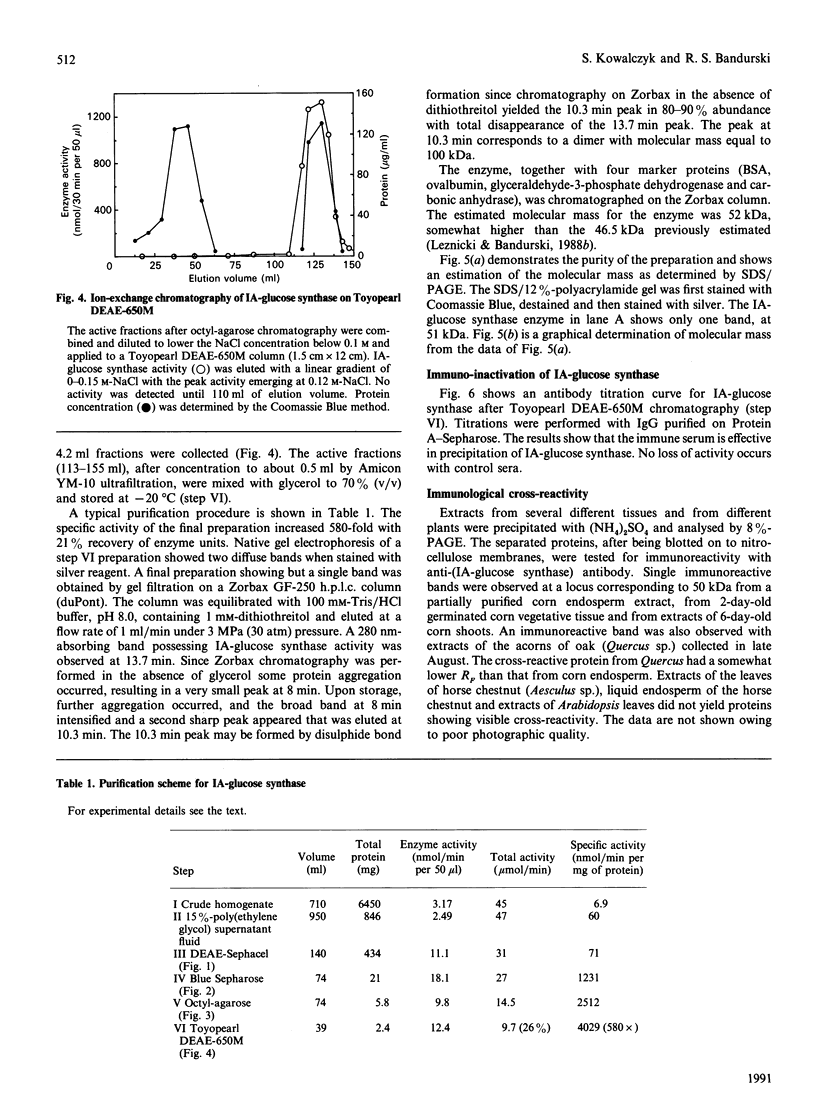
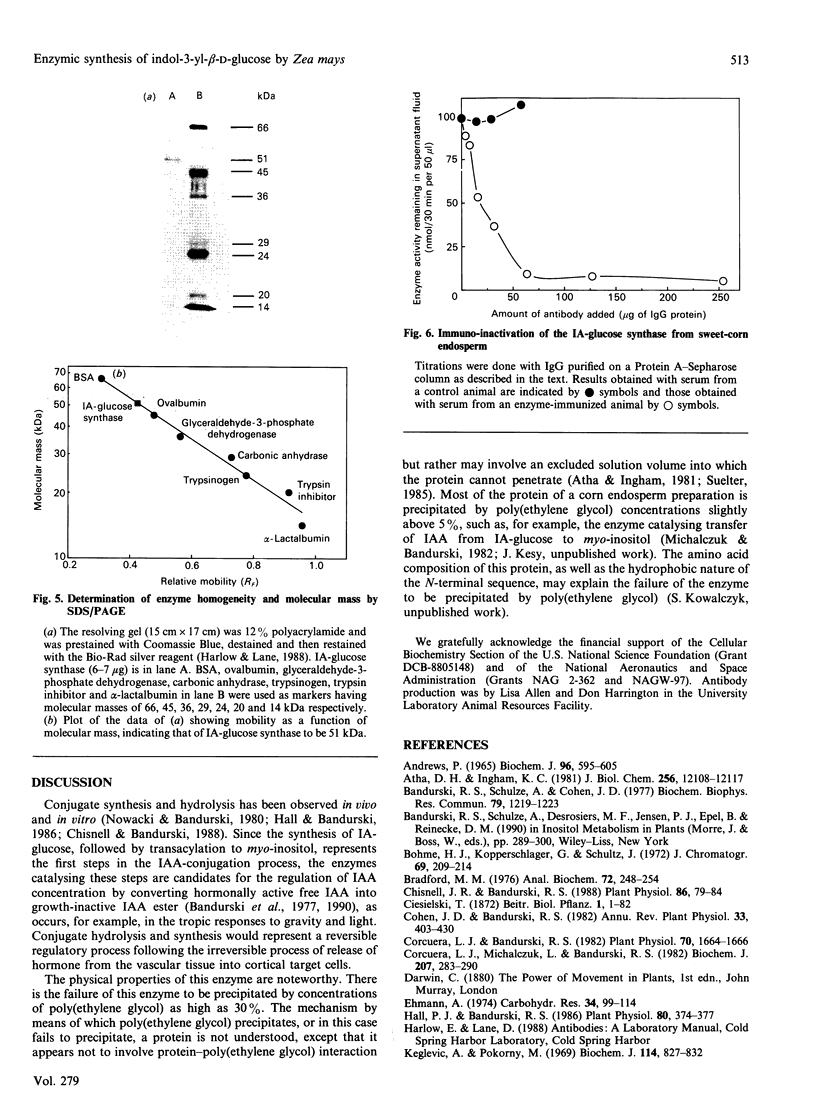

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atha D. H., Ingham K. C. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J Biol Chem. 1981 Dec 10;256(23):12108–12117. [PubMed] [Google Scholar]
- Bandurski R. S., Schulze A., Cohen J. D. Photo-regulation of the ratio of ester to free indole-3-acetic acid. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1219–1223. doi: 10.1016/0006-291x(77)91136-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Böhme H. J., Kopperschläger G., Schulz J., Hofmann E. Affinity chromatography of phosphofructokinase using Cibacron blue F3G-A. J Chromatogr. 1972 Jun 28;69(1):209–214. doi: 10.1016/s0021-9673(00)83103-9. [DOI] [PubMed] [Google Scholar]
- Chisnell J. R., Bandurski R. S. Translocation of radiolabeled indole-3-acetic acid and indole-3-acetyl-myo-inositol from kernel to shoot of Zea mays L. Plant Physiol. 1988;86:79–84. doi: 10.1104/pp.86.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcuera L. J., Bandurski R. S. Biosynthesis of Indol-3-yl-acetyl-myo-inositol Arabinoside in Kernels of Zea mays L. Plant Physiol. 1982 Dec;70(6):1664–1666. doi: 10.1104/pp.70.6.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcuera L. J., Michalczuk L., Bandurski R. S. Enzymic synthesis of indol-3-ylacetyl-myo-inositol galactoside. Biochem J. 1982 Nov 1;207(2):283–290. doi: 10.1042/bj2070283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann A. Identification of 2-O (indole-3-acetyl)-D-glucopyranose, 4-O-(indole-3-acetyl)-D-glucopyranose and 6-O-(indole-3-acetyl)-D-glucopyranose from kernels of Zea mays by gas-liquid chromatography-mass spectrometry. Carbohydr Res. 1974 May;34(1):99–114. doi: 10.1016/s0008-6215(00)80374-2. [DOI] [PubMed] [Google Scholar]
- Hall P. J., Bandurski R. S. [3H]Indole-3-acetyl-myo-inositol hydrolysis by extracts of Zea mays L. vegetative tissue. Plant Physiol. 1986;80:374–377. doi: 10.1104/pp.80.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keglević D., Pokorny M. The chemical synthesis of 1-O-(indol-3'-ylacetyl)-beta-D-glucopyranose. The higher activity of the glucoside in comparison with exogenous indol-3-ylacetic acid in plant-section elongation tests. Biochem J. 1969 Oct;114(4):827–832. doi: 10.1042/bj1140827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopcewicz J., Ehmann A., Bandurski R. S. Enzymatic Esterification of Indole-3-acetic Acid to myo-Inositol and Glucose. Plant Physiol. 