Abstract
While obesity, rapid weight loss, and sedentary lifestyles contribute to gallstones, the impacts of long-term weight patterns and physical activity on gallstone risk have not been extensively studied at the population level. Using Korea’s population-based health database, we created a cohort of 5,062,154 subjects who received over five consecutive biannual health check-ups from 2002 to 2018. Gallstone risk was calculated using hazard ratios (HRs) based on weight patterns (gain, loss, and cycling) and physical activity. Adjustments were made for covariates, such as body mass index (BMI), waist circumference, diabetes, and life style factors. Subgroup analysis was performed by sex and age groups (< 45 and ≥ 45 years). All groups with weight changes showed increased HRs 1.32 (95% CI 1.07–1.65) for > 20% weight loss, 1.13 (0.96–1.33) for > 20% weight gain, 1.04 (1.02–1.06) for weight cycling, 1.03 (1.01–1.05) for 5–20% weight gain, and 1.02 (1.00–1.04) for 5–20% weight loss. Risk increased with weight loss or gain in underweight or overweight BMI categories, respectively, and in any weight change for normal BMI. Males and older individuals had higher risks with weight loss, while females and younger individuals had higher risks with weight gain. Weight cycling increased risk for both sexes. Regular physical activity reduced gallstone risk in weight changers to levels similar to weight maintainers, with the highest reduction in those with > 20% weight gain (61%) and the lowest in weight cycling (1%). The risks of gallstones associated with weight gain, loss, or cycling can be mitigated by regular physical activity. The risks vary by sex and age.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77218-8.
Keywords: Gallstone, Weight pattern, Physical activity, Risk, Incidence, Sex
Subject terms: Gastroenterology, Risk factors
Introduction
Obesity is a global health concern, not only due to its prevalence but also because of the intense social pressures for weight reduction towards a lean body image. However, sustainable weight management is a challenge for most obese individuals, characterized by weight cycling—defined as intentional weight loss followed by unintentional weight regain—and gradual weight gain1. In South Korea, the prevalence of overweight and obesity among adults surged to 36.1% during 2005–2021, reflecting a growing public health issue2. Studies among Korean adults have shown an increasing trend of older adults overestimating their weight status, with significant percentages of non-overweight individuals attempting weight loss—15% of men and 25% of women over 60 years old2.
The prevalence of gallstones is increasing worldwide, affecting about 20% of the population in Western countries and 5% in Eastern countries3,4. Obesity not only increases the risk of developing gallstones due to excessive body weight but also through the mechanisms involved in body weight reduction5,6. Previous research has indicated that weight cycling, particularly the intensity and frequency of such fluctuations, can exacerbate the risk of symptomatic gallstones6,7. This association, well-documented in Western populations—who generally have higher rates of obesity, weight cycling, and gallstones—has not been extensively studied in Eastern contexts.
Recent shifts in health paradigms suggest that increasing physical fitness and activity may be more beneficial than focusing solely on weight loss for reducing health risks, including mortality and gallstone formation1,8. Indeed, engaging in physical activity is associated with reduced risks of cholecystectomy and gallstone development, suggesting that interventions aimed at promoting physical activity could be particularly effective9. However, the specific effects of physical activity in relation to long-term weight patterns on gallstone risk remain largely unexplored.
This study aims to assess the risks of gallstones relative to long-term weight patterns and physical activity using a population-based cohort from the National Health Insurance (NHI) and National Health Screening Program (NHSP) databases in South Korea. These databases provide comprehensive lifestyle and anthropometric data for all insured Korean populations, facilitating a detailed examination of these associations.
Results
Study population demographics
Table 1 summarized the demographics and covariates of individuals based on long-term weight patterns. Among the 5,062,154 individuals in the study, 31.6% maintained their weight and 27.5% experienced weight cycling over 10 years. Weight changes of 5–20% (gain or loss) were observed in 24.4% and 16.1% of subjects, respectively. Extreme weight changes of more than 20% (gain or loss) were observed in 0.2% and 0.1% of subjects, respectively. Compared to males, females had lower rates of weight maintenance (33.7% for males vs. 28.6% for females) and higher rates of weight cycling (24.5% for males vs. 31.9% for females). Among all groups, weight maintainers had the highest socioeconomic status (SES). Clinical characteristics varied distinctly by weight pattern. The mean ages (with standard deviations) of subjects ranged from the lowest in the group with more than 20% weight gain to the highest in the group with more than 20% weight loss, with ages of 38 (13.9) in more than 20% weight gain, 40 (12.1) in 5–20% weight gain, 44 (13.5) in weight cycling, 49 (12.1) in 5–20% weight loss, and 51 (14.3) years in more than 20% weight loss. The proportions of individuals with overweight and obesity, upper quartile (Q75–100) waist circumference (WC), liver dysfunction, hypertension, wide pulse pressure (PP), and high cholesterol levels increased progressively from the group with more than 20% weight gain to the group with more than 20% weight loss.
Table 1.
Demographic and health characteristics of the study population.
