Abstract
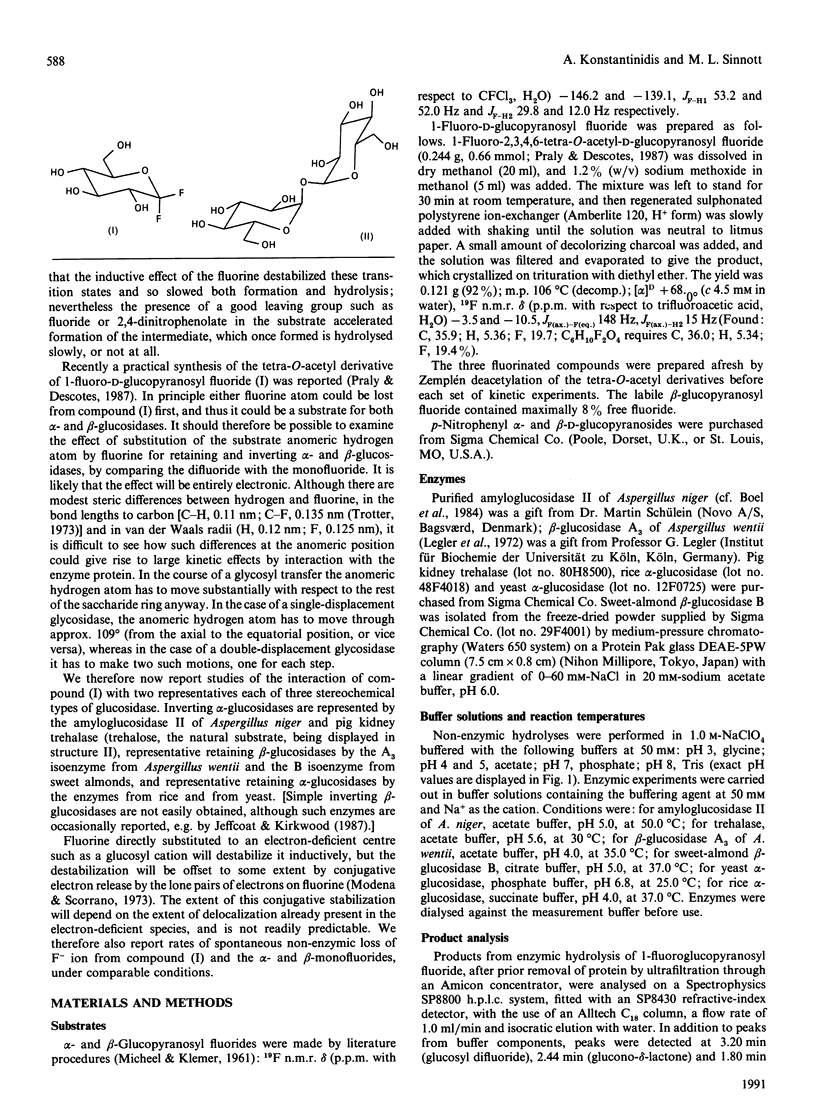
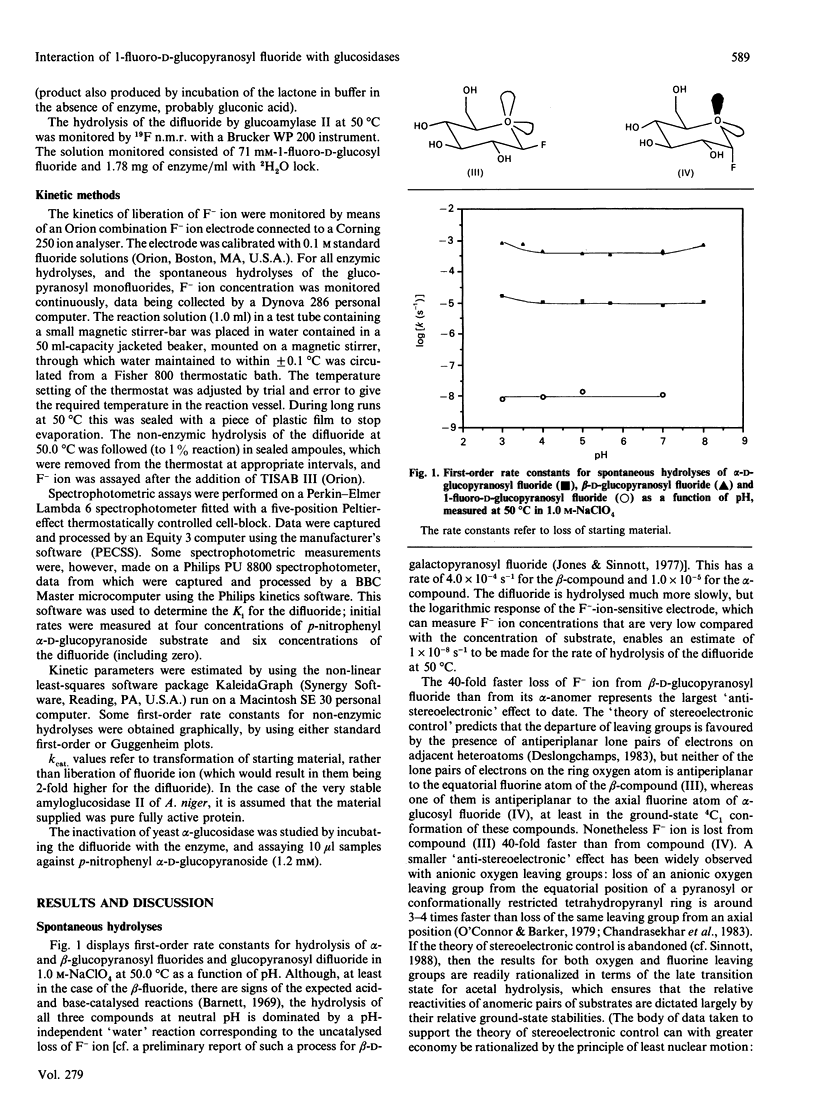
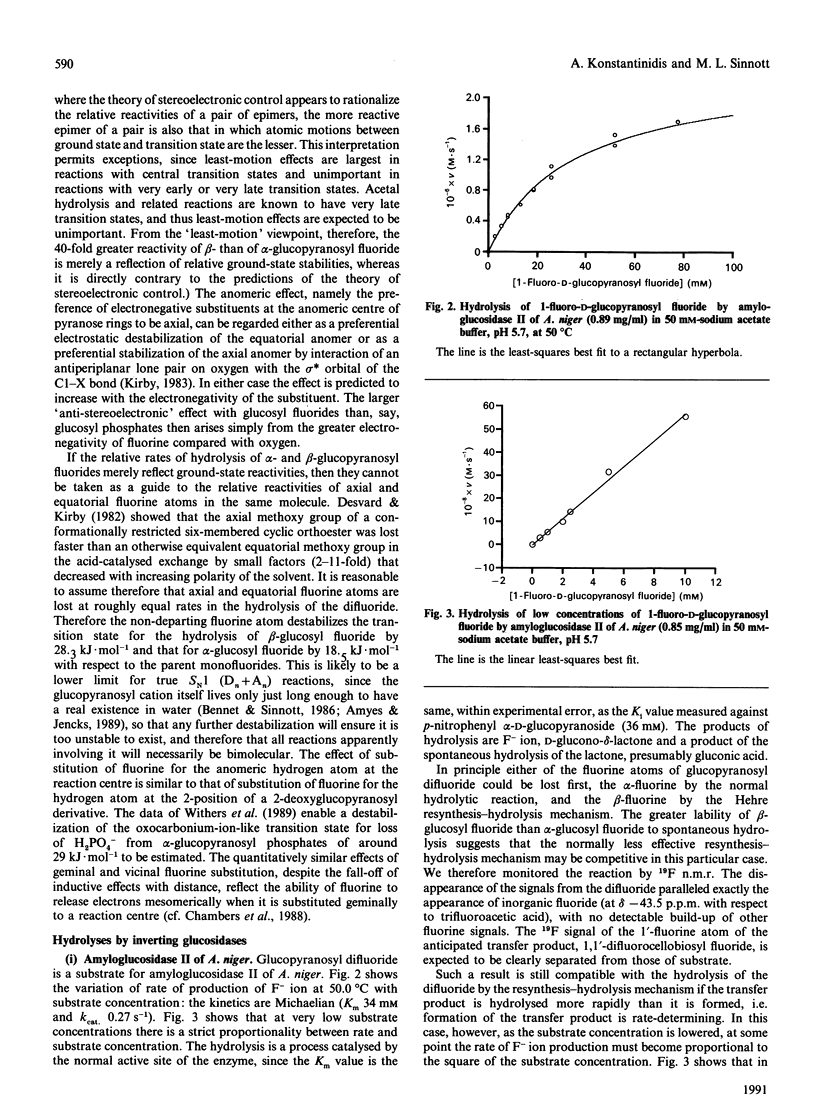
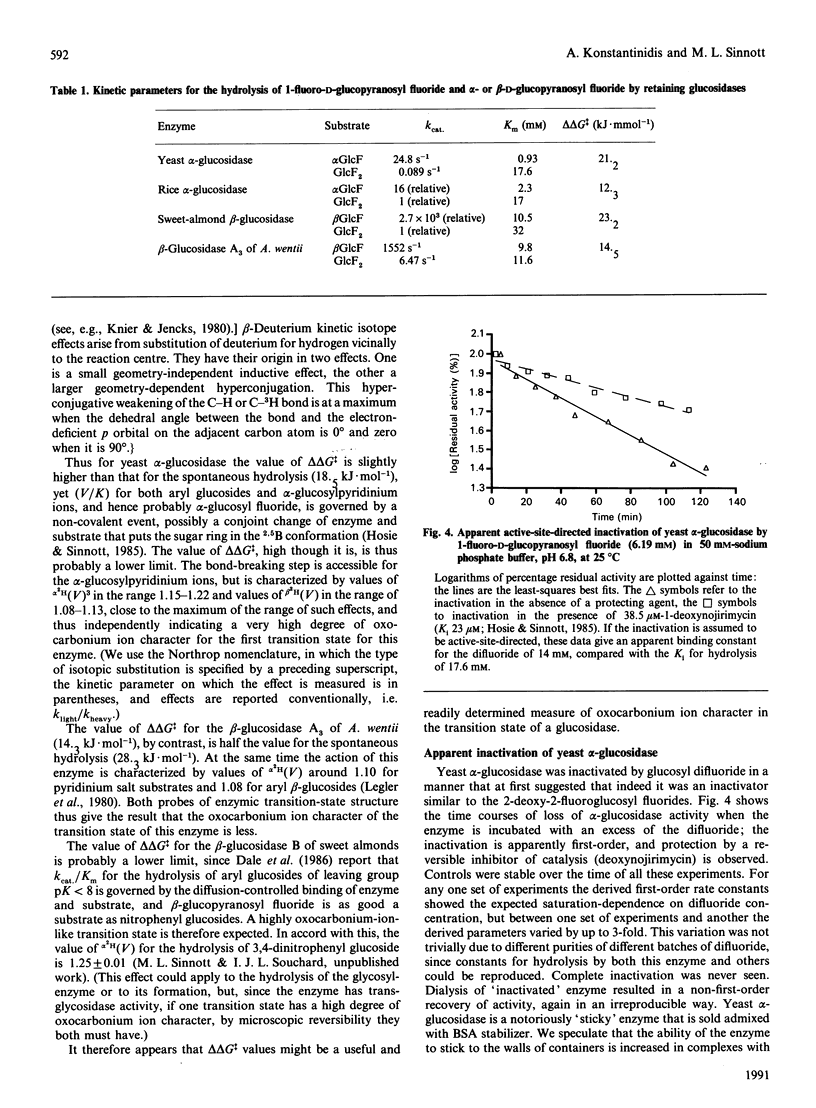
1. 1-Fluoro-D-glucopyranosyl fluoride undergoes pH-independent loss of F- ion at a rate of 1 x 10(-8) s-1 at 50.0 degrees C, some 10(3)-fold slower than alpha-D-glucopyranosyl fluoride and 4 x 10(4)-fold slower than beta-D-glucopyranosyl fluoride. 2. The (inverting) amyloglucosidase II of Aspergillus niger hydrolyses the difluoride according to Michaelis-Menten kinetics (Km 34 mM and kcat. 0.27 s-1), by apparently the same (simple) mechanism by which it hydrolyses alpha-D-glucopyranosyl fluoride (Km 38 mM and kcat. 730 s-1), rather than by the Hehre resynthesis-hydrolysis mechanism used to transform beta-D-glucopyranosyl fluoride. 3. The difluoride is also a substrate for the (inverting) trehalase of pig kidney [Km 17.3 mM and Vmax. 6.2 x 10(-4) relative to alpha-D-glucopyranosyl fluoride (Km 38 mM]). 4. The quantitatively similar effect of fluorine substitution on the one-step enzymic reactions and on the non-enzymic reactions suggests that they go through similar (oxocarbonium-ion-like) transition states. 