1974 Dec;54(6):846–851. doi: 10.1104/pp.54.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk S., Bandurski R. S. Isomerization of 1-O-indol-3-ylacetyl-beta-D-glucose. Enzymatic hydrolysis of 1-O, 4-O, and 6-O-indol-3-ylacetyl-beta-D-glucose and the enzymatic synthesis of indole-3-acetyl glycerol by a hormone metabolizing complex. Plant Physiol. 1990;94:4–12. doi: 10.1104/pp.94.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leznicki A. J., Bandurski R. S. Enzymic synthesis of indole-3-acetyl-1-O-beta-d-glucose. I. Partial purification and characterization of the enzyme from Zea mays. Plant Physiol. 1988;88:1474–1480. doi: 10.1104/pp.88.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leznicki A. J., Bandurski R. S. Enzymic synthesis of indole-3-acetyl-1-O-beta-d-glucose. II. Metabolic characteristics of the enzyme. Plant Physiol. 1988;88:1481–1485. doi: 10.1104/pp.88.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczuk L., Bandurski R. S. Enzymic synthesis of 1-O-indol-3-ylacetyl-beta-D-glucose and indol-3-ylacetyl-myo-inositol. Biochem J. 1982 Nov 1;207(2):273–281. doi: 10.1042/bj2070273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczuk L., Bandurski R. S. UDP-glucose: indoleacetic acid glucosyl transferase and indoleacetyl-glucose: myo-inositol indoleacetyl transferase. Biochem Biophys Res Commun. 1980 Mar 28;93(2):588–592. doi: 10.1016/0006-291x(80)91118-3. [DOI] [PubMed] [Google Scholar]
- Nowacki J., Bandurski R. S. Myo-Inositol Esters of Indole-3-acetic Acid as Seed Auxin Precursors of Zea mays L. Plant Physiol. 1980 Mar;65(3):422–427. doi: 10.1104/pp.65.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogita Z. I., Markert C. L. A miniaturized system for electrophoresis on polyacrylamide gels. Anal Biochem. 1979 Nov 1;99(2):233–241. doi: 10.1016/s0003-2697(79)80001-9. [DOI] [PubMed] [Google Scholar]
- Strack D., Gross W. Properties and Activity Changes of Chlorogenic Acid:Glucaric Acid Caffeoyltransferase From Tomato (Lycopersicon esculentum). Plant Physiol. 1990 Jan;92(1):41–47. doi: 10.1104/pp.92.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]