| Characteristics | Weight maintainers | Weight changers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight gainers 20%+ | Weight gainers 5 ~ 20% | Weight cyclers | Weight losers 5 ~ 20% | Weight losers 20%+ | ||||||||
| Number of subjects, % | 1,600,131 | 31.6 | 10,706 | 0.2 | 1,236,882 | 24.4 | 1,394413 | 27.5 | 815,680 | 16.1 | 4342 | 0.1 |
| Sex | ||||||||||||
| Male | 1,003,015 | 33.7 | 5904 | 0.2 | 799,923 | 26.9 | 728,089 | 24.5 | 435,794 | 14.6 | 2172 | 0.1 |
| Female | 597,116 | 28.6 | 4802 | 0.2 | 436,959 | 20.9 | 666,324 | 31.9 | 379,886 | 18.2 | 2170 | 0.1 |
| Ages (years, mean, SD) | 45 | 11.5 | 38 | 13.9 | 40 | 12.1 | 44 | 13.5 | 49 | 12.1 | 50 | 14.3 |
| BMI (kg/m2) | ||||||||||||
| < 18.5 | 37,078 | 2.3 | 2089 | 19.5 | 55,685 | 4.5 | 46,803 | 3.4 | 11,279 | 1.4 | 18 | 0.4 |
| 18.5–25.0 | 1,027,976 | 64.2 | 7763 | 72.5 | 909,669 | 73.5 | 915,539 | 65.7 | 470,727 | 57.7 | 1369 | 31.5 |
| 25.1–29.9 | 502,310 | 31.4 | 791 | 7.4 | 253,509 | 20.5 | 387,956 | 27.8 | 303,425 | 37.2 | 1783 | 41.1 |
| ≥ 30.0 | 32,767 | 2.0 | 63 | 0.6 | 18,019 | 1.5 | 44,115 | 3.2 | 30,249 | 3.7 | 1172 | 27.0 |
| Waist circumference | ||||||||||||
| Q1–25 | 410,843 | 25.7 | 1382 | 12.9 | 272,996 | 22.1 | 394,626 | 28.3 | 271,109 | 33.2 | 1963 | 45.2 |
| Q26–50 | 406,541 | 25.4 | 1839 | 17.2 | 305,360 | 24.7 | 340,922 | 24.4 | 210,260 | 25.8 | 904 | 20.8 |
| Q51–75 | 401,730 | 25.1 | 2458 | 23.0 | 313,764 | 25.4 | 329,197 | 23.6 | 186,099 | 22.8 | 754 | 17.4 |
| Q75–100 | 380,865 | 23.8 | 4989 | 46.6 | 344,535 | 27.9 | 329,411 | 23.6 | 148,140 | 18.2 | 719 | 16.6 |
| Socioeconomic status | ||||||||||||
| Medicare | 935 | 0.1 | 14 | 0.1 | 934 | 0.1 | 1429 | 0.1 | 753 | 0.1 | 3 | 0.1 |
| Q1–25 | 193,246 | 12.1 | 1361 | 12.7 | 146,704 | 11.9 | 212,688 | 15.3 | 123,447 | 15.1 | 637 | 14.7 |
| Q26–50 | 441,125 | 27.6 | 4166 | 38.9 | 394,759 | 31.9 | 472,074 | 33.9 | 250,067 | 30.7 | 1310 | 30.2 |
| Q51–75 | 732,176 | 45.8 | 4363 | 40.8 | 557,597 | 45.1 | 574,107 | 41.2 | 344,435 | 42.2 | 1924 | 44.3 |
| Q75–100 | 218,234 | 13.6 | 687 | 6.4 | 118,517 | 9.6 | 118,923 | 8.5 | 91,575 | 11.2 | 435 | 10.0 |
| Cholesterol (mg/dl) | ||||||||||||
| < 200 | 921,088 | 57.6 | 7422 | 69.3 | 809,939 | 65.5 | 836,609 | 60.0 | 437,439 | 53.6 | 2257 | 52.0 |
| 200–239 | 496,334 | 31.0 | 2463 | 23.0 | 324,098 | 26.2 | 405,931 | 29.1 | 267,943 | 32.8 | 1408 | 32.4 |
| ≥ 240 | 181,577 | 11.3 | 808 | 7.5 | 101,854 | 8.2 | 150,667 | 10.8 | 109,688 | 13.4 | 671 | 15.5 |
| Proteinuria | ||||||||||||
| Negative/equivocal | 1,575,132 | 98.4 | 10,502 | 98.1 | 1,218,990 | 98.6 | 1,369,963 | 98.2 | 799,991 | 98.1 | 4252 | 97.9 |
| Positive | 21,606 | 1.4 | 161 | 1.5 | 15,027 | 1.2 | 20,656 | 1.5 | 13,715 | 1.7 | 78 | 1.8 |
| FBG (mg/dl) | ||||||||||||
| ≤109 | 1,419,595 | 88.7 | 9696 | 90.6 | 1,139,000 | 92.1 | 1,232,504 | 88.4 | 682,741 | 83.7 | 3634 | 83.7 |
| 110–125 | 113,815 | 7.1 | 620 | 5.8 | 64,478 | 5.2 | 96,055 | 6.9 | 75,303 | 9.2 | 406 | 9.4 |
| ≥ 126 | 66,151 | 4.1 | 382 | 3.6 | 32,979 | 2.7 | 65,203 | 4.7 | 57,256 | 7.0 | 297 | 6.8 |
| ALT (U/L) | ||||||||||||
| ≤ 40 | 1,398,859 | 87.4 | 9741 | 91.0 | 1,118,041 | 90.4 | 1,240,180 | 88.9 | 704,316 | 86.3 | 3744 | 86.2 |
| ≥ 41 | 200,160 | 12.5 | 952 | 8.9 | 117,872 | 9.5 | 153,065 | 11.0 | 110,770 | 13.6 | 592 | 13.6 |
| AST (U/L) | ||||||||||||
| ≤ 40 | 1,503,911 | 94.0 | 10,143 | 94.7 | 1,176,507 | 95.1 | 1,308,466 | 93.8 | 756,223 | 92.7 | 4006 | 92.3 |
| ≥ 41 | 95,067 | 5.9 | 550 | 5.1 | 59,381 | 4.8 | 84,748 | 6.1 | 58,846 | 7.2 | 330 | 7.6 |
| GGT (U/L) | ||||||||||||
| ≤ 76 | 1,390,121 | 86.9 | 9753 | 91.1 | 1,116,748 | 90.3 | 1,227,843 | 88.1 | 693,977 | 85.1 | 3723 | 85.7 |
| ≥ 77 | 209,383 | 13.1 | 943 | 8.8 | 119,655 | 9.7 | 165,840 | 11.9 | 121,301 | 14.9 | 611 | 14.1 |
| SBP/DBP (mmHg) | ||||||||||||
| < 120/< 80 | 485,700 | 30.4 | 3903 | 36.5 | 435,776 | 35.2 | 455,308 | 32.7 | 222,987 | 27.3 | 1071 | 24.7 |
| 120–129/< 80 | 161,109 | 10.1 | 1039 | 9.7 | 129,737 | 10.5 | 139,896 | 10.0 | 79,361 | 9.7 | 415 | 9.6 |
| 130–139/80–89 | 574,283 | 35.9 | 3905 | 36.5 | 445,243 | 36.0 | 486,650 | 34.9 | 285,704 | 35.0 | 1448 | 33.3 |
