Summary
Chagas disease is a complex parasitic zoonosis that still threatens public health across the Americas. Initiatives to control Trypanosoma cruzi transmission via blood transfusion and non-native triatomine-bug vectors have yielded crucial advances; native vectors, however, actively bridge wild and domestic/peri-domestic transmission cycles throughout the region, and tens of thousands of people become infected each year. Oral-transmission outbreaks, urbanisation, and vertical transmission are additional/emerging issues calling for innovative strategic thinking. While critical for advocacy and sustained public health action, assessing Chagas disease burden remains difficult; the often-asymptomatic nature of T. cruzi infection, healthcare access limitations, pervasive underreporting, and other methodological hurdles inherent to reliably measuring incidence, prevalence, and disease progression all contribute to the difficulty. Whether and how parasite, vector, and host genetic makeups affect transmission dynamics and epidemiology is also unclear. Continued high-quality research and long-term, adaptive strategies combining vector control surveillance with enhanced case detection and integral patient care remain critical to effectively address the ethical and societal challenge of Chagas disease control.
This is the first in a Series of five papers about Chagas Disease. All papers in the Series are available at https://www.thelancet.com/series/chagasdisease.
Keywords: Chagas disease, Trypanosoma cruzi, Transmission, Disease burden, Control
Search strategy and selection criteria.
We searched PubMed for articles published in English using a search algorithm with “Chagas disease”, “Trypanosoma cruzi”, “Epidemiology”, and “Americas”. The final references were selected based on relevance to the scope of this Review.
Introduction
Chagas disease, caused by the multi-host zoonotic parasite Trypanosoma cruzi, was first identified in 1909 and has been endemic in the Americas for over nine millennia.1 Many factors contribute to shaping the complex epidemiology of the disease, including environmental conditions, host and vector biology, human behaviour, socioeconomic/political determinants, control programmes, and possibly parasite genetics.2, 3, 4 With seven genetically distinct “discrete typing units” (DTUs TcI to TcVI plus TcBat), T. cruzi is a remarkably diverse protozoan.3 In recent decades, declining vector-borne transmission (due mainly to vector-control efforts and improved housing) and increasing prevalence in previously non-endemic settings (due mainly to international and rural-to-urban migration) have substantially modified the disease landscape. These changes pose new challenges for understanding transmission dynamics and disease burden and for devising effective control and prevention strategies.5
Chagas disease primarily spreads to humans via blood-sucking true bugs of the subfamily Triatominae (Hemiptera: Reduviidae).6 Of about 160 known species across five tribes and 18 genera, the most important vectors are several American species of Triatoma, Rhodnius, and Panstrongylus.6, 7, 8 T. cruzi transmission cycles also involve over 150 domestic, synanthropic (i.e., living in close association with humans), and wild mammalian host species in at least eight orders—Didelphimorphia (opossums), Cingulata (armadillos), Pilosa (sloths, anteaters), Rodentia (rodents), Chiroptera (bats), Carnivora (e.g., dogs, cats), Artiodactyla (e.g., goats, pigs, donkeys), and Primates (monkeys and humans).9, 10, 11 Transmission occurs primarily when a vector feeds on the blood of a susceptible mammal and releases faeces and/or urine containing infective forms of the parasite, which then enter the host through skin lesions or mucous membranes.2, 3, 4, 5, 6,12 Human-vector contact is more frequent, and transmission more likely, when triatomine bugs stably infest houses.6 Other transmission routes include blood transfusion, organ transplantation, consumption of infected food or beverages, and vertical transmission from mother to foetus; predation of infected triatomines may be a relevant infection route among non-human mammals (Table 1).2,12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24
Table 1.
Trypanosoma cruzi transmission routes and probability of infection.
| Route | Details and likelihood of infection |
|---|---|
| Vector-borne | In Chagas disease-endemic areas from the southern USA to northern Argentina and Chile, Trypanosoma cruzi transmission is primarily mediated by vectors.6,25 Large-scale insecticide-spraying campaigns have drastically reduced transmission by non-native populations of Triatoma infestans, T. dimidiata, and Rhodnius prolixus.25 However, house-invading and house-infesting native populations of these three ‘primary’ and dozens of other ‘secondary’ vector species maintain transmission in rural and peri-urban communities throughout the Americas.25 Even though the underlying mechanism is rather inefficient,26 this persistent, geographically-widespread transmission mediated by native vectors still causes thousands of new infections per year and remains the primary driver of incidence.25 Most new vector-borne infections (up to ∼95%) are asymptomatic/oligosymptomatic and remain undiagnosed and unreported.13 |
| Vertical | Vertical transmission is the main source of new infections in non-endemic countries/territories and in urban settings where vectors are rare or absent.14,25 Frequency estimates, however, are sparse and heterogeneous. The probability of transmission from infected mother to child usually ranges between ∼1-2 and ∼5–6%, and may be higher in endemic than in non-endemic countries.15,16 High parasite loads likely increase, and anti-T. cruzi treatment of infected childbearing-aged women likely reduces, the odds of vertical transmission; the roles of maternal immune status, twin pregnancy, or parasite strains/genotypes should be better elucidated.14,25 Not all endemic countries consistently implement screening programs.17,25 |
| Oral | Oral transmission probably helps maintain enzootic infection among non-human mammals.18,19 It may also be a relevant source of human infections, with localised outbreaks and isolated cases reported with increasing frequency from several countries.18,20,25 Contamination of food or beverages by infected vectors (or, more rarely, opossums) is likely the main underlying mechanism, but consumption of infected mammals might also play a role.20 High infectious loads often result in a more severe clinical picture, including more severe acute myocarditis, than what is usually seen in classical vector-borne transmission.21 This conspicuous clinical picture, together with active contact-tracing during outbreaks, likely increases the fraction of incident cases that are diagnosed and reported.25 |
| Transfusion | Transfusion-associated T. cruzi transmission was once a major issue, but nearly-universal donor screening in blood banks across all endemic countries has effectively brought it under control.22,25 The risk of infection after transfusion of one unit of blood from an infected donor has been estimated at ∼10–20% in endemic countries; recent evidence from non-endemic settings suggests that risk may be ∼6–26% for platelets, but just ∼0–4% for packed red blood cells, plasma, and cryoprecipitates.23 |
| Organ transplantation | The risk of transmission associated with transplantation of an organ from an infected donor has been reported to vary between ∼13 and ∼75% in various cohorts and seems to be organ-specific, with transmission apparently less likely in kidney and liver recipients than in heart recipients.24 |
Despite the large reductions in transmission by house-infesting vectors, Chagas disease remains endemic across continental Latin America, with about 75 million people living at risk of infection.27,28 Estimates by the World Health Organization/Pan American Health Organization (WHO/PAHO) suggest that, between the early 1990s and 2010, prevalence fell from ∼18 to 6–8 million infected people, and incidence fell from ∼200,000 to ∼40,000 new cases per year.28,29 Estimates from the Global Burden of Disease (GBD) study,30 however, differ substantially for both past prevalence (∼7.3 million infected people in 1990; ∼6.7 million in 2010) and recent incidence (∼186,000 new cases in 2010; ∼240,000 in 1990).30 While these discrepancies highlight the hurdles inherent to getting robust estimates of key epidemiological parameters and merit detailed scrutiny, steady progress in Chagas disease control is nevertheless undeniable.25,31 Such progress owes mainly to national and regional public-health initiatives fostering vector control and blood- and organ-donor screening,32 likely with contributions from secular trends of housing-quality improvement and the rural exodus,33,34 and has shifted focus towards vertical and oral transmission.18,25
Crucially, however, the vast majority of new infections (likely >90%) go undiagnosed, and an estimated 70% of infected individuals are unaware of their condition.2,13,35 While early detection of T. cruzi infection significantly increases the odds of treatment success,36,37 only about 1% of those infected receive specific aetiological treatment (for which two drugs, benznidazole and nifurtimox, are available) in the Americas.38,39 If left untreated, Chagas disease—and in particular acute oral infections and chronic cardiac and digestive symptomatic forms—is associated with considerable morbidity, mortality, and overall burden.13,25 The wide gap between diagnostic/therapeutic needs and resources arises from a combination of (i) the asymptomatic/oligosymptomatic (i.e., without or with few signs or symptoms) nature of most infections and (ii) healthcare-related issues, including limited Chagas disease awareness among health staff and policymakers, under-resourced primary-care systems, inadequate availability of point-of-care testing and specific drugs, and low screening coverage.25,40 Bridging this gap, which disproportionately affects underserved communities and social groups,4,41 is vital for reducing the burden of Chagas disease and for enhancing patient health outcomes.25
In this series paper we review the current status of Chagas disease in the Americas. We focus primarily on T. cruzi transmission and spread, on the individual and societal burden associated with Chagas disease, and on the strategies used to alleviate such burden. Our aim is not only to provide an updated synthesis of current knowledge, but also to contribute to inform future research, interventions and policies. Other papers published in this Chagas disease series cover further relevant aspects including disease progression and severity,55 the potential effects of climate change on vector-borne transmission,56 diagnosis,57 and recent developments in vaccines and therapeutics.58
Transmission of Trypanosoma cruzi, past and present
Vector-borne transmission
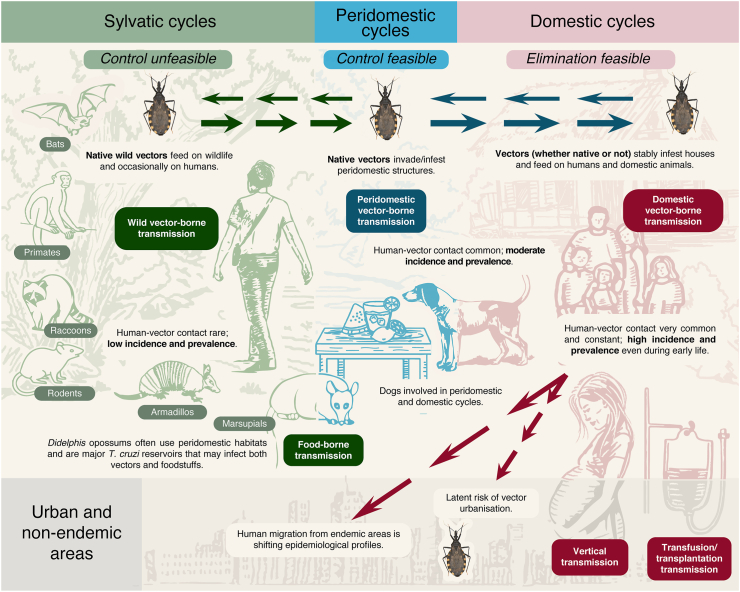
Chagas disease transmission dynamics involve complex interactions between T. cruzi, its insect vectors, and its mammalian hosts including humans (Fig. 1). Notably, however, many other components of the environment also play important roles in T. cruzi transmission. For example, climate/eco-regional patterns and human-mediated (passive) dispersal may dictate which vector and host species (co-)occur at coarse geographic scales;59 land-use patterns (e.g., deforestation, urbanisation) may modulate landscape-scale vector abundance and infection or host-community composition;60, 61, 62 and ecotope-scale vector abundance and infection often vary with, e.g., microhabitat structure (building materials, palm–crown complexity), microclimate, bloodmeal availability (including the blood of vertebrates that do not host T. cruzi, such as birds), human behaviour (e.g., insecticide use, husbandry of domestic animals), predation/pathogen pressure, or the bugs’ microbiota.62, 63, 64, 65, 66, 67 We emphasise, then, that Fig. 1 (and similar figures found in some textbooks and papers) is a simplified depiction of the intricate network of trophic/ecological interactions that—further modulated by parasite, vector, and host genetics—ultimately underpin vector-mediated T. cruzi transmission.
Fig. 1.
Ecoepidemiology and transmission dynamics of Chagas disease in the Americas.