5. The difluoride is a substrate for the (retaining) beta-glucosidases from Aspergillus wentii (A3 enzyme) and sweet-almond meal (B isoenzyme) and for the retaining alpha-glucosidase from rice: comparison with the appropriate monofluoride reveals a variable rate-retarding effect of the second fluorine atom on kcat./Km that correlates with other measures of oxocarbonium ion character in the transition state. 6. The difluoride is a substrate for the (retaining) alpha-glucosidase from yeast, but also gives an insidious mimicry of active-site-directed irreversible inhibition, which we tentatively attribute either to formation of the non-covalent complex or to the fluoroglucosyl-enzyme increasing the well-known tendency of this enzyme to come out of solution by adsorption on the walls of the vessel.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. E., Jarvis W. T., Munday K. A. The hydrolysis of glycosyl fluorides by glycosidases. Biochem J. 1967 Nov;105(2):669–672. doi: 10.1042/bj1050669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boel E., Hansen M. T., Hjort I., Høegh I., Fiil N. P. Two different types of intervening sequences in the glucoamylase gene from Aspergillus niger. EMBO J. 1984 Jul;3(7):1581–1585. doi: 10.1002/j.1460-2075.1984.tb02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale M. P., Kopfler W. P., Chait I., Byers L. D. Beta-glucosidase: substrate, solvent, and viscosity variation as probes of the rate-limiting steps. Biochemistry. 1986 May 6;25(9):2522–2529. doi: 10.1021/bi00357a036. [DOI] [PubMed] [Google Scholar]