| 140–179/90–119 | 370,226 | 23.1 | 1808 | 16.9 | 221,302 | 17.9 | 303,877 | 21.8 | 221,020 | 27.1 | 1356 | 31.2 |
| ≥ 180/≥ 120 | 8479 | 0.5 | 49 | 0.5 | 4588 | 0.4 | 8420 | 0.6 | 6447 | 0.8 | 50 | 1.2 |
| Pulse pressure (mmHg) | ||||||||||||
| < 39 | 239,970 | 15.0 | 1696 | 15.8 | 193,948 | 15.7 | 206,954 | 14.8 | 110,153 | 13.5 | 501 | 11.5 |
| 40–59 | 1,164,484 | 72.8 | 7908 | 73.9 | 918,273 | 74.2 | 1,007,390 | 72.2 | 578,934 | 71.0 | 3021 | 69.6 |
| ≥ 60 | 195,302 | 12.2 | 1100 | 10.3 | 124,391 | 10.1 | 179,768 | 12.9 | 126,398 | 15.5 | 817 | 18.8 |
| Smoking (Pack-Year) | ||||||||||||
| 0–10 | 1,100,425 | 68.8 | 6986 | 65.3 | 756,134 | 61.1 | 978,831 | 70.2 | 603,693 | 74.0 | 3318 | 76.4 |
| 10–19 | 355,899 | 22.2 | 2929 | 27.4 | 377,074 | 30.5 | 301,563 | 21.6 | 140,366 | 17.2 | 704 | 16.2 |
| ≥ 20 | 106,885 | 6.7 | 651 | 6.1 | 83,595 | 6.8 | 94,051 | 6.7 | 55,846 | 6.8 | 244 | 5.6 |
| Physical activity (≥ 1/week) | ||||||||||||
| None | 95,722 | 6.0 | 909 | 8.5 | 85,766 | 6.9 | 116,088 | 8.3 | 59,451 | 7.3 | 485 | 11.2 |
| Intermittent | 1,232,933 | 77.1 | 8543 | 79.8 | 967,274 | 78.2 | 1,103,328 | 79.1 | 640,035 | 78.5 | 3378 | 77.8 |
| Continent | 251,162 | 15.7 | 1104 | 10.3 | 167,308 | 13.5 | 149,581 | 10.7 | 102,592 | 12.6 | 370 | 8.5 |
| Alcohol drinking | ||||||||||||
| None | 525,019 | 32.8 | 3709 | 34.6 | 384,881 | 31.1 | 504,145 | 36.2 | 304,798 | 37.4 | 1773 | 40.8 |
| < 5 [4] drinks per week | 233,516 | 14.6 | 1668 | 15.6 | 182,688 | 14.8 | 203,421 | 14.6 | 113,581 | 13.9 | 567 | 13.1 |
| < 5 [4] drinks twice a week | 368,552 | 23.0 | 2658 | 24.8 | 332,383 | 26.9 | 283,673 | 20.3 | 147,609 | 18.1 | 685 | 15.8 |
| ≥ 5 [4] drinks twice a week | 99,230 | 6.2 | 539 | 5.0 | 70,667 | 5.7 | 75,629 | 5.4 | 47,835 | 5.9 | 203 | 4.7 |
All data represent number of patients (percent) or mean (standard deviation). Physical activity, none/intermittent (less than once per week) and continuous (more than once per week) over the decade; BMI, body mass index; FBG, Fasting blood glucose; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase: GGT, gamma-glutamyl transferase; SBP/DBP, systolic blood pressure/diastolic blood pressure.
Risk analysis of gallstones according to long-term weight patterns
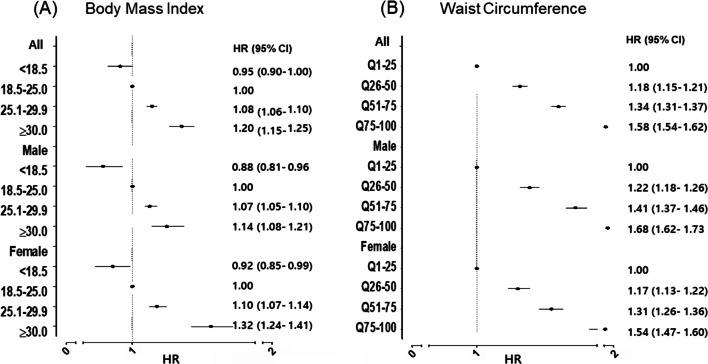
We analyzed gallstone risk according to body mass index (BMI) and WC (Fig. 1). Both overall and central obesity are correlated with gallstone risk, with a greater risk associated with central obesity, hazard ratio (HR) 1.32 (95% confidence interval [CI], 1.24–1.41) for BMI ≥ 30 kg/m2 and HR 1.58 (95% CI 1.54–1.62) for WC75-100. The association between gallstone risk and overall obesity was stronger in females (HR 1.32 for females compared to 1.14 for males). However, the association between gallstone risk and central obesity was more pronounced in males (HR 1.68 for males compared to 1.54 for females).
Fig. 1.
Analysis of gallstone risk associated with obesity in males and females. (A) Body mass index, (B) Waist circumference. Adjusted by age, blood pressure, pulse pressure, cholesterol, fasting blood glucose, proteinuria, AST/ALT, r-GTP, socioeconomic status, physical activity, drinking and smoking.
Table 2 illustrated the incidence rates, incidence rate ratio (IRR) and HR of gallstones by weight patterns. The highest gallstone rates were in subjects with either more than 20% weight gain or loss (50.7 per 10,000 person-years for both), followed by those with 5–20% weight loss (38.1 per 10,000), weight cycling (35.3 per 10,000), and the lowest in those with 5–20% weight gain (31.6 per 10,000). After adjusting for covariates, all weight change groups showed increased HRs compared to those who maintained weight, with the following values: 1.32 (95% CI 1.07–1.65) in more than 20% weight loss, 1.13 (0.96–1.33) in more than 20% weight gain, 1.04 (1.02–1.06) in weight cycling, 1.03 (1.01–1.05) in 5–20% weight gain, and 1.02 (1.00–1.04) in 5–20% weight loss.
Table 2.
Risks of gallstone disease according to long-term weight patterns.