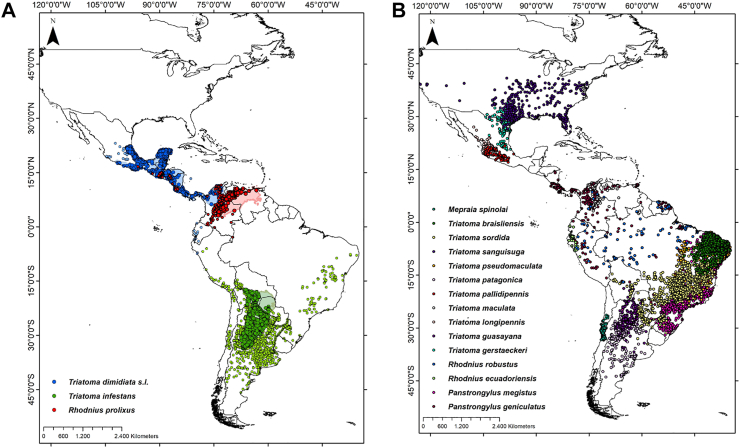
A key historical event in the transition of T. cruzi from wildlife parasite to widespread human pathogen was the accidental introduction of a few highly synanthropic populations of Triatoma infestans, T. dimidiata, and Rhodnius prolixus beyond their native ranges.29 These non-native triatomines heavily infested homes but did not spread back into wild habitats, and became known as ‘primary’, ‘domestic’, or ‘strictly domiciliated’ vectors. Non-native ‘primary’ vectors were successfully targeted by large-scale insecticide-spraying campaigns (see below), but still persist in northern Colombia (R. prolixus), coastal Ecuador (T. dimidiata), and parts of Peru, Argentina, Paraguay and, to a lesser extent, Chile and Brazil (T. infestans).25 Importantly, all ‘primary’ vector species are native to some subregion of Latin America: T. infestans to the dry Chaco and the Bolivian montane dry forests, R. prolixus to the Orinoco basin, and T. dimidiata to Mesoamerica-northern South America (Fig. 2).6, 7, 8,68, 69, 70, 71, 72 These three and 140+ further native-American triatomine bug species maintain T. cruzi transmission cycles in highly diverse microhabitats (from underground to forest canopy) and ecoregions (from hyper-arid deserts to hyper-moist rainforests) over most of the Americas (Fig. 2).6, 7, 8
Fig. 2.
Distribution of Trypanosoma cruzi vector species in the Americas. Main domiciliated vector species (Panel A): larger and darker points correspond to records within the ecoregion-level native range of each species (light-coloured shapes), and smaller and lighter points to recordsof non-native populations (some of which have already been eliminated).7,25 Other important vector species (Panel B). Data sourced mainly from DataTri 2022.71,72
Many such native species also invade and infest houses and peridomestic structures in rural, periurban, and urban settings across the region (Fig. 2).6, 7, 8,68, 69, 70 The prevention of recurrent/persistent T. cruzi transmission to humans by these native vectors is a critical standing challenge.25 Strictly wild vector populations involved in enzootic transmission cycles are not targets for control, but, to the extent that they occasionally enter houses, should be the objects of surveillance.68, 69, 70, 71, 72, 73
The distinction between ‘native’ and ‘non-native’ vector species—which, we note, can only be made after specifying a geographic unit (e.g., country or ecoregion) of reference—has major practical implications: simply put, non-native populations can be locally eliminated, whereas native vectors with widespread wild populations cannot.68, 69, 70,73 Thus, the native/non-native dichotomy was central to the insecticide-based elimination of non-native T. infestans and R. prolixus from large swathes of, respectively, the Southern Cone and Mexico/Central America,25,74,75 but more flexible/adaptive strategies—emphasising the long-term control-surveillance of native vectors and fully integrating case detection and patient care—will be required as the remaining non-native populations dwindle.25,69,70 Critical to the design and operation of such sustainable strategies is the issue of shrinking vector- and case-detection probabilities.73 First, the ‘classical’ pattern of spatially-clustered, heavy house infestations by a single non-native vector species is replaced by a pattern of recurrent house invasions and small infestation foci scattered over vast geographic areas and involving many, often little-known native species.25,68,70 Second, primary-healthcare workers become less familiar with acute Chagas disease as new vector-borne infections, which are almost always asymptomatic or oligosymptomatic,13 become rarer, further lowering the chances of case detection.73 As risk and incidence become less visible, policymakers may feel justified in diverting limited resources away from Chagas disease surveillance, which can only reduce visibility even further and may lead to a perverse, positive feedback loop undermining disease prevention.68
Vertical transmission
Vertical (mother-to-child) transmission of T. cruzi is the leading driver of Chagas disease incidence in vector-free non-endemic countries and in Latin American cities or territories where vector- and transfusion-mediated transmission are effectively under control.76, 77, 78, 79 In 2010, WHO/PAHO estimated that ∼1.13 million women of childbearing age (15–44 y old) were infected with T. cruzi, leading to approximately 8700 new cases of congenital Chagas disease each year.28 More recently, PAHO suggested that at least 15,000 cases occur annually in Latin America.25,80
Meta-analytic summaries of the average rate of vertical T. cruzi transmission (or ‘risk’, measured as the percent of infected children among those born to infected mothers) have ranged from ∼4.7% (95% CI 3.9–5.6; studies published in 1975–2012) to ∼2.0% (1.0–2.0; studies published in 2004–2019).15,16 Transmission risk appears to vary widely among subregions, and may be higher in endemic (∼5%) than in non-endemic countries (∼3%).15,78,81 A recent prospective, multicentric observational study illustrates inter-country variation within Latin America.82 Seropositive mothers (73.2% of which also tested positive by blood PCR) were enrolled in Mexico (n = 32), Honduras (n = 182), and Argentina (n = 136); while Argentina and Mexico reported vertical transmission rates of 6.6% (95% CI 3.1–12.2) and 6.3% (0.8–20.8), respectively, there were no confirmed congenital cases in Honduras.82 The factors driving this variation in vertical-transmission risk remain overall poorly understood.14 Higher maternal bloodstream parasite loads probably increase vertical-transmission risk, and risk may also be higher when previous pregnancies resulted in vertical transmission and when the mother's infection was itself congenital.81,83, 84, 85, 86, 87 The evidence is weaker or inconclusive in the case of other putative transmission-risk drivers/modulators—including characteristics of mothers (e.g., age, parity, residence in urban vs rural areas, exposure to vectors, immune-effector levels), newborns (e.g., sex, number of siblings, birth order, immune effectors), and parasites (DTUs, strains, virulence factors)—, and further research is needed to clarify their effects and relative importance.14,78,81
Crucially, mounting evidence suggests that treatment of infected childbearing-aged girls and women with specific anti-T. cruzi drugs reduces the odds of vertical transmission in subsequent pregnancies.25,88, 89, 90, 91, 92, 93 Furthermore, the early detection of congenital infections virtually guarantees treatment success, with cure rates close to 100%.77,78 These findings have galvanised efforts towards expanding maternal and newborn Chagas disease screening, including the PAHO-led ‘EMTCT plus Framework for Elimination of Mother-To-Child Transmission of HIV, Syphilis, Hepatitis B, and Chagas’—which advocates for the inclusion of T. cruzi-infection testing in routine ante/perinatal screening and seeks to (i) achieve 90% T. cruzi-screening coverage of pregnant women and children born to seropositive mothers, and (ii) provide treatment to 90% of seropositive women after delivery and infant breast-feeding.80,94 Economic evaluations suggest that ante/perinatal screening for T. cruzi infection, with specific treatment offered when needed, would be clearly cost-effective in both endemic and non-endemic settings.77,95 Routine screening, however, has yet to be widely implemented; substantial variation in diagnostic guidelines/algorithms across countries and territories reflects both technical/operational hurdles and a lack of expert consensus.25,77, 95 Universal screening of childbearing-aged women born in (or to mothers from) endemic regions might hold the key to the primary prevention of congenital Chagas disease.25,77,81
Oral transmission
Oral transmission of T. cruzi is much more efficient than ‘classical’ (percutaneous) vector-borne transmission96 and may represent the ancestral transmission route.21,97, 98, 99 It is probably common among wild insectivorous/omnivorous mammals that eat infected triatomines, among carnivores that eat infected prey, and among didelphid opossums exposed to anal scent-gland secretions of infected conspecifics.10,19,98,100 Similarly, transmission to humans may be the result of eating/drinking (i) raw or undercooked meat/organs/blood of infected mammals or, more frequently, (ii) food/beverages contaminated by infected vectors (or their faeces/urine) or by the scent-gland secretions of infected opossums.20 Oral transmission of human Chagas disease has been documented in Colombia, Venezuela, Brazil, French Guiana, Ecuador, Bolivia, and Argentina, but probably occurs elsewhere too.81,98,99,101, 102, 103 Foodborne transmission tends to yield spatial–temporal case-clusters or ‘outbreaks’ of acute Chagas disease involving people who shared an infected meal, although isolated cases have also been reported and many might go undiagnosed.101 There is no conclusive evidence about transmission of T. cruzi through breast-feeding—which should only be discontinued in acute or reactivated Chagas disease or if there is nipple bleeding.104
While outbreaks of foodborne Chagas disease were first reported in the late 1960s,105 some recent, well-studied instances—such as the 2007 Caracas outbreak resulting in 103 infections among students and staff at a local school—have highlighted the importance of this transmission route.106 An investigation of 49 outbreaks across South America up to 2019 identified 15 outbreaks associated with the consumption of açaí (Euterpe spp.) palm-fruit pulp—a staple food in the eastern Brazilian Amazon.107 Sugar-cane juice, fruit juices (guava, mango, orange, or rose-apple), palm products (mainly from Attalea and Oenocarpus spp.), and even water and soup, have also been linked to outbreaks; the evidence, though, is often circumstantial, and in many outbreaks no specific source of infection was identified.107 Genotyping of T. cruzi isolates from a small number of foodborne outbreaks revealed TcI in Colombia, Venezuela, French Guiana, and northern Brazil; TcII in southern Brazil; and TcIII and TcIV in the Brazilian Amazon.101,102,106,108, 109, 110, 111
Foodborne T. cruzi infections are often more clinically patent, more severe, and more deadly than vector-borne infections; this has been linked to overall larger infectious loads, and may result in a particularly high per-case disease burden.20 Importantly, increased disease severity and active contact tracing/testing—which usually ensue when oral transmission is suspected— both enhance the visibility of orally-transmitted acute infections, thus helping reduce underreporting. This is a sharp contrast to the often asymptomatic vector-borne infections.25,107
Although prevention of foodborne Chagas disease is still an open issue, current knowledge suggests that it will require considering distinct transmission scenarios in (mainly) urban vs rural settings.111, 112, 113, 114, 115, 116 Urban outbreaks may involve (i) infected vectors accidentally carried within fruit cargos from harvesting or distribution sites into nearby cities; (ii) local vector populations breeding in, e.g., urban parks, forest remnants, or human dwellings; or (iii) synanthropic opossums (that live in close association with humans and thrive in anthropic environments).113, 114, 115 Contamination of commercially-distributed foodstuffs is of greater concern in urban than in rural settings. Rural outbreaks (and likely also isolated transmission events) are often linked to family- or community-based production of non-commercial food or beverages that become contaminated when vectors fall into (or infest) food-processing gear.113,114 In both rural and urban settings, foodborne Chagas disease prevention and early case detection and management require strong inter-sectoral health education, promotion, and communication initiatives aimed at all stakeholders—food producers, distributors, vendors, and consumers; healthcare providers; public-health officials and decision-makers; and the general public.25,96,112 Food-safety regulation is seen as key to preventing urban outbreaks linked to food retail, but rural transmission may be less sensitive to regulation. In rural settings, prevention should focus instead on creating the conditions for safer food production and management at the household level, including, e.g., insect-screening of food-processing premises/equipment and improved hygiene along the entire production process—from food handling, processing, and storing to the maintenance of food-related gear.25,96,112,116
Transfusion- and transplantation-mediated transmission