- Hehre E. J., Brewer C. F., Genghof D. S. Scope and mechanism of carbohydrase action. Hydrolytic and nonhydrolytic actions of beta-amylase on alpha- and beta-maltosyl fluoride. J Biol Chem. 1979 Jul 10;254(13):5942–5950. [PubMed] [Google Scholar]
- Hehre E. J., Matsui H., Brewer C. F. Hydrolysis of beta-D-glucopyranosyl fluoride to alpha-D-glucose catalyzed by Aspergillus niger alpha-D-glucosidase. Carbohydr Res. 1990 Apr 2;198(1):123–132. doi: 10.1016/0008-6215(90)84282-y. [DOI] [PubMed] [Google Scholar]
- Hehre E. J., Sawai T., Brewer C. F., Nakano M., Kanda T. Trehalase: stereocomplementary hydrolytic and glucosyl transfer reactions with alpha- and beta-D-glucosyl fluoride. Biochemistry. 1982 Jun 22;21(13):3090–3097. doi: 10.1021/bi00256a009. [DOI] [PubMed] [Google Scholar]
- Hosie L., Sinnott M. L. Effects of deuterium substitution alpha and beta to the reaction centre, 18O substitution in the leaving group, and aglycone acidity on hydrolyses of aryl glucosides and glucosyl pyridinium ions by yeast alpha-glucosidase. A probable failure of the antiperiplanar-lone-pair hypothesis in glycosidase catalysis. Biochem J. 1985 Mar 1;226(2):437–446. doi: 10.1042/bj2260437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffcoat R., Kirkwood S. Implication of histidine at the active site of exo-beta-(1-3)-D-glucanase from Basidiomycete sp. QM 806. J Biol Chem. 1987 Jan 25;262(3):1088–1091. [PubMed] [Google Scholar]