| All | Male | Female | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight pattern | Person-years | Number of cases | Rates (per 10,000)* | IRR (95% CI) | HRs (95% CI)a | Person-years | Number of cases | Rates (per 10,000)* | IRR (95% CI) | HRs (95% CI)a | Person-years | Number of cases | *Rates (per 10,000) | IRR (95% CI) | HRs (95% CI)a |
| Weight maintenance | 8,334,198 | 29,102 | 34.9 | 1 | 5,444,576 | 19,223 | 35.3 | 1 | 1 | 2,889,621 | 9879 | 34.2 | 1 | 1 | |
| Weight gain 20%+ | 21,694 | 110 | 50.7 | 1.45 (0.85–1.14) | 1.13 (0.96–1.33) | 30,132 | 86 | 28.5 | 0.80 (0.65–1.00) | 0.93 (0.73–1.17) | 1,845,059 | 6735 | 36.5 | 1.07 (1.04–1.10) | 1.36 (1.08–1.70) |
| Weight gain 5 ~ 20% | 6,268,751 | 19,815 | 31.6 | 0.91 (0.89–0.92) | 1.03 (1.01–1.05) | 4,184,177 | 12,724 | 30.4 | 0.86 (0.84–0.88) | 1.02 (0.99–1.05) | 10,473 | 49 | 46.8 | 1.37 (1.01–1.81) | 1.06 (1.02–1.10) |
| Weight cycling | 6,973,168 | 24,582 | 35.3 | 1.01 (0.99–1.03) | 1.04 (1.02–1.06) | 3,764,356 | 13,309 | 35.4 | 1.00 (0.98–1.02) | 1.03 (1.01–1.06) | 3,208,812 | 11,273 | 35.1 | 1.03 (1.00–1.06) | 1.04 (1.01–1.07) |
| Weight loss 5 ~ 20% | 4,153,529 | 15,833 | 38.1 | 1.09 (1.07–1.11) | 1.02 (1.00–1.04) | 2,308,470 | 9098 | 39.4 | 1.12 (1.09–1.14) | 1.04 (1.01–1.07) | 1,845,059 | 6735 | 36.5 | 1.07 (1.04–1.10) | 1 (0.96–1.03) |
| Weight loss 20%+ | 21,694 | 110 | 50.7 | 1.45 (1.19–1.75) | 1.32 (1.07–1.65) | 11,221 | 61 | 54.4 | 1.54 (1.18–1.98) | 1.34 (1.00–1,78) | 10,473 | 49 | 46.8 | 1.37 (1.01–1,81) | 1.33 (0.97–1.83) |
*No. of events per 10,000 person-years; aadjusted by age, sex, body mass index, waist circumference, blood pressure, pulse pressure, cholesterol, fasting blood glucose, proteinuria, AST/ALT, r-GTP, socioeconomic status, physical activity, drinking and smoking. IRR, incidence rate ratio; HR, hazard ratio; CI: confidence interval.
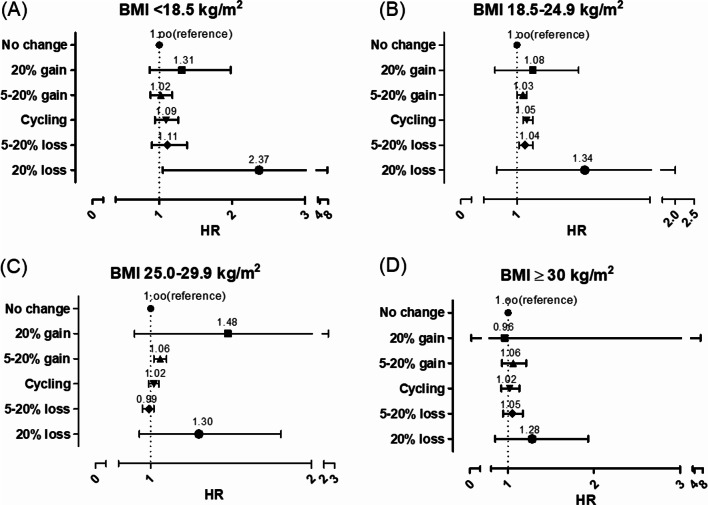
We analyzed gallstone relative risk to long-term weight patterns and BMI categories (Fig. 2). In the underweight BMI group (< 18.5 kg/m2), an increased risk of gallstones was associated with more than 20% weight loss. For those with a normal BMI (18.5–25.0 kg/m2), any weight change was a risk factor for gallstones. In the overweight category (25.0–29.9 kg/m2), only weight gain was a risk factor. However, for those with obesity (> 30.0 kg/m2), no long-term weight patterns were significantly associated with gallstone risk.
Fig. 2.
Analysis of gallstone risk associated with long-term weight patterns by body mass index. (A) BMI < 18.5 kg/m2, (B) BMI 18.5–24.9 kg/m2, (C) BMI 25.0–29.9 kg/m2, (D) BMI 18.5–24.9 kg/m2. BMI, body mass index; HR, hazard ratio. Adjusted by age, sex, waist circumference, blood pressure, pulse pressure, cholesterol, fasting blood glucose, proteinuria, AST/ALT, r-GTP, socioeconomic status, physical activity, drinking, and smoking.
Subgroup analyses on gallstone risks by sex and age
Subgroup analyses, as presented in Table 2, indicated that the risks associated with different weight patterns varied between males and females. Males primarily showed increased risks with weight loss, while females showed increased risks with weight gain. Weight cycling posed an increased risk for both sexes.
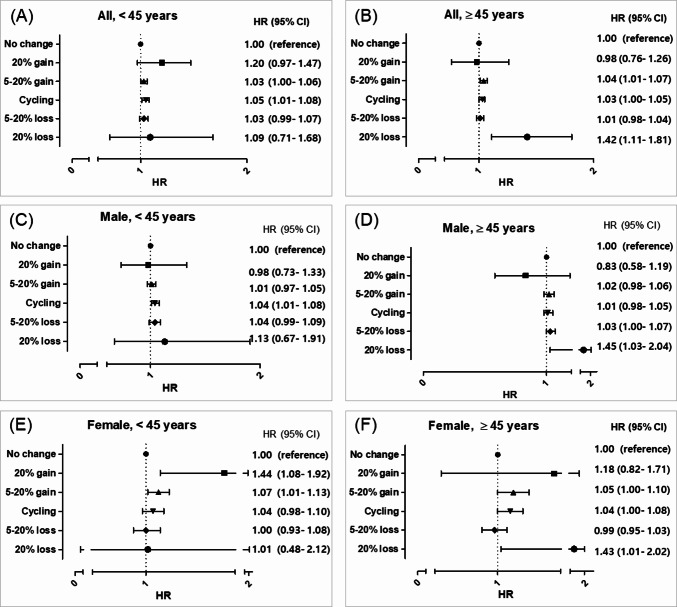
When analyzing HRs according to age at enrollment, younger individuals (under 45 years) displayed the highest risks associated with weight cycling, whereas older individuals (45 years and above) faced the highest risks with more than 20% weight loss (Fig. 3). In particular, younger males were most at risk in the weight cycling category, while older males were predominantly at risk in the weight loss category. Notably, weight gain did not significantly affect the risk of gallstones in males. In contrast, females exhibited different patterns: younger females showed the highest risk with more than 20% weight gain, while older females were most at risk with more than 20% weight loss.
Fig. 3.
Analysis of gallstone risk associated with long-term weight patterns by sex and age. (A) All, < 45 years old, (B) All, ≥ 45 years old, (C) Male, < 45 years old, (D) Male, ≥ 45 years old, (E) Female, < 45 years old, (F) Female, ≥ 45 years old. HR, hazard ratio. Adjusted by body mass index, waist circumference, blood pressure, pulse pressure, cholesterol, fasting blood glucose, proteinuria, AST/ALT, r-GTP, socioeconomic status, physical activity, drinking, and smoking.