Transfusion-associated T. cruzi transmission caused tens of thousands of new infections per year up to the early 1990s.117, 118, 119 Since then, nearly-universal donor screening in endemic countries has effectively brought it under control.25,22,120 Screening is now mandatory in all countries where the parasite circulates naturally, including the USA.12,25 However, the sheer amount of donations and transfusions, the often non-negligible prevalence of infection among donors, and the slightly suboptimal performance of screening tests, taken together, mean that mandatory screening likely misses a non-trivial number of infected donations. For example, WHO/PAHO estimates that ∼93 per 10,000 (0.93%) blood donations made in Latin America in 2010 contained T. cruzi28; assuming that all donations were screened with a highly (99%) sensitive test, then ∼930 infected donations yielded (false-)negative screening-test results, and hence were labelled as safe, per million blood donations. This suggests that T. cruzi infection likely went undetected in over 9000 of the ∼9.9 million blood donations that, according to PAHO, were made in Chagas disease-endemic countries in 2017.121 Although not all infected donations necessarily result in T. cruzi transmission (see Table 1), clinicians and public-health officials should keep in mind that sporadic transfusion-related infections likely occur each year across the Americas in spite of universal screening.25,120
According to WHO/PAHO estimates for 2010, the average prevalence of T. cruzi infection among blood donors (given here as number of positives per 10,000) in 17 Latin American countries ranged from 45 in Costa Rica to ∼3000 in Argentina (median, 390; IQR 190–1340).28 Prevalence was >2000 in Argentina, Paraguay, and Bolivia; >1000 in El Salvador and Guatemala; and relatively low (<200) in Costa Rica, Chile, Honduras, Brazil, and Ecuador.28 Prevalence was intermediate (∼350 per 10,000 donors) in Guyana by the late 2000s.122 In the USA, recent estimates vary from 3.3 (ref. 12) to 6.4 (ref. 123), with much higher values reported for, e.g., Florida (∼26), California (∼12 overall but up to ∼36 in the southern part of the state), and Texas (∼15 per 10,000).123, 124, 125 Data from Canada suggest a prevalence slightly above 3 per 10,000 donors.126 In 2005, Schmuñis and Cruz noted that, technically, universal screening for T. cruzi infection may not be cost-effective when donor infection prevalence is very low; quite aptly, they then observed that “it is far more difficult to explain this concept to the recipient of a T. cruzi-infected blood unit”.120
Transmission of T. cruzi can also occur through transplantation of organs or bone marrow from infected donors, and may lead to severe disease in immunosuppressed patients.29,97,127,128 Transmission can occur when infected donors are not screened; when screening yields false-negative results; or when transplantation from a known infected donor proceeds because the benefits for the recipient are deemed to outweigh the risks—the management of which heavily relies on timely diagnosis and treatment.29,81,128 Although the evidence is weak overall, the probability of transmission seems to be higher in heart (>75%) than in liver (∼20%) or kidney (∼10–15%) transplantation.29,127,129 Immunosuppressive therapy can also lead to (potentially severe) reactivation of Chagas disease in T. cruzi-infected individuals receiving organ transplantations, especially in heart recipients.97,127,128,130 Again, pre-transplantation serological screening and risk management play critical roles in improving patient outcomes.29,81,97,127,128 As with blood transfusion, international migration has outspread the risk of transplantation-mediated T. cruzi transmission to non-endemic settings.12,129,131
The genetic diversity of Trypanosoma cruzi
Trypanosoma cruzi is a genetically highly diverse parasite.3,132 Extensive genotyping and phylogenetic studies have so far led to the recognition of seven discrete typing units (DTUs): TcI to TcVI plus Tcbat.3 Understanding DTU distribution patterns, both in space and among vectors and vertebrate hosts including humans, is paramount for elucidating the epidemiological landscape of Chagas disease. Despite early indications and some suggestive patterns, there is no definite evidence for any clear–cut association between T. cruzi DTUs and distinct transmission cycles (e.g., ‘sylvatic’ vs ‘domestic’ or arboreal vs terrestrial), different transmission routes (e.g., vector-borne vs foodborne vs vertical), or the severity of human Chagas disease.8,133,134 To our knowledge, no broad-scale representative survey looking at such putative associations has so far been conducted, and the evidence is therefore patchy; furthermore, the methods used for genotyping in the overwhelming majority of studies (from allozyme electrophoresis to PCR-based techniques) may not capture the true within-host diversity and dynamics of parasite strains.135,136
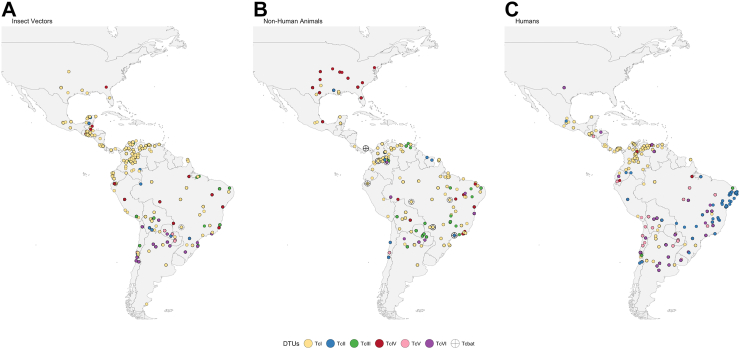
Current knowledge suggests that all DTUs from TcI to TcVI have a broad geographical distribution across the Americas.3,132, 133, 134, 135, 136, 137 While certain DTUs may be more frequently found in some regions, no DTU can be considered strictly endemic to a particular area, and their overlapping distributions (Fig. 3) should be kept in mind when evaluating the possibility of mixed infections.137 Overall, TcI emerges as the DTU most often detected in samples drawn from infected humans, but TcII, TcV, and TcVI also contribute to an important proportion of infections.133 Notably, almost all DTUs are implicated in congenital transmission.111 From a clinical perspective, the relations between DTUs and disease outcomes show high variability, and efforts are underway to establish whether there are robust associations (Table 2).133,134,138
Fig. 3.
Distribution of DTUs in the Americas in vectors, non-human animals, and humans. Distribution of DTUs identified in vectors (Panel A), non-human animals (Panel B), and humans (Panel C). The size or shape of the symbols does not indicate the exact geographic location or frequency as some points may be overlapped. Map created with R Software. Data source.137
Table 3.
Model-based estimates of Chagas disease prevalence and incidence in the Americas.
| Country | Source |
|||||
|---|---|---|---|---|---|---|
| WHO/PAHO 201028 |
GBD 2010a |
GBD 2021a |
||||
| Prevalence (%) | Incidence (rate per 100 k)b | Prevalence (%) | Incidence (rate per 100 k) | Prevalence (%) | Incidence (rate per 100 k) | |
| Canada | NR | NR | 4474 (0.01) | NR | 4413 (0.01) | NR |
| United Statesc | NR | NR | 216,649 (0.07) | NR | 241,691 (0.08) | NR |
| Mexico | 876,458 (0.78) | 6135/1788 (5/89) | 912,265 (0.83) | 28,198 (24.55) | 1,182,286 (0.96) | 32,469 (25.12) |
| Belize | 1040 (0.33) | 10/25 (3/333) | 35 (0.01) | 2 (0.51) | 52 (0.01) | 2 (0.55) |
| Guatemala | 166,667 (1.23) | 1275/164 (9/35) | 92,591 (0.71) | 3554 (26.10) | 101,726 (0.67) | 3460 (21.94) |
| Honduras | 73,333 (0.92) | 933/257 (11/126) | 65,328 (0.85) | 2523 (31.59) | 78,686 (0.81) | 2805 (27.74) |
| El Salvador | 90,222 (1.30) | 972/234 (13/187) | 37,344 (0.64) | 1215 (19.96) | 37,367 (0.61) | 1127 (17.47) |
| Nicaragua | 29,300 (0.52) | 383/138 (6/124) | 37,382 (0.67) | 1341 (23.39) | 41,101 (0.64) | 1362 (19.88) |
| Costa Rica | 7667 (0.17) | 10/61 (0.2/80) | 20,587 (0.49) | 618 (14.09) | 21,486 (0.47) | 571 (12.03) |
| Panama | 18,337 (0.52) | 175/40 (4/56) | 20,219 (0.60) | 601 (17.18) | 21,111 (0.51) | 605 (14.09) |
| Colombiad | 437,960 (0.96) | 5274/1046 (11/114) | 121,954 (0.29) | 3875 (8.71) | 129,569 (0.27) | 3772 (7.69) |
| Ecuador | 199,872 (1.38) | 2042/696 (14/317) | 137,537 (0.95) | 4106 (27.32) | 144,098 (0.83) | 3995 (22.12) |
| Venezuela | 193,339 (0.71) | 873/665 (3/110) | 479,699 (1.78) | 15,857 (57.05) | 461,326 (1.79) | 12,398 (46.56) |
| French Guiana, Guyana, and Suriname | 12,600 (0.84) | 280/18 (18/75) | NR | NR | NR | NR |
| Guyana | NR | NR | 33 (<0.01) | 2 (0.24) | 31 (<0.01) | 2 (0.22) |
| Suriname | NR | NR | 40 (0.01) | 2 (0.35) | 42 (0.01) | 2 (0.34) |
| Brazil | 1,156,821 (0.61) | 46e/571 (0.08/20) | 1,857,485 (0.96) | 58,955 (29.81) | 2,058,211 (0.96) | 60,162 (27.30) |
| Peru | 127,282 (0.44) | 2055/232 (7/38) | 188,055 (0.66) | 5686 (19.67) | 196,295 (0.55) | 5594 (15.42) |
| Bolivia | 607,186 (6.10) | 8087/616 (81/235) | 559,626 (5.77) | 17,142 (170.42) | 469,788 (4.12) | 13,589 (115.19) |
| Paraguay | 184,669 (2.13) | 297/525 (3/340) | 31,794 (0.53) | 1064 (17.30) | 32,427 (0.47) | 1019 (14.22) |
| Uruguay | 7852 (0.24) | 0f/20 (0/40) | 25,016 (0.78) | 0f | 18,748 (0.57) | 0f |
| Argentina | 1,505,235 (3.64) | 1078/1457 (2/210) | 1,103,871 (2.80) | 25,422 (61.84) | 768,416 (1.76) | 17,417 (38.29) |
| Chileg | 119,660 (0.70) | 0f/115 (0/46) | 288,220 (1.81) | 0f | 236,228 (1.31) | 0f |
NR, not reported.
GBD estimates originally include measures of uncertainty not presented here; see30 for details.
Overall/congenital (new cases per 100,000 population/new congenital cases per 100,000 live births).
Prevalence estimated at 288,000 in 2014–2018.139
Prevalence estimated at 506,000 in 2020.42
This is likely a gross underestimate and should be interpreted with caution; official notification records for 2010 include 130 new, confirmed cases of acute Chagas disease—1 vertical, 30 vector-borne, 63 foodborne, and 36 in which the transmission route was either unknown or not reported (https://datasus.saude.gov.br/informacoes-de-saude-tabnet/; accessed June 11 2024). If underreporting was similar for vertical (1/571, or ∼0.175%) and non-vertical transmission, then 26,266 non-vertical transmission-mediated cases would have had to occur to yield the ‘46 new cases’ estimate.
Assumed zero (i.e., not modelled) based on PAHO certificates of interruption of transmission.
In Chile, incidence was nonzero in each year between 2007 and 2021, with, respectively, 44, 115, 268, 438, 520, 24, 1, 2, 2, 1, 2, 4, 14, 18, and 7 new, confirmed cases of acute Chagas disease reported by the Ministry of Health (1460 new cases overall); based on these official data, the incidence rate was ∼2.6 per 100,000 population in 2010, but just ∼0.04 per 100,000 in 2021.140 We did not find any official data from Uruguay.
TcI is itself also genetically highly diverse and has been linked to human Chagas disease in the USA, Mexico, Central America, and the Andean and Amazon regions; TcI infections can cause severe cardiomyopathy and meningoencephalitis, particularly in immunocompromised individuals, but have rarely been associated with digestive outcomes.111,134 TcI has been found infecting mammals in the orders Didelphimorphia, Cingulata, Pilosa, Rodentia, Chiroptera, Carnivora, Artiodactyla, and Primates.132,142 TcBat was first found in bats and later detected in a few humans and triatomine bugs; it seems to be phylogenetically closer to TcI than to other DTUs, and has so far been reported from Panama, Colombia, Ecuador, Chile, and Brazil.134
TcII and putative TcII/TcIII hybrid lineages (TcV and TcVI) are overall less genetically diverse than TcI and have been linked to chronic infections with cardiac and digestive disease in southern and central South America.111 TcV is common in the Gran Chaco (which covers parts of Argentina, Paraguay, and Bolivia), where it may cause digestive and (relatively mild) heart disease.143,144 Besides humans, there are records of TcV and/or TcVI in domestic (dogs, cats, guinea pigs, goats, sheep, pigs), wild (opossums, armadillos, rodents, bats), and captive mammals (macaques), as well as in Triatoma, Panstrongylus, Rhodnius, and Mepraia vectors.132,135,141,142 Both TcV and TcVI have wide geographic distributions, with records spanning from the southern USA to Argentina.132,135,142
TcIII has been found circulating in sylvatic transmission cycles mainly among armadillos, but also infects rodents, marsupials, carnivores, bats, and non-human primates.132,142,145, 146, 147, 148 Species of Panstrongylus, Triatoma, and Rhodnius maintain TcIII transmission in both terrestrial and arboreal habitats.8,132,142 TcIII infections have been reported in both foodborne-acute and chronic-indeterminate human Chagas disease patients, as well as in domestic dogs.141,147,149 Patients coinfected with TcIII plus TcV or TcVI may develop chronic Chagas heart disease.148,150 Overall, available TcIII records cover the whole of continental Latin America, from Mexico to Argentina and from semi-arid to rainforest environments.132,142
Trypanosoma cruzi TcIV infects humans, non-human primates, carnivores (including domestic dogs), bats, armadillos, and rodents, and can be transmitted by species of Triatoma, Panstrongylus, Rhodnius, and Dipetalogaster.132,135,142,151 TcIV has been implicated in acute vector-borne infections and human foodborne outbreaks, and can also cause chronic infections and heart disease.113,146,152, 153, 154, 155, 156
The striking levels of genetic diversity in T. cruzi have implications for our understanding of critical aspects of Chagas disease including clinical outcomes, parasite tissue tropism and pathogenesis, diagnosis (especially through serology), and epidemiology.3,133,138,157,158 The complex interactions between parasite genetics, host genetic background and immune responses, and environmental factors likely contribute to the diverse clinical outcomes observed across different regions.3,138,159 Continued interdisciplinary research will be required to unravel the intricacies of T. cruzi DTU transmission dynamics and their implications for public health.