- Kasumi T., Brewer C. F., Reese E. T., Hehre E. J. Catalytic versatility of trehalase: synthesis of alpha-D-glucopyranosyl alpha-D-xylopyranoside from beta-D-glucosyl fluoride and alpha-D-xylose. Carbohydr Res. 1986 Jan 15;146(1):39–49. doi: 10.1016/0008-6215(86)85022-4. [DOI] [PubMed] [Google Scholar]
- Kasumi T., Tsumuraya Y., Brewer C. F., Kersters-Hilderson H., Claeyssens M., Hehre E. J. Catalytic versatility of Bacillus pumilus beta-xylosidase: glycosyl transfer and hydrolysis promoted with alpha- and beta-D-xylosyl fluoride. Biochemistry. 1987 Jun 2;26(11):3010–3016. doi: 10.1021/bi00385a009. [DOI] [PubMed] [Google Scholar]
- Kitahata S., Brewer C. F., Genghof D. S., Sawai T., Hehre E. J. Scope and mechanism of carbohydrase action. Stereocomplementary hydrolytic and glucosyl-transferring actions of glucoamylase and glucodextranase with alpha- and beta-D-glucosyl fluoride. J Biol Chem. 1981 Jun 25;256(12):6017–6026. [PubMed] [Google Scholar]
- Legler G. Untersuchungen zum Wirkungsmechanismus glykosidspaltender Enzyme. 3. Markierung des aktiven Zentrums einer beta-Glucosidase aus Aspergillus wentii mit (14C) Condurit-B-epoxid. Hoppe Seylers Z Physiol Chem. 1968 Jun;349(6):767–774. [PubMed] [Google Scholar]