Risk analysis of gallstones by the physical activity and long-term weight patterns
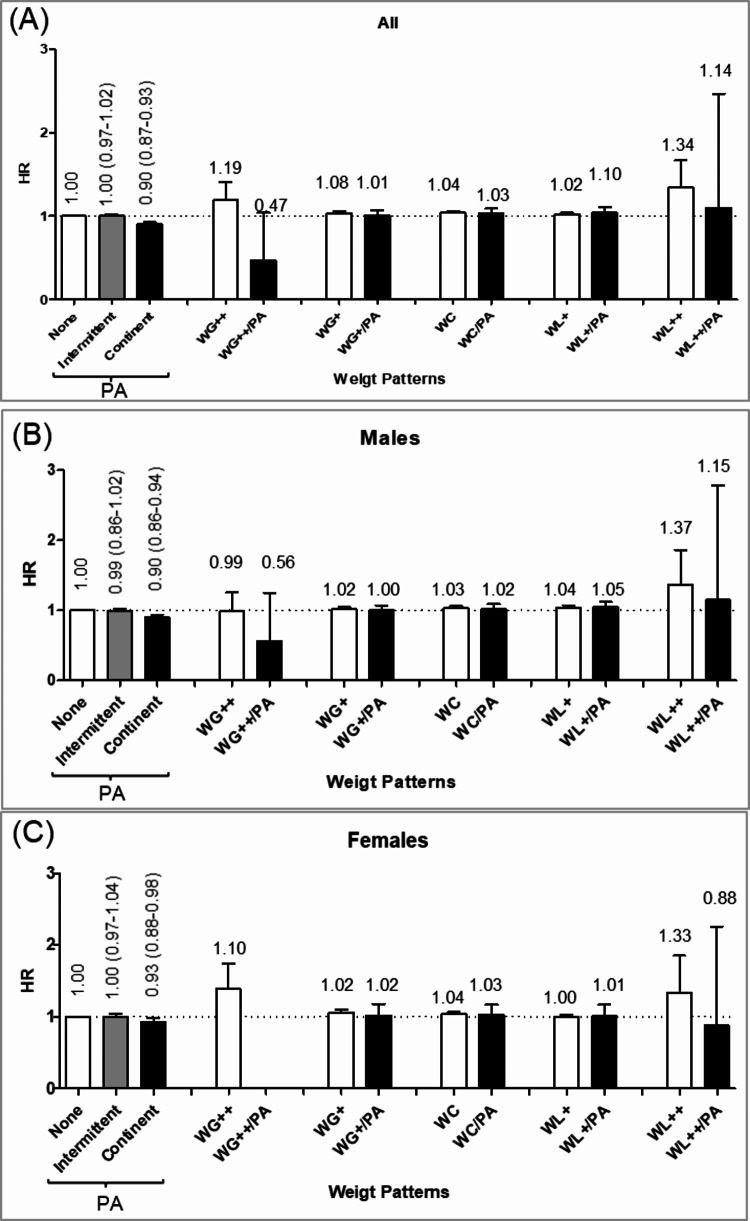
Risks of gallstones by the physical activity and long-term weight patterns were presented in Fig. 4. Consistent physical activity reduced the gallstone risk, revealing an HR of 0.92 (95% CI 0.89–0.96) in both males (0.90; 0.86–0.94) and females (0.93; 0.88–0.98). In individuals with consistent physical activity, all weight changers reduced their gallstone risk to levels similar to weight maintainers. Conversely, those with no or intermittent physical activity showed increased risks for all types of weight changes except the 5–20% weight loss group. The most significant risk reductions attributed to regular physical activity were observed in those gaining more than 20% of their weight (61%), followed by those losing more than 20% (17%), those gaining between 5 and 20% (3%), and those experiencing weight cycling (1%), in descending order. The risk reduction by physical activity manifested in both males and females.
Fig. 4.
Analysis of gallstone risk associated with long-term weight patterns by physical activity. (A) All, (B) Male, (C) Female. PA, physical activity; WG++, > 20% weight gain; WG+, 5–20% weight gain; wc, weight cycling; WL+, 5–20% weight loss; WL++, > 20% weight loss. Adjusted by age, sex, body mass index, waist circumference, blood pressure, pulse pressure, cholesterol, fasting blood glucose, proteinuria, AST/ALT, r-GTP, socioeconomic status, drinking, and smoking.
Risk analysis of covariates associated with gallstones
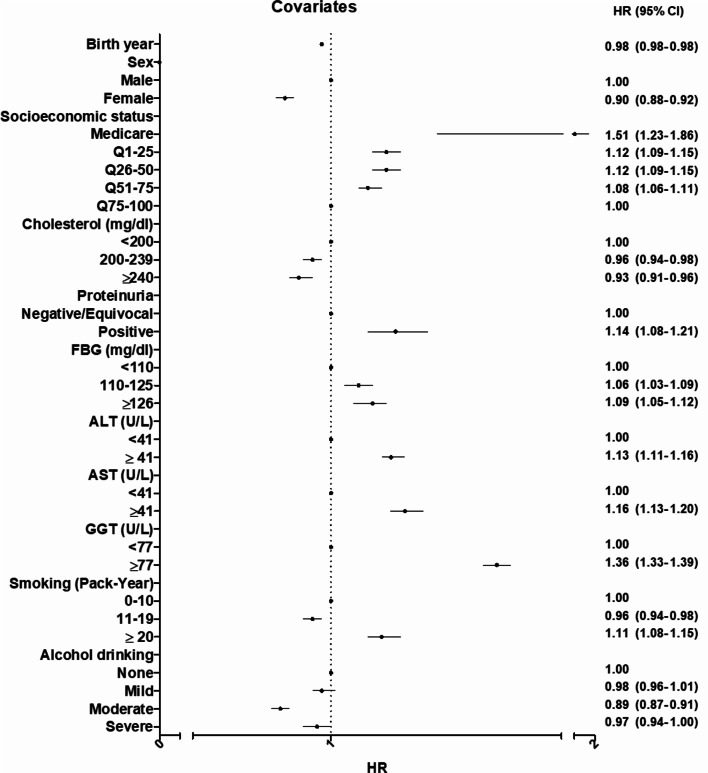
Covariates that increased the risk of gallstones included older age, male sex, low SES, proteinuria, prediabetes or diabetes, abnormal liver function tests, and heavy smoking. In addition, levels of cholesterol, blood pressure (BP), and PP did not show a correlation with the risk of gallstones, as illustrated in Fig. 5.
Fig. 5.
Risk analysis of covariates associated with gallstones.
Discussion
This study demonstrated that the risks associated with gallstones correlate with long-term weight patterns and physical activity. Subjects experiencing weight changes had an increased risk of gallstones compared to those maintaining stable weight, regardless of overall or central obesity. However, consistent physical activity mitigated the gallstone risk associated with weight changes, bringing it to the level of those who maintained their weight.