The epidemiology and burden of Chagas disease
Over the last two decades, the complexities of accurately estimating the burden of Chagas disease across the Americas have become increasingly apparent and understood. These complexities include substantial geographical variability, evolving epidemiological patterns, the extended period between initial infection (which is itself asymptomatic in about 95% of the cases) and chronic-symptom onset (which occurs in only 30–40% of those infected), gross underreporting of both cases and deaths and, in general, the scarcity of comprehensive data across both endemic and non-endemic regions.160 The main challenges to estimating Chagas disease burden are summarised in Box 1.
Box 1. Challenges and opportunities for estimating Chagas disease burden.
Challenges
Asymptomatic acute phase:
Most new infections (about 95% of those mediated by vectors) do not produce any signs or symptoms; when they do, clinical manifestations are mostly non-specific (local skin inflammation [‘chagoma’] at the site of parasite entry, fever, oedema, rash, malaise, asthenia, etc.), with only Romaña's sign (unilateral conjunctivitis with palpebral oedema) clearly helpful for clinical diagnosis. This leads to massive underdetection and underreporting of new cases and hampers the estimation of disease incidence.
Asymptomatic chronic infection:
The indeterminate form of chronic Chagas disease lasts decades, with 60–70% of patients never evolving towards symptomatic forms. This means that most patients likely remain undiagnosed until they either donate blood or develop chronic disease. Delayed symptom onset hampers the estimation of both incidence and prevalence.
Surveillance systems and diagnosis access:
Diagnostic approaches vary widely, with some countries relying on specific services like blood banks and others offering broader diagnosis access. The limited availability of diagnostic tests undermines the reliability of traditional surveillance methods for estimating disease burden and hampers the comparability of data across different regions.
Serosurvey limitations:
While seroprevalence surveys are valuable, their focus on specific populations (often those deemed at higher risk) and/or age groups, as well as their variable methodological and testing approaches, limit generalisability and may introduce biases. Crucially, age-structured serological surveys can aid in estimating the historical force-of-infection (the rate at which susceptible individuals become infected) and contribute to parameterising infection-dynamics models.
Incidence calculations:
True incidence is essentially impossible to measure and is hugely underreported. We can, however, use mathematical modelling and seroprevalence data to derive incidence, for instance using force-of-infection models.42,43 Even simple methods can provide insights into the magnitude of underreporting. In Brazil, for example, contrasting data from the second National Serological Survey44 with compulsory-notification records45 suggests that only about 8.5% of new vector-mediated infections were reported to the Ministry of Health in 2001–2008.
Integration with demographic data:
Accurately modelling Chagas disease burden requires integrating knowledge on infection dynamics with historical demographic and migration profiles at sub-national levels. The lack of detailed historical demographics for some countries in the Americas makes it difficult to achieve this integration.
Variability in transmission and progression parameters:
Geographic, demographic, social, environmental, or (parasite, vector, and host) genetic factors may all affect the relative importance of different transmission routes and may all modulate disease progression and severity, further challenging the accurate estimation of Chagas disease burden; adaptable models that reflect local realities are needed to confront this challenge.
Opportunities for integrated modelling
Mathematical models of Chagas disease were first developed in the 1960s.46 Currently, over 100 mathematical models47 simulate different pieces of the epidemiological landscape including, e.g., parasite genetics,48 vector life cycles,49,50 routes and dynamics of transmission,49,51 human dispersal,49,52 and control interventions.53 Despite these efforts, mathematical models have found very limited practical application. The primary concern is the absence of a comprehensive model integrating all relevant components related to Chagas disease transmission (including, e.g., domestic/peridomestic vector ecotopes, domestic and synanthropic mammal populations, and human demographics and behaviour) in changing ecological settings. The development of such a model would provide key guidance to decision-makers in specific situations, particularly if it is applied within an “adaptive management” framework.54
Burden on individuals
Acute phase
Chagas disease incubation (i.e., the time interval between exposure to T. cruzi and the onset of symptoms—in the few symptomatic patients) generally lasts between one and two weeks for most transmission routes, but may extend to several weeks or months when transmission is mediated by solid-organ transplantation or blood transfusion.13 The hallmark of acute Chagas disease is patent parasitaemia; the acute phase is asymptomatic or associated with mild, non-specific signs and symptoms in the vast majority of cases (see Box 1), and typically lasts between four and eight weeks.13 Vector-borne, full-term congenital, and transfusion-related acute infections are often silent and rarely severe.161 In contrast, foodborne infections often present with distinct and severe signs and symptoms including fever, malaise, oedema, anorexia, gastrointestinal illness, lymphadenopathy, hepatosplenomegaly, and acute myocarditis.97,101 Case-fatality is high in foodborne acute Chagas disease, with recent reviews reporting crude rates of ∼4% (2470 cases and 97 deaths; only peer-reviewed studies),107 ∼6.5% (568 cases and 37 deaths; peer-reviewed and unpublished studies),98 or 8–35% (expert opinion).161 The few peer-reviewed studies with N > 100 cases report ∼1–7% case fatality, with upper CI limits ranging from ∼5% to ∼12%.107 A meta-analysis of published and unpublished data reported a substantially lower case-fatality rate estimate (∼1%, 95% CI ∼0–4%).98
Inoculum size (i.e., the number of parasites entering the host at the point of infection), which is usually larger in foodborne than in vector-borne cases,20 is likely a key driver of disease severity and progression, with higher parasite loads linked to increased myocardial damage.162,163 Patient age, T. cruzi strain/DTU,164 and the promptness of medical treatment may also modulate the severity of acute T. cruzi infections. Pharmacological immunosuppression can lead to severe acute infections in transmission mediated by organ transplantation.29,97,127,128
Chronic phase
After the acute phase, most patients remain infected but asymptomatic; this ‘indeterminate form’ of chronic Chagas disease usually lasts for decades, and only about 30–40% of patients develop symptomatic chronic disease (Box 1). Cardiac and digestive manifestations are distinct clinical outcomes of chronic Chagas disease.55,97 While heart disease is widely reported across the Americas, digestive disease is much more common in southern South America (including Argentina, Bolivia, Chile, Paraguay, Uruguay, and parts of Brazil) than in northern South America, Central America, or Mexico. These geographical patterns are currently mainly attributed to variations in the predominant genotypes of T. cruzi.2 Both heart and digestive forms of chronic symptomatic Chagas disease can be highly debilitating; heart failure and enlargement cause chest pain, dyspnoea, oedema, and thromboembolism (including brain and pulmonary embolisms); arrhythmias cause palpitations, dizziness, syncope, and sudden death (mainly from ventricular fibrillation); enlargement/dysfunction of the oesophagus leads to megaoesophagus and causes chronic regurgitation and dysphagia (which can lead to malnutrition), whereas enlargement/dysfunction of the colon leads to megacolon and causes severe constipation.2,29,119
About one-third of individuals infected with T. cruzi are expected to develop chronic heart disease, with an annual rate of progression of ∼2–7%.162 A recent systematic review and meta-analysis examining original studies of patients with confirmed Chagas disease (mainly from Brazil) found that the prevalence of chronic heart disease varies from 42.3% to 53.8%, while the prevalence of the digestive form varies between 18.4% and 25.4% in the Southern Cone of South America.165 These variations may be driven by T. cruzi strain diversity, patient age and genetic background, or the level of endemicity (hence exposure to the parasite) within a given region, underscoring the complexity of clinical progression.165
The risk of death is 1.74 times higher (95% CI 1.5–2.0) in patients with chronic heart Chagas disease than in patients with comparable clinical profiles but without T. cruzi infection.29,166 Sudden death is not a rare event and can be the first manifestation of the disease in patients with no previous signs or symptoms.128,167 Mortality from chronic Chagas heart disease reaches 8.0% per year, mostly due to cardiovascular events (6.3%) including heart failure, arrhythmias, and thromboembolism; annual mortality is thus higher than in HIV/AIDS (7.0%), and similar to that seen in leukaemia (7.9%).168,169 The degree of cardiomyopathy highly correlates with mortality, and there is some evidence suggesting that TcII may be associated with severe heart disease.156 Between 2000 and 2019, Chagas disease was reported as the underlying cause of death in nearly 95,000 death certificates in Brazil; of those deaths, about 80% (77,000) were due to chronic heart disease and about 12–13% (12,000) to digestive chronic forms.170
Reactivation
Reactivation of Chagas disease is a severe, life-threatening acute condition that may occur in individuals infected with T. cruzi who later become immunocompromised, either as a result of immunosuppressive therapy (in, e.g., organ transplantation or cancer chemotherapy) or due to coinfection with HIV. It is estimated that around 13–20% of patients co-infected with HIV and T. cruzi may undergo Chagas disease reactivation, with the frequency depending mainly on HIV loads and CD4+ cell counts.171, 172, 173 Meningoencephalitis and myocarditis are often seen in reactivation,174 and male sex and low CD4+ counts may be associated with increased mortality.173
Burden on society: estimates of prevalence and incidence
Despite many challenges (see Box 1), there have been some efforts to model and estimate the burden of Chagas disease. In the early 2010s, when about 7.7–8.3 million people were likely infected with T. cruzi worldwide, the global costs of Chagas disease were estimated at US$ 7.2 billion per year and US$ 189 billion per lifetime, and the burden of disease was quantified at ∼810,000 disability-adjusted life years (DALYs) lost annually.175 At about the same time, the WHO/PAHO published model-based estimates suggesting that approximately 5.7 million people were infected, with nearly 1.2 million suffering from Chagas heart disease, in 21 Latin American (i.e., excluding the USA) countries or territories where Chagas disease is endemic.28 The Global Burden of Disease (GBD)30 Study has produced model-based estimates of prevalence since 1990; by 2010, global prevalence was estimated at 6.26 (5.46–7.13) million infected individuals, with a slight increase to 6.30 (5.42–7.21) million by 2021.176 The GBD estimates a modest 11% decrease in global prevalence from 1990 to 2019.30,177 Prevalence remains high in many rural areas of Latin America where recurrent invasion and infestation of (usually substandard) dwellings by T. cruzi-infected triatomines maintain endemic transmission.178 Annual incidence estimates vary much more widely, from ∼40,000 (WHO/PAHO)28 to >150,000 (GBD)176 new infections per year. This large discrepancy likely stems from differences in how demographic data were integrated into incidence models; for example, if country-specific populations are split into higher-risk (rural) and lower-risk (urban) strata, GBD-based estimates decrease to about 24,000 to 50,000 new cases per year, broadly matching the WHO/PAHO estimate. In Table 3 we present country-level estimates of Chagas disease prevalence and incidence within the Americas, and below we briefly comment on disease burden across subregions with enzootic/endemic T. cruzi transmission cycles.
Table 2.
Trypanosoma cruzi discrete typing units (DTUs): chronic clinical outcomes and transmission routes.