- Legler G., von Radloff M., Kempfle M. Composition, N-terminal amino acids, and chain length of a -glucosidase from Aspergillus wentii. Biochim Biophys Acta. 1972 Jan 26;257(1):40–48. doi: 10.1016/0005-2795(72)90252-8. [DOI] [PubMed] [Google Scholar]
- MICHEEL F., KLEMER A. Glycosyl fluorides and azides. Adv Carbohydr Chem. 1961;16:85–103. doi: 10.1016/s0096-5332(08)60260-x. [DOI] [PubMed] [Google Scholar]
- Matsui H., Blanchard J. S., Brewer C. F., Hehre E. J. Alpha-secondary tritium kinetic isotope effects for the hydrolysis of alpha-D-glucopyranosyl fluoride by exo-alpha-glucanases. J Biol Chem. 1989 May 25;264(15):8714–8716. [PubMed] [Google Scholar]
- Nakano M., Brewer C. F., Kasumi T., Hehre E. J. Steric course of the hydrolysis of alpha,alpha-trehalose and alpha-D-glucosyl fluoride catalyzed by pig kidney trehalase. Carbohydr Res. 1989 Dec 1;194:139–144. doi: 10.1016/0008-6215(89)85013-x. [DOI] [PubMed] [Google Scholar]
- Street I. P., Rupitz K., Withers S. G. Fluorinated and deoxygenated substrates as probes of transition-state structure in glycogen phosphorylase. Biochemistry. 1989 Feb 21;28(4):1581–1587. doi: 10.1021/bi00430a024. [DOI] [PubMed] [Google Scholar]
- Withers S. G., Rupitz K., Street I. P. 2-Deoxy-2-fluoro-D-glycosyl fluorides. A new class of specific mechanism-based glycosidase inhibitors. J Biol Chem. 1988 Jun 15;263(17):7929–7932. [PubMed] [Google Scholar]