In this study, weight changers, both weight loss and weight gain as well as weight cycling, heightened the risk of gallstones relative to stable weight, at equivalent levels of obesity. The magnitude of risk varied with the amount of weight change. Subjects with weight changes exceeding 20% exhibited a higher risk compared to those with weight changes between 5% and 20% (increased gallstone risk: 13% vs. 3% for weight gain, and 32% vs. 2% for weight loss, respectively).
While having a low BMI or low WC is associated with a reduced risk of developing gallstones, rapid or excessive weight loss can paradoxically increase the risk of gallstone formation. This is because rapid weight loss triggers several metabolic changes that create a prolithogenic state—a condition that favors the formation of gallstones10. During weight loss, the bile cholesterol saturation index increases and gallbladder stasis occurs11. Weight gain may also increase gallstone risk, potentially related to a fat-dominant body composition, microbial changes, or dietary factors12.
Among the individuals with weight loss or gain, the risk of gallstones was greater in the individuals with severe weight loss (more than 20% of body weight) than severe weight gain (more than 20% of body weight). These results are consistent with previous study, such as one that found the risk of cholecystectomy in women increasing by 14% and 61% with weight gain or loss of more than 5 pounds, respectively, compared to weight maintainers6.
Weight cycling presents another independent risk for gallstones, regardless of obesity status. It alters body composition, leading to fat accumulation, muscle loss, and ectopic fat deposition, which can damage the intestinal barrier, increase epithelial permeability, and cause lipotoxicity13. Subsequent weight loss following obesity can prime adipose macrophages for enhanced inflammation upon weight regain, potentially worsening glucose tolerance14. However, the increased gallstone risk due to weight cycling in this study was only 4%, which is lower compared to 11–51% in Western men7 and 20–68% in Western women6. The lesser impact in Asian populations may be due to less severe weight fluctuations and a lower prevalence of cholesterol stones, although the increasing rates of weight cycling and obesity in recent years suggest that this risk may rise2.
This study also revealed demographic differences in gallstone risk: the increased risk associated with weight gain was more pronounced in females and younger individuals, consistent with previous studies3,10. The gallstone risk from weight loss was more distinct in males and older individuals. Weight loss induces a catabolic state through caloric restriction, which significantly elevates the accumulation of excess fat, possibly linked to the gallstone risk, particularly among the elderly15. The reasons for linking weight loss and males are not clarified. Weight cycling, despite being more frequent in females16, increased the gallstone risk across both sexes but was more significant in younger males. The reasons for sex-predominant gallstone risk by weight patterns need further studies.
An inverse association between physical activity and gallstone was also confirmed in this study9,17,18. Engaging in regular physical activity more than once per week can reduce the risk of gallstones among individuals with weight changes to the level of weight maintainers. Their protective effects were more substantial in those gaining weight compared to those losing weight or cycling in this study. The risk reduction by physical activity is effective in both sexes consistent with previous studies17,18. Physical activity directly reduces gallstone risk by decreasing biliary cholesterol supersaturation and enhancing gallbladder motility10,19. In addition, it reduces the level of deoxycholic acid by accelerating colonic transit. It is also effective in improving glucose tolerance and reducing insulin levels through the enhancement of glucose utilization20.
This study also confirmed that both overall (BMI) and central adiposity obesity (WC) are correlated with gallstone risk, with a greater risk associated with central obesity21,22. Consistent with previous studies8,23, the gallstone risk associated with overall obesity was greater in females. However, the effect of central obesity was greater in males in this study, whereas it was higher in females in Mexican-American24. Additionally, various types of weight changes were linked with gallstone risk across different BMI categories. A new finding in this study was that all types of weight changes were associated with gallstone risk in individuals with a normal BMI. Therefore, maintaining a stable weight is essential to prevent gallstones, even in non-obese individuals.
This study identified several risk factors for gallstones, including older age, male sex, low SES, the presence of proteinuria, prediabetes or diabetes, abnormal liver function tests, and heavy smoking. Conversely, moderate alcohol consumption seemed to have a protective effect against gallstones25,26. Diabetes is recognized as a risk factor for gallstones18,27, with insulin levels playing a role in gallbladder kinetics28. Smoking may influence gallstone formation by promoting endothelial dysfunction and thrombosis, as well as affecting the immune response and microbial composition8,27. Older age contributes to gallstone risk due to slowed metabolism and reduced physical activity. In this study, males exhibited a 10% higher risk of gallstones than females, contrary to trends in Western populations where females predominantly suffer from gallstone12. Low SES, proteinuria, and abnormal liver function tests were also identified as risk factors.
This study offers several advantages over previous research. Firstly, it employs a cohort study design, tracking both weight changers and maintainers to provide a time-based estimate of gallstone risk. Secondly, unlike studies drawing cases from specialized clinics or medical centers, our population-based study included a broader spectrum of the general population in Korea. Thirdly, this is the first study to explore the long-term effects of weight patterns and physical activity on gallstone risk within an Asian context, where the prevalence of gallstone and the predominance of cholesterol stones are lower compared to Western countries. Furthermore, a wide range of covariates related to gallstone risk, including anthropometric measurements, metabolic factors, lifestyle factors, and comorbidities, were adjusted for in the analysis.
However, this study has several limitations. Firstly, symptomatic and asymptomatic gallstones could not be differentiated, as the identification of gallstones relied solely on ICD-10 codes. Some asymptomatic cases may have been overlooked. Additionally, we could not differentiate between intentional and unintentional weight loss. Given the demographics of those experiencing weight loss, who were more likely to be obese and exhibit metabolic risk factors, we suggest a significant proportion were likely attempting intentional weight loss. Lastly, we were unable to identify other potential risk factors such as diet29, genetics, medications, and microbiome influences in this study.
In conclusion, the risks of gallstones associated with weight gain, loss, or cycling can be mitigated by regular physical activity. Maintaining stable body weight with normal BMI combined with regular physical activity are particularly crucial to reduce the risk of gallstones.
Methods
Data sources
Data for this study were sourced from the NHI database of Korea, which contains comprehensive healthcare utilization records, including diagnoses (International Classification of Disease, 10th Revision, ICD-10 codes), screenings, and demographics.
The NHSP, a population-based health screening program, mandates biannual standardized health check-ups for all insured individuals and their dependents. These check-ups capture data through a standard questionnaire on lifestyle habits such as alcohol consumption, smoking, and physical activity. Additionally, they include anthropometric measurements like BMI, WC, and BP, along with basic laboratory tests for fasting blood glucose (FBG) levels, lipid profiles, proteinuria, and liver enzymes.