| DTU | Chronic clinical manifestations |
Transmission |
||
|---|---|---|---|---|
| Heart disease | Digestive disease | Vertical | Foodborne | |
| TcI | X | X | ? | X |
| TcII | X | X | X | ? |
| TcIII | X | ? | ? | X |
| TcIV | X | X | ? | X |
| TcV | X | X | X | ? |
| TcVI | X | X | X | X |
| TcBat | ? | ? | ? | ? |
‘X’ marks indicate known outcomes and routes; question marks (‘?’) emphasise that the absence of evidence does not necessarily mean that no association exists between specific DTUs and different clinical outcomes or transmission routes. Note that all DTUs can cause acute Chagas disease and can potentially be transmitted by triatomine bug vectors or infected blood/organs. Modified from.141
Chagas disease burden in the United States of America
The USA has well-documented enzootic transmission of T. cruzi, involving 11 triatomine species and various mammalian hosts.12 Nearly 30 human cases due to local, vector-borne transmission were documented between 1950 and 2015179; Arizona, Arkansas, California, Louisiana, Mississippi, Missouri, Tennessee, and Texas have all reported autochthonous cases.180 The majority of T. cruzi infections in the USA, however, correspond to Latin American immigrants who became infected in their home countries.12 Recent estimates from age-structured models indicate that ∼288,000 infected adults were living in the USA in 2014–2018, with ∼10,000 locally acquired cases.139 GBD estimates for 2021 suggest that nearly 242,000 people (95% uncertainty interval, 202,000–284,000) may be infected in the country (Table 3).30
Chagas disease burden in Mexico
Mexico faces a considerable Chagas disease burden, with estimates suggesting that ∼900,000–1.2 million people are infected (Table 3).28,176 Official reports, however, acknowledge only a few thousand cases annually.181 Historically, T. cruzi infection was predominantly noted in Mexico's southern states, but recent research highlights the presence of the disease also in the northern regions—with both migration and local transmission likely contributing to this trend.182,183 Annual incidence estimates vary between ∼8000 and > 30,000 (Table 3).28,176 The most recent incidence estimates (for 2021) range from 41.9 (Chiapas) to 19.3 (Campeche) new cases per 100,000 population.30
Chagas disease burden in Central America
Approximately 12% of the population of Central America (Belize, Guatemala, El Salvador, Honduras, Nicaragua, Costa Rica, and Panama) lives at risk of Chagas disease.28 Identified in El Salvador as early as 1913, the disease remains underreported across the region.28,184 Current estimates suggest that between 300,000 and 400,000 individuals are infected in Central America, with annual incidence varying from ∼5000 to ∼10,000 (Table 3).28,176
Chagas disease burden in South America
South America faces the highest global burden of Chagas disease, with ∼4–5 million infected people—mainly in Brazil, Argentina, Bolivia, and Venezuela (Table 3).28,176 Trypanosoma cruzi strains, triatomine vectors, mammalian hosts, and the clinical manifestations of the disease are all particularly diverse in South America.185 Incidence estimates vary between ∼26,000 and ∼120,000 new cases per year, with rates as high as ∼80–115 per 100,000 population in Bolivia (Table 3).28,176
Chagas disease burden among Latin American migrants
By 2020, it was estimated that ∼32 million Latin American and Caribbean (LAC) migrants were living in countries or territories where Chagas disease is not endemic—about 26 million in the USA and 5 million in Europe.186 Although population-representative data are lacking, studies using convenience sampling have reported prevalence values ranging from 0.2% to 20%.187 Understanding the prevalence in the country of origin is crucial. For example, in the USA the frequency of T. cruzi infection is substantially higher among immigrants from El Salvador than among those from Mexico.139 In Europe, seroprevalence among LAC migrants was estimated at 4.2% on average (up to 18.1% among Bolivians) in 2012.188 Other surveys have found infection frequencies from 0.3% among LAC blood donors (France, 2007–2008)189 to 12.8% for LAC migrants in general (Switzerland, 2008),190 with intermediate values in LAC migrants living in, e.g., London (1.27% in 2011)191 or Spain (2.1% in 2018),192 and among pregnant LAC women living in Switzerland (2.0% to 9.7% in 2007–2008).193,194
Chagas disease prevention and management
Primary prevention: vector control-surveillance, donor screening, and the multinational initiatives
Efforts to eliminate non-native ‘primary’ vectors, and in particular Triatoma infestans and Rhodnius prolixus, have relied heavily on residual-insecticide spraying, with some programmes also including housing improvement—mainly by replacing mud walls, earthen floors, and thatched roofs—and community-oriented initiatives.25,75,195 In spite of major advances, continuous surveillance remains crucial to control transmission mediated by native vectors—of which 140+ species occur in the Americas.25,70,73,196 In 1991, the health ministers of Argentina, Brazil, Bolivia, Chile, Paraguay, and Uruguay launched the Initiative of the Southern Cone Countries for the control of Chagas disease (INCOSUR), which aimed primarily at eliminating domestic T. infestans populations and interrupting transfusion-related T. cruzi transmission.197 Similar initiatives were later established throughout the region, including Central America and Mexico, the Andean countries, and Amazonia.25 The progress of these subregional initiatives is regularly reviewed by PAHO.198,199 Up to the present, PAHO has issued certifications of interruption of T. cruzi transmission by non-native populations of two vector species: T. infestans in Uruguay, Chile, Brazil, Paraguay, and parts of Argentina, Bolivia, and Peru; and R. prolixus in Mexico, Guatemala, Belize, Honduras, El Salvador, Nicaragua, Costa Rica, and parts of Colombia.73,200
In the complex epidemiological landscape of Chagas disease, involving native and non-native vector species and diverse transmission routes, PAHO has recently revised its certification approach to also consider the ‘elimination of Chagas disease as a public health problem’.201 The process of certification in this case entails (i) demonstrating successful control efforts against all vector species and transmission routes (leading to “zero incidence” of vector-borne and transfusion-mediated infections), and (ii) having a structured and functional programme for Chagas disease surveillance, diagnosis, and treatment with guaranteed adequate coverage levels (with, e.g., screening of all pregnant women and all blood or organ donors; diagnosis offered to all childbearing-aged women at risk and to all babies born to seropositive mothers; and ‘timely and suitable’ treatment of all cases detected—whether congenital, acute, or chronic). Certifications of interruption of transmission and elimination of the disease as a public health problem are subject to review every five years.201 Below we provide a brief overview of the regional initiatives, with a focus on vector-borne transmission (see25 for further details).
The Initiative of Central America and Mexico (IPCAM) includes Mexico, Belize, Guatemala, El Salvador, Honduras, Nicaragua, Costa Rica, and Panama, and primarily targets non-native R. prolixus and native T. dimidiata sensu lato (s.l.).25 Notably, R. prolixus was found infesting houses and peridomestic structures in rural Oaxaca, Mexico after the region was certified as free from transmission by this non-native vector in 2009.202 More recently, a focus of infestation by T. infestans (which is native to the Chaco and central Andes in South America) has been reported in coastal Colima, Mexico.203 Triatoma dimidiata s.l. is native to the region and often reinvades and reinfests dwellings—as do, to different degrees, several other species including T. ryckmani, T. nitida, T. barberi, T. pallidipennis, T. longipennis, T. huehuetenanguensis, or R. pallescens. The long-term control of vector-borne Chagas disease in the subregion will probably require integrating insecticide spraying with household-level environmental management and community involvement.25
The Initiative of the Andean Countries (IPA) includes Colombia, Venezuela, Ecuador and Peru. Non-native R. prolixus occur in north-western Colombia, non-native T. dimidiata in coastal Ecuador, and non-native T. infestans in parts of south-western Peru. These introduced populations can, in principle, be eliminated through large-scale control campaigns.25 However, R. prolixus is native to the Orinoco savannahs and adjacent dry ecoregions,204,205 and T. dimidiata sensu stricto (s.s.) to most of western Colombia and likely parts of northern Venezuela7,206,207; persistent reinvasion and reinfestation of dwellings by these and several other native vectors (e.g., T. maculata in Colombia and Venezuela or R. ecuadoriensis and Panstrongylus chinai in Ecuador and Peru) is common, and long-term control-surveillance will be required to effectively curb transmission.25
The Amazon Countries (AMCHA) Initiative includes parts of Colombia, Ecuador, Peru, Bolivia, Brazil, and Venezuela plus Guyana, Suriname, and French Guiana. Although stable house infestation by triatomines is not common in the region, there is an extensive list of sylvatic vectors that often invade human dwellings in both rural and urban areas.25 Transmission mediated by these vectors can be either direct, leading to an overall prevalence of human infection of about 1–2% (but up to 4–5% in some subregions), or through contamination of food or beverages, with clustered outbreaks of orally-transmitted disease.25,68,208 House-infestation foci, however, are common in northernmost Brazil (T. maculata), in the middle-upper Marañón Valley in Peru (P. lignarius/herreri), and in parts of Bolivia (R. stali).25,73,208
The Initiative of the Southern Cone Countries (INCOSUR) includes Brazil, Peru, Bolivia, Chile, Argentina, Paraguay, and Uruguay. Non-native T. infestans populations were once widespread in rural settings across the region; extensive control campaigns eliminated them from Uruguay, most of Brazil, and parts of Paraguay, Argentina, Peru, Bolivia, and Chile, leading to substantial reductions in the incidence and prevalence of human T. cruzi infections.25,75 In spite of major advances, prevalence remains high in parts of Bolivia and, to a lesser extent, Paraguay, Brazil, or Argentina, and native vectors maintain transmission throughout the Southern Cone.25 Some T. infestans populations have adapted to urban/periurban environments in, e.g., Arequipa, Peru209; San Juan, Argentina210; and Cochabamba, Bolivia.211 The control of urban infestations and transmission is particularly challenging and will require innovative tactics.212,213 Crucially, T. infestans is native to the dry Chaco and the Bolivian montane dry forests,7 and other locally native species (T. brasiliensis, P. megistus, T. sordida, T. pseudomaculata and many more) often infest houses and peridomestic structures. Control of these domestic/peridomestic native vectors is challenging, with reinfestations leading to persistent transmission.25,214
Secondary/tertiary prevention: diagnosis, treatment, and integral patient care
As we have emphasised throughout this review, millions of people across the Americas (and thousands in Europe, Asia, and Oceania) carry chronic T. cruzi infections, and tens of thousands probably become infected each year—mainly via direct or indirect contact with vectors but also vertically or, in a minority of cases, through blood transfusion, organ transplantation, or laboratory accidents. Timely diagnosis and adequate, integral care are critical to the fundamental rights to life, health, and well-being215 of people infected with T. cruzi, whatever their age, gender, origins, background, or means—and no matter whether the infection is acute or chronic; symptomatic or silent; vector-borne, foodborne, congenital, blood-borne, or acquired through transplantation; or caused by one particular T. cruzi DTU or another. Below we present a quick overview of Chagas disease diagnosis and treatment; for more comprehensive reviews of these and related topics, see the other series papers on clinical progression and severity,55 diagnosis,57 and vaccines and therapeutics.58
The diagnosis of Chagas disease is challenging. First, the likelihood of clinical suspicion is low, both because most infections (acute and chronic) are asymptomatic or cause nonspecific signs/symptoms and because many caregivers lack awareness about Chagas disease.216 When there is clinical suspicion and laboratory diagnosis is sought, several biological and technical shortcomings weaken the performance of available procedures. In acute and congenital infections, diagnosis usually aims at detecting parasites or their DNA (the ‘targets for detection’) in blood samples; even if parasitaemia is common in the acute phase, there is no guarantee that all samples drawn from infected patients will contain those targets—and, when they do, neither parasitological nor molecular tests have perfect (i.e., exactly 100%) sensitivity.217,218 The problem of imperfect target availability only worsens in chronic infections, where parasitaemia is sparser and likely intermittent.217,219 Serological testing has the advantage of targeting a widely bio-available class of molecules—antibodies—that are present in essentially any serum sample drawn from an infected patient.217,219 Serology has, however, several major drawbacks; first, most serological tests lack validation across different populations and may have low specificity, so that at least two positive serological tests based on different antigenic principles are required for diagnosis29; in addition, sensitivity and specificity can also vary with T. cruzi genotype/DTU and/or antigenic makeup.220 Rapid diagnostic (immunochromatographic) tests, while promising and especially well suited for point-of-care testing and large-scale screening/surveys in resource-limited settings, have variable sensitivity and may perform very poorly in some subregions.221,222 Although new biomarkers with high specificity and wide bio-availability would likely enhance diagnosis and perhaps inform prognosis,217,219 the most critical issue is still the lack of equitable access to available testing methods and algorithms—and, when needed, to integral patient care.25,223
Treatment of Chagas disease involves not only the use of specific anti-T. cruzi drugs, but also the treatment and management of chronic heart and digestive disease and their complications.97 Two drugs, benznidazole and nifurtimox, are indicated in all acute infections, congenital infections, chronically infected children, and disease reactivation. Treatment of women of childbearing age with chronic asymptomatic infections seems to reduce the risk of vertical transmission and is therefore recommended, whereas the benefits are less clear for other adults with indeterminate chronic disease. Currently, PAHO recommends that benznidazole and nifurtimox should not be used in clinical practice for treating adult patients with moderate or severe symptomatic chronic forms; this recommendation, however, is ‘conditional’ and based on evidence of ‘moderate’ strength.97,224 We note that, in general, designing clinical trials for trypanocidal drug efficacy is complex, as critical endpoints (chronic heart or digestive disease, death) may take decades to come about; clinical findings, moreover, can be confounded by many factors including parasite genetics, human population peculiarities, access to healthcare, reinfection, or re-treatment.225 Consequently, the strength of the evidence supporting the recommendations about anti-T. cruzi treatment we have just outlined is at best moderate.224 Although benznidazole is overall well tolerated, an estimated 17–35% of chronically infected adult patients interrupt their treatment due to side effects.224 Recent evidence suggests that shorter regimens, which may enhance adherence and increase treatment coverage, are non-inferior to standard regimens in terms of parasitological response as measured by qPCR.226 While new trypanocidal drugs are sorely needed, there has been virtually no progress over the last half-century; an exception is a benzoxaborole prodrug (AN15368) that has yielded promising results in non-human primate chronic-infection models.227,228 One critical issue is that the effective treatment of chronic Chagas disease requires drugs that kill extra- and intracellular parasites including ‘dormant’ forms that are protected from, and hence resistant to, the trypanocidal action of standard doses of benznidazole or nifurtimox.229 Preclinical research suggests that AN15368 and modified benznidazole regimens might be useful against both bloodstream and intracellular (active and dormant) T. cruzi forms.227,230
Importantly, timely anti-T. cruzi treatment can prevent the evolution of asymptomatic or mild infections towards the chronic symptomatic forms of Chagas disease, which can be highly debilitating and impose a heavy burden on patients and society.97,128 Patients with chronic symptomatic forms of Chagas heart disease may need additional specialised care for heart failure (from diet to diuretic, antihypertensive, or beta-blocker drugs to heart transplantation), arrhythmias (from amiodarone or beta blockers to catheter ablation, implantable cardioverter-defibrillators, or pacemakers), and thromboembolism (acetylsalicylic acid, warfarin).97 The management of chronic digestive forms spans from diet and counselling to surgery. Megaoesophagus treatment aims at relieving dysphagia by facilitating the passage of food through the lower oesophageal sphincter; this may involve diet/counselling and/or the use of drugs that relax smooth-muscle fibres, physical (pneumatic-balloon) dilatation, or surgery. Treating megacolon may also involve diet (with a focus on increasing water intake), counselling (on, e.g., avoiding food or drugs that may induce constipation), the use of laxatives or enemas, manual removal of faecalomas, or surgery—including emergency surgery in cases of volvulus, haemorrhage, obstruction, or perforation.97
Conclusions and outlook: a future free of Chagas disease?