Study population
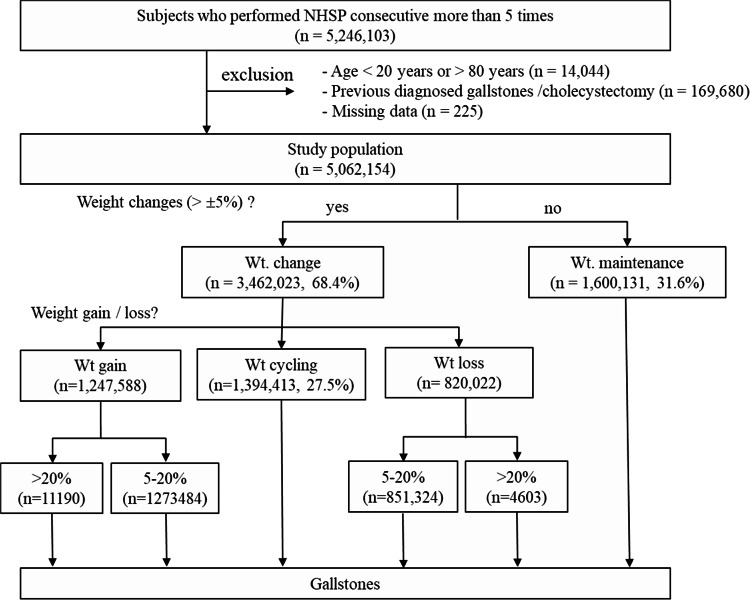
From the NHI and NHSP databases, we identified 5,246,103 individuals who underwent consecutive biannual standardized health checkups more than five times from 2002 to 2018. We excluded individuals with prior histories of gallstones or cholecystectomy (ICD-10 codes K80, or cholecystectomy codes Q7380 and Q7410; n = 169,680), those outside the age range of 20–80 years (n = 14,044), and those with missing data (n = 225). Consequently, 5,062,154 individuals were included in the study (Fig. 6). We focused on individuals newly diagnosed with gallstones (ICD-10 code K80) within the study timeframe.
Fig. 6.
Flow chart of the study. Wt, weight; NHSP, National Health Screening Program.
Assessment of weight patterns, physical activity, and covariates
Weight patterns over the 10-year observation period were categorized based on biennial health check-up data: weight maintainers (individuals whose net weight change was within ± 5%), weight gainers (individuals who experienced more than 5% net weight gain, further subdivided into 5–20% and more than 20%), weight losers (individuals who experienced more than 5% net weight loss, similarly subdivided), and weight cyclers (individuals who gained more than 5% of body weight followed by a loss of more than 5%, while those whose net weight change remained within ± 5%). Physical activity was assessed as light to moderate-intensity activity for at least 30 min per day, or vigorous-intensity activity for at least 20 min per day30. Consistency over the decade was classified as none/intermittent (less than once per week) or continuous (more than once per week).
The covariates for the adjusted model related to gallstone risk were selected based on previous studies, which include obesity (BMI and WC), metabolic factors (FBS, cholesterol level, BP, and PP), lifestyle factors (smoking and alcohol consumption), SES, and comorbidities (liver function tests and proteinuria31). We have included a Directed Acyclic Graph (DAG) in the supplementary Fig. 1 to illustrate the minimal adjustment model.
BMI was calculated as underweight (< 18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥ 30 kg/m2). WC at last health check-up was divided into quartiles: Q1–25, Q26–50, Q51–75, and Q75–100. Based on their NHSP response at enrollment, smoking was categorized according to pack-years of < 10, 10 to < 20, and ≥ 20. Alcohol consumption was categorized according to never, < 5 [4] drinks per week, < 5 [4] drinks twice a week, or ≥ 5 [4] drinks twice a week; numbers in parentheses represent the amount of alcohol consumption in women. Serum biochemical parameters, including FBG (categorized as < 100 mg/dl for normal, 100–125 mg/dl for prediabetes, and ≥ 126 mg/dl for diabetes), total cholesterol (categorized as < 200 mg/dl for normal, 200–239 mg/dl for borderline high, and ≥ 240 mg/dl for high), aspartate aminotransferase (AST) or alanine aminotransferase (ALT) (≤ 40 U/L for normal and > 41 U/L for high), and gamma-glutamyl transferase (GGT) (≤ 76 U/L for normal and > 76 U/L for high), were also included as covariates. Systolic and diastolic BP (SBP/DBP, < 120/<80, 120–129/<80, 130–139/80–89, 140–179/90–119, 180+/120 + mmHg) and PP (< 40, 40–59, 60 + mmHg) were also categorized. Proteinuria was categorized (negative/equivocal or positive). SES was determined by insurance premium categories ranging from below the poverty line to the highest quintile.
Statistical analysis
The study population was followed from enrollment until a diagnosis of gallstone, death, or the end of the study period, calculating person-years accordingly. Cox proportional hazards regression models estimated the IRR and HR with CI for gallstones, adjusted for the identified covariates and stratified by age (< 45 or ≥45 years) and sex. The proportional hazards assumption was verified using Schoenfeld residuals. We used Stata version 15.0 (Stata Corp) in the execution of all statistical analyses.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board Ethics Committee of Korea University College of Medicine (KUIRB-2021-0403-01). Given the retrospective and anonymous nature of the data, informed consent was waived. The data were accessed through the NHIS database of Korea (https://nhiss.nhis.or.kr).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Source of funding; This research was supported by the BK21 FOUR of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 5199990614277). All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author contributions
S.M.P. and H.J.K. contributed to the study design, data interpretation, drafting the manuscript, and finalizing it. K.T.U., M.J.K., and S.H. were responsible for obtaining and processing the data and methodological support.All authors participated in reviewing the manuscript.