Chagas disease remains a major public health challenge in the Americas. Over six million people are infected, and most of them face substantial barriers to accessing essential healthcare, including diagnosis and treatment. Despite considerable progress in controlling transmission through universal blood-bank and organ-donor screening and the elimination of many non-native, domiciliated vector populations, active transmission—still mainly mediated by vectors—persists. While current intervention strategies have led to major advances, they are unlikely to deliver complete success. Widespread native vector populations continue to invade, infest, and reinfest human dwellings, bridging sylvatic, peridomestic, and domestic T. cruzi transmission cycles (Fig. 1). A highly diverse array of vectors and non-human hosts maintain sylvatic transmission of T. cruzi from the USA to southern South America (Fig. 1, Fig. 2, Fig. 3); eradication of Chagas disease is, therefore, biologically implausible: even if non-native vector populations are effectively eliminated, dozens of native vector species will continue transmitting T. cruzi across tropical-subtropical America. All estimates suggest that tens of thousands of new vector-borne human infections occur each year in the Americas, yet, crucially, most of them remain invisible to surveillance systems—and the alarms remain, therefore, silent. Vertical and foodborne transmission have become increasingly prominent in the current epidemiological landscape, but this should not obscure the fact that the majority of new cases is most likely vector-borne. Congenital Chagas disease presents unique challenges; specific diagnostic and treatment protocols for pregnant women (and, ideally, childbearing-aged women with a history of potential exposure) and newborns are needed to prevent transmission. The complex nature of Chagas disease transmission, in sum, demands a holistic healthcare strategy covering from vector surveillance to integral patient care, and highlights the critical need for in-depth ecological, epidemiological, clinical, and public-health research.25,128 Such research should aim to i) understand the complex interplay between sylvatic and domestic/peridomestic transmission cycles, including the roles played by native vectors across the Americas; ii) assess the impact of T. cruzi DTUs on transmission patterns, disease severity, and treatment outcomes; iii) develop enhanced diagnostic and therapeutic approaches; iv) generate more accurate disease burden estimates (e.g., using age-structured dynamic models); and v) use implementation science to develop and test integrative control-surveillance strategies within the diverse ecological, socio-economic and climatic contexts where transmission still occurs. Bridging these knowledge gaps is vital for crafting more effective long-term interventions, for consolidating the substantial achievements of control programmes and initiatives, and perhaps for developing adaptive strategies in which living close to T. cruzi vectors and hosts does not necessarily translate into full-blown Chagas disease.
Contributors
ZMC led the conceptualisation, writing of the original draft, and data visualisations. FA-F co-led the conceptualisation and writing of the manuscript. SAGR validated information against the original datasets and sources, conducted data curation, prepared the initial draft, and assisted with visualisations. JDR and NVO did data curation for molecular data and drafted the corresponding sections. SC led data curation and validation for vector distribution information and vector visualisations. GPH assisted with the vector-borne transmission section. AHM verified clinical data and conducted writing checks. MGB, JR, and PN guided the overall content direction and revised the manuscript. All authors contributed to writing and editing and approved the final version.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
M.G.B. acknowledges funding from the MRC Centre for Global Infectious Disease Analysis (MR/X020258/1), funded by the UK Medical Research Council (MRC). This UK-funded award is carried out in the framework of the Global Health EDCTP3 Joint Undertaking.
Contributor Information
Zulma M. Cucunubá, Email: zulma.cucunuba@javeriana.edu.co.
Fernando Abad-Franch, Email: abadfranch@ufmg.br.
References
- 1.Aufderheide A.C., Salo W., Madden M., et al. A 9000-year record of Chagas disease. Proc Natl Acad Sci U S A. 2004;101:2034–2039. doi: 10.1073/pnas.0307312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bern C. Chagas' disease. N Engl J Med. 2015;373:456–466. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 3.Zingales B., Miles M.A., Campbell D.A., et al. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Ventura-Garcia L., Roura M., Pell C., et al. Socio-cultural aspects of Chagas disease: a systematic review of qualitative research. PLoS Negl Trop Dis. 2013;7 doi: 10.1371/journal.pntd.0002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Q., Chen J., Zhou X.-N. Preparedness for Chagas disease spreading worldwide. Infect Dis Poverty. 2020;9:44. doi: 10.1186/s40249-020-00658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lent H., Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas’ disease. Bull Am Mus Nat History. 1979;163:125–520. [Google Scholar]
- 7.Monteiro F.A., Weirauch C., Felix M., Lazoski C., Abad-Franch F. Evolution, systematics, and biogeography of the Triatominae, vectors of Chagas disease. Adv Parasitol. 2018;99:265–344. doi: 10.1016/bs.apar.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Abad-Franch F., Gurgel-Gonçalves R. In: Triatominae - The biology of Chagas disease vectors. Entomology in Focus. Guarneri A.A., Lorenzo M.G., editors. Vol. 5. Springer; Cham: 2021. The ecology and natural history of wild Triatominae in the Americas; pp. 387–445. [Google Scholar]
- 9.Rodríguez-Monguí E., Cantillo-Barraza O., Prieto-Alvarado F.E., Cucunubá Z.M. Heterogeneity of Trypanosoma cruzi infection rates in vectors and animal reservoirs in Colombia: a systematic review and meta-analysis. Parasit Vectors. 2019;12:308. doi: 10.1186/s13071-019-3541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noireau F., Diosque P., Jansen A.M. Trypanosoma cruzi: adaptation to its vectors and its hosts. Vet Res. 2009;40:26. doi: 10.1051/vetres/2009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen A.M., Xavier S.C.D.C., Roque A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasit Vectors. 2018;11:502. doi: 10.1186/s13071-018-3067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bern C., Messenger L.A., Whitman J.D., Maguire J.H. Chagas disease in the United States: a public health approach. Clin Microbiol Rev. 2019;33 doi: 10.1128/CMR.00023-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rassi A., Jr., Rassi A., Marin-Neto J.A. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 14.Klein M.D., Proaño A., Noazin S., Sciaudone M., Gilman R.H., Bowman N.M. Risk factors for vertical transmission of Chagas disease: a systematic review and meta-analysis. Int J Infect Dis. 2021;105:357–373. doi: 10.1016/j.ijid.2021.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard E.J., Xiong X., Carlier Y., Sosa-Estani S., Buekens P. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121:22–33. doi: 10.1111/1471-0528.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santana K.H., Oliveira L.G.R., Barros de Castro D., Pereira M. Epidemiology of Chagas disease in pregnant women and congenital transmission of Trypanosoma cruzi in the Americas: systematic review and meta-analysis. Trop Med Int Health. 2020;25:752–763. doi: 10.1111/tmi.13398. [DOI] [PubMed] [Google Scholar]
- 17.Lynn M.K., Rodriguez Aquino M.S., Cornejo Rivas P.M., et al. Chagas disease maternal seroprevalence and maternal-fetal health outcomes in a parturition cohort in Western El Salvador. Trop Med Infect Dis. 2023;8 doi: 10.3390/tropicalmed8040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coura J.R. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions- a comprehensive review. Mem Inst Oswaldo Cruz. 2015;110:277–282. doi: 10.1590/0074-0276140362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roellig D.M., Ellis A.E., Yabsley M.J. Oral transmission of Trypanosoma cruzi with opposing evidence for the theory of carnivory. J Parasitol. 2009;95:360–364. doi: 10.1645/GE-1740.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson L.J., Havelaar A.H., Keddy K.H., Devleesschauwer B., Sripa B., Torgerson P.R. The importance of estimating the burden of disease from foodborne transmission of Trypanosoma cruzi. PLoS Negl Trop Dis. 2024;18 doi: 10.1371/journal.pntd.0011898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shikanai-Yasuda M.A., Carvalho N.B. Oral transmission of Chagas disease. Clin Infect Dis. 2012;54:845–852. doi: 10.1093/cid/cir956. [DOI] [PubMed] [Google Scholar]
- 22.Schmunis G.A. Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):75–85. doi: 10.1590/s0074-02762007005000093. [DOI] [PubMed] [Google Scholar]
- 23.Benjamin R.J., Stramer S.L., Leiby D.A., Dodd R.Y., Fearon M., Castro E. Trypanosoma cruzi infection in North America and Spain: evidence in support of transfusion transmission. Transfusion. 2012;52:1913–1921. doi: 10.1111/j.1537-2995.2011.03554.x. [DOI] [PubMed] [Google Scholar]
- 24.Huprikar S., Bosserman E., Patel G., et al. Donor-derived Trypanosoma cruzi infection in solid organ recipients in the United States, 2001-2011. Am J Transplant. 2013;13:2418–2425. doi: 10.1111/ajt.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rojasde Arias A., Monroy C., Guhl F., Sosa-Estani S., Santos W.S., Abad-Franch F. Chagas disease control-surveillance in the Americas: the multinational initiatives and the practical impossibility of interrupting vector-borne Trypanosoma cruzi transmission. Mem Inst Oswaldo Cruz. 2022;117 doi: 10.1590/0074-02760210130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nouvellet P., Dumonteil E., Gourbière S. The improbable transmission of Trypanosoma cruzi to human: the missing link in the dynamics and control of Chagas disease. PLoS Negl Trop Dis. 2013;7 doi: 10.1371/journal.pntd.0002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan American Health Organization Chagas disease. https://www.paho.org/en/topics/chagas-disease
- 28.World Health Organization Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015;6:33–43. [PubMed] [Google Scholar]
- 29.World Health Organization . Second report of the WHO Expert Committee. World Health Organ Tech Rep Ser; 2002;905. pp. i–vi. (Control of chagas disease). 1–109. [Google Scholar]
- 30.Institute for Health Metrics and Evaluation (IHME) GBD results. 2024. https://vizhub.healthdata.org/gbd-results/
- 31.Salvatella R., Irabedra P., Castellanos L.G. Interruption of vector transmission by native vectors and ‘the art of the possible’. Mem Inst Oswaldo Cruz. 2014;109:122–125. doi: 10.1590/0074-0276140338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dias J.C.P., Silveira A.C., Schofield C.J. The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- 33.Rural population - Latin America & Caribbean. World bank open data. https://data.worldbank.org/indicator/SP.RUR.TOTL?locations=ZJ
- 34.Naciones Unidas Portal de desigualdades en América Latina. CEPALSTAT. https://statistics.cepal.org/portal/inequalities/housing-and-basic-services.html?lang=es&indicator=260
- 35.Pan American Health Organization . Pan American Health Organization; 2021. 70% of people with Chagas don’t know they’re infected.https://www.paho.org/en/news/13-4-2021-70-people-chagas-dont-know-theyre-infected [Google Scholar]
- 36.Sgambatti de Andrade A.L.S., Zicker F., de Oliveira R.M., et al. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348:1407–1413. doi: 10.1016/s0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- 37.Cançado J.R. Long term evaluation of etiological treatment of Chagas disease with benznidazole. Rev Inst Med Trop Sao Paulo. 2002;44:29–37. [PubMed] [Google Scholar]
- 38.Chaves G.C., Abi-Saab Arrieche M., Rode J., et al. Estimación de la demanda de medicamentos antichagásicos: una contribución para el acceso en América Latina. Rev Panam Salud Publica. 2017;41:e45. doi: 10.26633/RPSP.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manne-Goehler J., Reich M.R., Wirtz V.J. Access to care for Chagas disease in the United States: a health systems analysis. Am J Trop Med Hyg. 2015;93:108–113. doi: 10.4269/ajtmh.14-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cucunubá Z.M., Manne-Goehler J.M., Díaz D., et al. How universal is coverage and access to diagnosis and treatment for Chagas disease in Colombia? A health systems analysis. Soc Sci Med. 2017;175:187–198. doi: 10.1016/j.socscimed.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Briceño-León R. La enfermedad de Chagas en las Américas: una perspectiva de ecosalud. Cad Saúde Pública. 2009;25:S71–S82. doi: 10.1590/s0102-311x2009001300007. [DOI] [PubMed] [Google Scholar]
- 42.Ledien J., Cucunubá Z.M., Parra-Henao G., et al. From serological surveys to disease burden: a modelling pipeline for Chagas disease. Philos Trans R Soc Lond B Biol Sci. 2023;378 doi: 10.1098/rstb.2022.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cucunubá Z.M., Nouvellet P., Conteh L., et al. Modelling historical changes in the force-of-infection of Chagas disease to inform control and elimination programmes: application in Colombia. BMJ Glob Health. 2017;2 doi: 10.1136/bmjgh-2017-000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostermayer A.L., Passos A.D.C., Silveira A.C., Ferreira A.W., Macedo V., Prata A.R. O inquérito nacional de soroprevalência de avaliação do controle da doença de Chagas no Brasil (2001-2008) Rev Soc Bras Med Trop. 2011;44(Suppl 2):108–121. doi: 10.1590/s0037-86822011000800015. [DOI] [PubMed] [Google Scholar]
- 45.DATASUS – Ministério da Saúde. https://datasus.saude.gov.br/
- 46.Silva GR da. Sôbre o modêlo catalítico reversível aplicado ao estudo da cinética epidemiológica da infecção chagásica. Rev Saude Publica. 1969;3:23–29. [PubMed] [Google Scholar]
- 47.Nouvellet P., Cucunubá Z.M., Gourbière S. Ecology, evolution and control of Chagas disease: a century of neglected modelling and a promising future. Adv Parasitol. 2015;87:135–191. doi: 10.1016/bs.apar.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Devillers H., Raymond Lobry J., Menu F. An agent-based model for predicting the prevalence of Trypanosoma cruzi I and II in their host and vector populations. J Theor Biol. 2008;255:307–315. doi: 10.1016/j.jtbi.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 49.Pelosse P., Kribs-Zaleta C.M., Ginoux M., Rabinovich J.E., Gourbière S., Menu F. Influence of vectors' risk-spreading strategies and environmental stochasticity on the epidemiology and evolution of vector-borne diseases: the example of Chagas' disease. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens L., Rizzo D.M., Lucero D.E., Pizarro J.C. Household model of Chagas disease vectors (Hemiptera: Reduviidae) considering domestic, peridomestic, and sylvatic vector populations. J Med Entomol. 2013;50:907–915. doi: 10.1603/me12096. [DOI] [PubMed] [Google Scholar]
- 51.Ramalho E., Albuquerque J., Cristino C., et al. Congenital and blood transfusion transmission of Chagas disease: a framework using mathematical modeling. Complexity. 2018;2018 [Google Scholar]
- 52.Lozada-Yavina R., Marchant C., Cancino-Faure B., Hernández-Rodríguez E.W., Córdova-Lepe F. A description of the epidemiological dynamics of Chagas disease via mathematical modeling. Acta Trop. 2023;243 doi: 10.1016/j.actatropica.2023.106930. [DOI] [PubMed] [Google Scholar]
- 53.Spagnuolo A.M., Shillor M., Stryker G.A. A model for Chagas disease with controlled spraying. J Biol Dynam. 2011;5:299–317. [Google Scholar]
- 54.Shea K., Possingham H.P., Murdoch W.W., Roush R. Active adaptative management in insect pest and weed control: intervention with a plan for learning. Ecol Appl. 2002;12:927–936. [Google Scholar]
- 55.Nunes MCP, Bern C, Clark EH, Teixeira AL, Molina I. Clinical aspects of Chagas disease progression and severity. Lancet Reg Health Am.
- 56.Forsyth CJ, Ni AH, Hamer S, et al. Climate change and trypanosoma cruzi transmission in north America, Central America, and the Caribbean. Lancet Microbe, In press. [DOI] [PubMed]
- 57.Schijman A.G., Alonso-Padilla J., Britto C., Cp H.B. Retrospect, Advances and challenges in Chagas disease diagnosis: a comprehensive review. Lancet Reg Health Am. 2024;36 doi: 10.1016/j.lana.2024.100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinazo MJ, Malchiodi EL, Ioset JR, Bivona AE, Gollob KJ Dutra W.O Challenges and advancements in human Chagas disease vaccine and therapy development: from Bench to Field. Lancet Microbe. In press. [DOI] [PubMed]
- 59.Rengifo-Correa L., Rodríguez-Moreno Á., Becker I., et al. Risk of a vector-borne endemic zoonosis for wildlife: hosts, large-scale geography, and diversity of vector-host interactions for Trypanosoma cruzi. Acta Trop. 2024;251 doi: 10.1016/j.actatropica.2024.107117. [DOI] [PubMed] [Google Scholar]
- 60.Gottdenker N.L., Calzada J.E., Saldaña A., Carroll C.R. Association of anthropogenic land use change and increased abundance of the Chagas disease vector Rhodnius pallescens in a rural landscape of Panama. Am J Trop Med Hyg. 2011;84:70–77. doi: 10.4269/ajtmh.2011.10-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santos W.S., Gurgel-Gonçalves R., Garcez L.M., Abad-Franch F. Deforestation effects on Attalea palms and their resident Rhodnius, vectors of Chagas disease, in eastern Amazonia. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varian C.P., Saldaña A., Calzada J.E., et al. Food web structure and microenvironment affect Chagas disease vector infection and abundance in a rural landscape. Ecosphere. 2023;14 doi: 10.1002/ecs2.4347. [DOI] [Google Scholar]
- 63.Cohen J.E., Gürtler R.E. Modeling household transmission of American trypanosomiasis. Science. 2001;293:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- 64.Valença-Barbosa C., Lima M.M., Sarquis O., Bezerra C.M., Abad-Franch F. Modeling disease vector occurrence when detection is imperfect II: drivers of site-occupancy by synanthropic Triatoma brasiliensis in the Brazilian northeast. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schaub G.A. Interaction of, triatomines and the microbiota of the vectors-A review. Microorganisms. 2024;12 doi: 10.3390/microorganisms12050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gürtler R.E., Fernandez M.P, Cardinal M.V. In: Triatominae: the biology of Chagas disease vectors. Guarneri A.A., editor. Springer International Publishing; 2021. Eco-epidemiology of the domestic vector-borne transmission of Trypanosoma cruzi; pp. 447–489. [Google Scholar]
- 67.Salcedo-Porras N., Lowenberger C. In: Triatominae - The biology of Chagas disease vectors, Entomology in Focus, vol 5. Guarneri A.A., editor. Cham: Springer; 2021. The immune system of triatomines; pp. 307–344. [Google Scholar]
- 68.Abad-Franch F., Diotaiuti L., Gurgel-Gonçalves R., Gürtler R.E. Certifying the interruption of Chagas disease transmission by native vectors: cui bono? Mem Inst Oswaldo Cruz. 2013;108:251–254. doi: 10.1590/0074-0276108022013022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abad-Franch F. A simple, biologically sound, and potentially useful working classification of Chagas disease vectors. Mem Inst Oswaldo Cruz. 2016;111:649–651. doi: 10.1590/0074-02760160203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waleckx E., Gourbière S., Dumonteil E. Intrusive versus domiciliated triatomines and the challenge of adapting vector control practices against Chagas disease. Mem Inst Oswaldo Cruz. 2015;110:324–338. doi: 10.1590/0074-02760140409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ceccarelli S., Balsalobre A., Cano M.E., et al. Occurrence Dataset; 2022. Datos de ocurrencia de triatominos americanos del Laboratorio de Triatominos del CEPAVE (CONICET-UNLP). Version 1.6. [DOI] [Google Scholar]
- 72.Ceccarelli S., Balsalobre A., Cano M.E., et al. Occurrence Dataset; 2020. Datos de ocurrencia de triatominos presentes en Argentina del Laboratorio de Triatominos del CEPAVE (CONICET-UNLP). Version 1.2. [DOI] [Google Scholar]
- 73.Abad-Franch F., Diotaiuti L., Gurgel-Gonçalves R., Gürtler R.E. On bugs and bias: improving Chagas disease control assessment. Mem Inst Oswaldo Cruz. 2014;109:125–130. [PubMed] [Google Scholar]
- 74.Hashimoto K., Schofield C.J. Elimination of Rhodnius prolixus in central America. Parasit Vectors. 2012;5:45. doi: 10.1186/1756-3305-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schofield C.J., Jannin J., Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22:583–588. doi: 10.1016/j.pt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 76.Oliveira I., Torrico F., Muñoz J., Gascon J. Congenital transmission of Chagas disease: a clinical approach. Expert Rev Anti Infect Ther. 2014;8:945–956. doi: 10.1586/eri.10.74. [DOI] [PubMed] [Google Scholar]
- 77.Abras A., Ballart C., Fernández-Arévalo A., et al. Worldwide control and management of Chagas disease in a new era of globalization: a close look at congenital Trypanosoma cruzi infection. Clin Microbiol Rev. 2022;35 doi: 10.1128/cmr.00152-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Messenger L.A., Bern C. Congenital Chagas disease: current diagnostics, limitations and future perspectives. Curr Opin Infect Dis. 2018;31:415–421. doi: 10.1097/QCO.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 79.Picado A., Cruz I., Redard-Jacot M., et al. The burden of congenital Chagas disease and implementation of molecular diagnostic tools in Latin America. BMJ Glob Health. 2018;3 doi: 10.1136/bmjgh-2018-001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pan American Health Organization . PAHO; Whasington, DC: 2017. EMTCT Plus. Framework for elimination of mother-to-child transmission of HIV, Syphilis, Hepatitis B, and Chagas.https://iris.paho.org/handle/10665.2/34306 [Google Scholar]
- 81.Shikanai Yasuda M.A. Emerging and reemerging forms of Trypanosoma cruzi transmission. Mem Inst Oswaldo Cruz. 2022;117 doi: 10.1590/0074-02760210033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buekens P., Cafferata M.L., Alger J., et al. Congenital transmission of Trypanosoma cruzi in Argentina, Honduras, and Mexico: an observational prospective study. Am J Trop Med Hyg. 2018;98:478–485. doi: 10.4269/ajtmh.17-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carlier Y., Sosa-Estani S., Luquetti A.O., Buekens P. Congenital Chagas disease: an update. Mem Inst Oswaldo Cruz. 2015;110:363–368. doi: 10.1590/0074-02760140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carlier Y., Truyens C. Congenital Chagas disease as an ecological model of interactions between Trypanosoma cruzi parasites, pregnant women, placenta and fetuses. Acta Trop. 2015;151:103–115. doi: 10.1016/j.actatropica.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 85.Amorín B., Pérez L. Chagas congénito de segunda generación en Uruguay: Primer caso sintomático descrito en el país. Arch Pediatr Urug. 2016;87:245–252. [Google Scholar]
- 86.Sánchez Negrette O., Mora M.C., Basombrío M.A. High prevalence of congenital Trypanosoma cruzi infection and family clustering in Salta, Argentina. Pediatrics. 2005;115:e668–e672. doi: 10.1542/peds.2004-1732. [DOI] [PubMed] [Google Scholar]
- 87.Danesi E., Fabbro D.L., Segura E.L., Sosa-Estani S. Higher congenital transmission rate of Trypanosoma cruzi associated with family history of congenital transmission. Rev Soc Bras Med Trop. 2020;53 doi: 10.1590/0037-8682-0560-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fabbro D.L., Danesi E., Olivera V., et al. Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murcia L., Simón M., Carrilero B., Roig M., Segovia M. Treatment of infected women of childbearing age prevents congenital Trypanosoma cruzi infection by eliminating the parasitemia detected by PCR. J Infect Dis. 2017;215:1452–1458. doi: 10.1093/infdis/jix087. [DOI] [PubMed] [Google Scholar]
- 90.Sosa-Estani S., Cura E., Velazquez E., Yampotis C., Segura E.L. Etiological treatment of young women infected with Trypanosoma cruzi, and prevention of congenital transmission. Rev Soc Bras Med Trop. 2009;42:484–487. doi: 10.1590/s0037-86822009000500002. [DOI] [PubMed] [Google Scholar]
- 91.Moscatelli G., Moroni S., García-Bournissen F., et al. Prevention of congenital Chagas through treatment of girls and women of childbearing age. Mem Inst Oswaldo Cruz. 2015;110:507–509. doi: 10.1590/0074-02760140347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Álvarez M.G., Vigliano C., Lococo B., Bertocchi G., Viotti R. Prevention of congenital Chagas disease by Benznidazole treatment in reproductive-age women. An observational study. Acta Trop. 2017;174:149–152. doi: 10.1016/j.actatropica.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 93.Carlier Y., Altcheh J., Angheben A., et al. Congenital Chagas disease: updated recommendations for prevention, diagnosis, treatment, and follow-up of newborns and siblings, girls, women of childbearing age, and pregnant women. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Organización Panamericana de la Salud . Washington DC: OPS; 2019. Nuevas generaciones sin la infección por el VIH, la sífilis, la hepatitis B y la enfermedad de Chagas en las Américas 2018. ETMI Plus.https://iris.paho.org/bitstream/handle/10665.2/50993/9789275320679_spa.pdf?sequence=1&isAllowed=y [Google Scholar]
- 95.Cucunubá Z.M., Valencia-Hernández C.A., Puerta C.J., et al. First Colombian consensus on congenital Chagas and clinical approach for women of child-bearing age diagnosed with Chagas. Infection. 2017;21:255–266. [Google Scholar]
- 96.Eickhoff C.S., Dunn B.A., Sullivan N.L., Hoft D.F. Comparison of the infectivity of Trypanosoma cruzi insect-derived metacyclic trypomastigotes after mucosal and cutaneous contaminative challenges. Mem Inst Oswaldo Cruz. 2013;108:508–511. doi: 10.1590/0074-0276108042013018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dias J.C.P., Ramos A.N., Jr., Gontijo E.D., et al. 2nd Brazilian Consensus on Chagas disease, 2015. Rev Soc Bras Med Trop. 2016;40 (Suppl 1):3–60. doi: 10.1590/0037-8682-0505-2016. [DOI] [PubMed] [Google Scholar]
- 98.López-García A., Gilabert J.A. Oral transmission of Chagas disease from a One Health approach: a systematic review. Trop Med Int Health. 2023;28:689–698. doi: 10.1111/tmi.13915. [DOI] [PubMed] [Google Scholar]