Data availability
The data used in this study were acquired from the Korean National Health Insurance (KNHI) Service (https://www.nhis.or.kr/english/index.do) and the Korean National Health and Nutrition Examination Survey (KNHANES, https://knhanes.cdc.go.kr/knhanes/eng/sub03/sub03_01.do). Due to restrictions on the availability of the data, it was used with permission for the current study and is therefore not publicly available. However, the date is available from the corresponding author on reasonable request and with the permission of KNHI and KNHANES.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gaesser, G. A. & Angadi, S. S. Obesity treatment: Weight loss versus increasing fitness and physical activity for reducing health risks. iScience. 24, 102995. 10.1016/j.isci.2021.102995 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee, K. Trends in prevalence of overweight and obesity, self-perceived overweight or obesity, and weight loss efforts among older adults in South Korea, 2005–2021. Prev. Med.180, 107854. 10.1016/j.ypmed.2024.107854 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Colvin, H. S. et al. Risk factors for gallstones and cholecystectomy: A large-Scale Population-based prospective cohort study in Japan. Dig. Dis.40, 385–393. 10.1159/000517270 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Gu, Q., Zhou, G. & Xu, T. Risk factors for gallstone disease in Shanghai: An observational study. Med. (Baltim).99, e18754. 10.1097/md.0000000000018754 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everhart, J. E. Contributions of obesity and weight loss to gallstone disease. Ann. Intern. Med.119, 1029–1035. 10.7326/0003-4819-119-10-199311150-00010 (1993). [DOI] [PubMed] [Google Scholar]
- 6.Syngal, S. et al. Long-term weight patterns and risk for cholecystectomy in women. Ann. Intern. Med.130, 471–477. 10.7326/0003-4819-130-6-199903160-00003 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Tsai, C. J., Leitzmann, M. F., Willett, W. C. & Giovannucci, E. L. Weight cycling and risk of gallstone disease in men. Arch. Intern. Med.166, 2369–2374. 10.1001/archinte.166.21.2369 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Shi, C. et al. Lifestyle factors and the risk of gallstones: Results from the national health and nutrition examination survey 2018–2020 and mendelian randomization analysis. Scand. J. Gastroenterol.58, 1021–1029. 10.1080/00365521.2023.2197093 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Leitzmann, M. F. et al. Recreational physical activity and the risk of cholecystectomy in women. N. Engl. J. Med.341, 777–784. 10.1056/nejm199909093411101 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Stokes, C. S. & Lammert, F. Excess body weight and gallstone disease. Visc. Med.37, 254–260. 10.1159/000516418 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebhard, R. L. et al. The role of gallbladder emptying in gallstone formation during diet-induced rapid weight loss. Hepatology. 24, 544–548. 10.1002/hep.510240313 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Sun, H. et al. Factors influencing gallstone formation: A review of the literature. Biomolecules. 1210.3390/biom12040550 (2022). [DOI] [PMC free article] [PubMed]
- 13.Li, W. & Chen, W. Weight cycling based on altered immune microenvironment as a result of metaflammation. Nutr. Metab. (Lond.). 20, 13. 10.1186/s12986-023-00731-6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou, H. et al. Association between weight cycling and risk of developing diabetes in adults: A systematic review and meta-analysis. J. Diabetes Investig.12, 625–632. 10.1111/jdi.13380 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coker, R. H. & Wolfe, R. R. Weight loss strategies in the Elderly: A clinical conundrum. Obes. (Silver Spring). 26, 22–28. 10.1002/oby.21961 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiffman, M. L., Sugerman, H. J., Kellum, J. M., Brewer, W. H. & Moore, E. W. Gallstone formation after rapid weight loss: A prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am. J. Gastroenterol.86, 1000–1005 (1991). [PubMed] [Google Scholar]
- 17.Zhang, Y. P. et al. Physical activity and the risk of gallstone disease: A systematic review and meta-analysis. J. Clin. Gastroenterol.51, 857–868. 10.1097/mcg.0000000000000571 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Wang, H. et al. Gallstone is associated with metabolic factors and exercise in Korea. Healthcare (Basel). 10. 10.3390/healthcare10081372 (2022). [DOI] [PMC free article] [PubMed]
- 19.Sari, R., Balci, N. & Balci, M. K. Effects of exercise on gallbladder volume and motility in obese women. J. Clin. Ultrasound. 33, 218–222. 10.1002/jcu.20117 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Qian, Q., Jiang, H., Cai, B., Chen, D. & Jiang, M. Physical activity and risk of gallstone disease: A mendelian randomization study. Front. Genet.13, 943353. 10.3389/fgene.2022.943353 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou, L. et al. Anthropometric measurements, physical activity, and the risk of symptomatic gallstone disease in Chinese women. Ann. Epidemiol.19, 344–350. 10.1016/j.annepidem.2008.12.002 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aune, D., Norat, T. & Vatten, L. J. Body mass index, abdominal fatness and the risk of gallbladder disease. Eur. J. Epidemiol.30, 1009–1019. 10.1007/s10654-015-0081-y (2015). [DOI] [PubMed] [Google Scholar]
- 23.Lim, J. et al. Obesity, adiposity, and risk of symptomatic gallstone disease according to genetic susceptibility. Clin. Gastroenterol. Hepatol.20, e1083–e1120. 10.1016/j.cgh.2021.06.044 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haffner, S. M., Diehl, A. K., Stern, M. P. & Hazuda, H. P. Central adiposity and gallbladder disease in Mexican americans. Am. J. Epidemiol.129, 587–595. 10.1093/oxfordjournals.aje.a115171 (1989). [DOI] [PubMed] [Google Scholar]
- 25.Wang, J., Duan, X., Li, B. & Jiang, X. Alcohol consumption and risk of gallstone disease: A meta-analysis. Eur. J. Gastroenterol. Hepatol.29, e19–e28. 10.1097/meg.0000000000000803 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Maclure, K. M. et al. Weight, diet, and the risk of symptomatic gallstones in middle-aged women. N. Engl. J. Med.321, 563–569. 10.1056/nejm198908313210902 (1989). [DOI] [PubMed] [Google Scholar]
- 27.Yuan, S., Gill, D., Giovannucci, E. L., Larsson, S. C. & Obesity Type 2 diabetes, lifestyle factors, and risk of Gallstone disease: A mendelian randomization investigation. Clin. Gastroenterol. Hepatol.20, e529–e537. 10.1016/j.cgh.2020.12.034 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Mathus-Vliegen, E. M., Van Ierland-Van Leeuwen, M. L. & Terpstra, A. Determinants of gallbladder kinetics in obesity. Dig. Dis. Sci.49, 9–16. 10.1023/b:ddas.0000011595.39555.c0 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Di Ciaula, A. et al. The role of diet in the pathogenesis of cholesterol gallstones. Curr. Med. Chem.26, 3620–3638. 10.2174/0929867324666170530080636 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun, M. Y. Validity and reliability of Korean version of international physical activity questionnaire short form in the elderly. Korean J. Fam. Med.33, 144–151. 10.4082/kjfm.2012.33.3.144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park, S. K. et al. The level of urine dipstick proteinuria and its relation to the risk of incident cholelithiasis. J. Epidemiol.31 (1), 59–64 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study were acquired from the Korean National Health Insurance (KNHI) Service (https://www.nhis.or.kr/english/index.do) and the Korean National Health and Nutrition Examination Survey (KNHANES, https://knhanes.cdc.go.kr/knhanes/eng/sub03/sub03_01.do). Due to restrictions on the availability of the data, it was used with permission for the current study and is therefore not publicly available. However, the date is available from the corresponding author on reasonable request and with the permission of KNHI and KNHANES.