- 99.Dias J.C.P. Notas sobre o Trypanosoma cruzi e suas catacterísticas bio-ecológicas, como agente de enfermidades transmitidas por alimentos. Rev Soc Bras Med Trop. 2006;39:370–375. doi: 10.1590/s0037-86822006000400010. [DOI] [PubMed] [Google Scholar]
- 100.Deane M.P., Lenzi H.L., Jansen A. Trypanosoma cruzi: invertebrate cycles in the same mammal host, the oppsoum Didelphis marsupialis. Mem Inst Oswaldo cruz. 1984;79:513–515. doi: 10.1590/s0074-02761984000400021. [DOI] [PubMed] [Google Scholar]
- 101.Beatty N.L., Arango-Ferreira C., Gual-Gonzalez L., Zuluaga S., Nolan M.S., Cantillo-Barraza O. Oral Chagas disease in Colombia-confirmed and suspected routes of transmission. Trop Med Infect Dis. 2024;9 doi: 10.3390/tropicalmed9010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramírez J.D., Montilla M., Cucunubá Z.M., Floréz A.C., Zambrano P., Guhl F. Molecular epidemiology of human oral Chagas disease outbreaks in Colombia. PLoS Negl Trop Dis. 2013;7 doi: 10.1371/journal.pntd.0002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andrade D.V., Gollob K.J, Dutra W.O. Acute Chagas disease: new global challenges for an old neglected disease. PLoS Negl Trop Dis. 2014;8:e3010. doi: 10.1371/journal.pntd.0003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Norman F.F., López-Vélez R. Chagas disease and breast-feeding. Emerg Infect Dis. 2013;19:1561–1566. doi: 10.3201/eid1910.130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.da Silva N.N., Clausell D.T., Nólibos H., et al. Surto epidêmico da doença de Chagas com provável contaminação oral. Rev Inst Med Trop Sao Paulo. 1968;10:265–276. [PubMed] [Google Scholar]
- 106.Noya B.A., Díaz-Bello Z., Colmenares C., et al. Update on oral Chagas disease outbreaks in Venezuela: epidemiological, clinical and diagnostic approaches. Mem Inst Oswaldo Cruz. 2015;110:377–386. doi: 10.1590/0074-02760140285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bruneto E.G., Fernandes-Silva M.M., Toledo-Cornell C., et al. Case-fatality from orally-transmitted acute Chagas disease: a systematic review and meta-analysis. Clin Infect Dis. 2021;72:1084–1092. doi: 10.1093/cid/ciaa1148. [DOI] [PubMed] [Google Scholar]
- 108.Blanchet D., Brenière S.F., Schijman A.G., et al. First report of a family outbreak of Chagas disease in French Guiana and posttreatment follow-up. Infect Genet Evol. 2014;28:245–250. doi: 10.1016/j.meegid.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 109.Freitas V.L.T., Piotto M.R., Esper H.R., et al. Detection of Trypanosoma cruzi DTUs TcI and TcIV in two outbreaks of orally-transmitted Chagas disease in the Northern region of Brazil. Rev Inst Med Trop Sao Paulo. 2023;65:e7. doi: 10.1590/S1678-9946202365007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barbosa M.D., Ferreira J.M., Arcanjo A.R., et al. Chagas disease in the State of Amazonas: history, epidemiological evolution, risks of endemicity and future perspectives. Rev Soc Bras Med Trop. 2015;48(Suppl 1):27–33. doi: 10.1590/0037-8682-0258-2013. [DOI] [PubMed] [Google Scholar]
- 111.Messenger L.A., Miles M.A., Bern C. Between a bug and a hard place: Trypanosoma cruzi genetic diversity and the clinical outcomes of Chagas disease. Expert Rev Anti Infect Ther. 2015;13:995–1029. doi: 10.1586/14787210.2015.1056158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Olivera M.J., Rincón Acevedo C.Y., Olivera A.J., Mendez-Cardona S., Vera Soto M.J. Addressing Chagas disease from a One Health perspective: risk factors, lessons learned and prevention of oral transmission outbreaks in Colombia. Sci One Health. 2024;3 doi: 10.1016/j.soh.2024.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Valente S.A., da Costa Valente V., das Neves Pinto A.Y., et al. Analysis of an acute Chagas disease outbreak in the Brazilian Amazon: human cases, triatomines, reservoir mammals and parasites. Trans R Soc Trop Med Hyg. 2009;103:291–297. doi: 10.1016/j.trstmh.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 114.Xavier SC das C., Roque A.L.R., Bilac D., et al. Distantiae transmission of Trypanosoma cruzi: a new epidemiological feature of acute Chagas disease in Brazil. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cantillo-Barraza O., Bedoya S.C., Xavier S.C.C., et al. Trypanosoma cruzi infection in domestic and synanthropic mammals such as potential risk of sylvatic transmission in a rural area from north of Antioquia, Colombia. Parasite Epidemiol Control. 2020;11 doi: 10.1016/j.parepi.2020.e00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.de Sousa D.R.T., de Oliveira Guerra J.A., Ortiz J.V., et al. Acute Chagas disease associated with ingestion of contaminated food in Brazilian western Amazon. Trop Med Int Health. 2023;28:541–550. doi: 10.1111/tmi.13899. [DOI] [PubMed] [Google Scholar]
- 117.Dias J.C.P., Brener S. Chagas' disease and blood transfusion. Mem Inst Oswaldo Cruz. 1984;79:139–147. [Google Scholar]
- 118.Schmuñis G.A. Trypanosoma cruzi, the etiologic agent of Chagas’ disease: status in the blood supply in endemic and nonendemic countries. Transfusion. 1991;31:547–557. doi: 10.1046/j.1537-2995.1991.31691306255.x. [DOI] [PubMed] [Google Scholar]
- 119.World Health Organization. Control of Chagas disease Report of a WHO expert committee. World Health Organ Tech Rep Ser. 1991;811:1–95. [PubMed] [Google Scholar]
- 120.Schmunis G.A., Cruz J.R. Safety of the blood supply in Latin America. Clin Microbiol Rev. 2005;18:12–29. doi: 10.1128/CMR.18.1.12-29.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pan American Health Organization . Washington DC: PAHO; 2020. Supply of blood for transfusion in Latin America and Caribbean countries 2016-2017. [DOI] [Google Scholar]
- 122.Bwititi P.T., Browne J. Seroprevalence of Trypanosoma cruzi in blood donors at the national blood transfusion services – Guyana. West Indian Med J. 2012;61:559–563. [PubMed] [Google Scholar]
- 123.Dodd R.Y., Groves J.A., Townsend R.L., et al. Impact of one-time testing for Trypanosoma cruzi antibodies among blood donors in the United States. Transfusion. 2019;59:1016–1023. doi: 10.1111/trf.15118. [DOI] [PubMed] [Google Scholar]
- 124.Bern C., Montgomery S.P., Katz L., Caglioti S., Stramer S.L. Chagas disease and the US blood supply. Curr Opin Infect Dis. 2008;21:476–482. doi: 10.1097/QCO.0b013e32830ef5b6. [DOI] [PubMed] [Google Scholar]
- 125.Garcia M.N., Woc-Colburn L., Rossmann S.N., et al. Trypanosoma cruzi screening in Texas blood donors, 2008-2012. Epidemiol Infect. 2016;144:1010–1013. doi: 10.1017/S0950268814002234. [DOI] [PubMed] [Google Scholar]
- 126.O’Brien S.F., Scalia V., Goldman M., et al. Selective testing for Trypanosoma cruzi: the first year after implementation at Canadian blood services. Transfusion. 2013;53:1706–1713. doi: 10.1111/j.1537-2995.2012.03950.x. [DOI] [PubMed] [Google Scholar]
- 127.Pérez-Molina J.A., Molina I. Chagas disease. Lancet. 2018;391:82–94. doi: 10.1016/S0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- 128.de Sousa A.S., Vermeij D., Ramos A.N., Jr., Luquetti A.O. Chagas disease. Lancet. 2024;403:203–218. doi: 10.1016/S0140-6736(23)01787-7. [DOI] [PubMed] [Google Scholar]
- 129.Pierrotti L.C., Carvalho N.B., Amorin J.P., Pascual J., Kotton C.N., López-Vélez R. Chagas disease recommendations for solid-organ transplant recipients and donors. Transplantation. 2018;102:S1–S7. doi: 10.1097/TP.0000000000002019. [DOI] [PubMed] [Google Scholar]
- 130.Altclas J.D., Barcan L., Nagel C., Lattes R., Riarte A. Organ transplantation and Chagas disease. JAMA. 2008;299:1134–1135. doi: 10.1001/jama.299.10.1134-a. [DOI] [PubMed] [Google Scholar]
- 131.Gonzalez-Sanz M., Crespillo-Andújar C., Chamorro-Tojeiro S., Monge-Maillo B., Perez-Molina J.A., Norman F.F. Chagas disease in Europe. Trop Med Infect Dis. 2023;8 doi: 10.3390/tropicalmed8120513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brenière S.F., Waleckx E., Barnabé C. Over six thousand Trypanosoma cruzi strains classified into discrete typing units (DTUs): attempt at an inventory. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zingales B., Bartholomeu D.C. Trypanosoma cruzi genetic diversity: impact on transmission cycles and Chagas disease. Mem Inst Oswaldo Cruz. 2022;117 doi: 10.1590/0074-02760210193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Magalhães L.M.D., Gollob K.J., Zingales B., Dutra W.O. Pathogen diversity, immunity, and the fate of infections: lessons learned from Trypanosoma cruzi human-host interactions. Lancet Microbe. 2022;3:e711–e722. doi: 10.1016/S2666-5247(21)00265-2. [DOI] [PubMed] [Google Scholar]
- 135.Dumonteil E., Desale H., Tu W., et al. Intra-host Trypanosoma cruzi strain dynamics shape disease progression: the missing link in Chagas disease pathogenesis. Microbiol Spectr. 2023;11 doi: 10.1128/spectrum.04236-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Herrera C., Majeau A., Didier P., Falkenstein K.P., Dumonteil E. Trypanosoma cruzi diversity in naturally infected nonhuman primates in Louisiana assessed by deep sequencing of the mini-exon gene. Trans R Soc Trop Med Hyg. 2019;113:281–286. doi: 10.1093/trstmh/try119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Velásquez-Ortiz N., Herrera G., Hernández C., Muñoz M., Ramírez J.D. Discrete typing units of Trypanosoma cruzi: geographical and biological distribution in the Americas. Sci Data. 2022;9:360. doi: 10.1038/s41597-022-01452-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zingales B. Trypanosoma cruzi genetic diversity: something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018;184:38–52. doi: 10.1016/j.actatropica.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 139.Irish A., Whitman J.D., Clark E.H., Marcus R., Bern C. Updated estimates and mapping for prevalence of Chagas disease among adults, United States. Emerg Infect Dis. 2022;28:1313–1320. doi: 10.3201/eid2807.212221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Departamento de Epidemiología del Ministerio de Salud de Chile Bases ENO. Bases ENO. https://epi.minsal.cl/bases-de-datos-eno/
- 141.Zingales B., Macedo A.M. Fifteen years after the definition of Trypanosoma cruzi DTUs: what have we learned? Life. 2023;13 doi: 10.3390/life13122339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Izeta-Alberdi A., Ibarra-Cerdeña C.N., Moo-Llanes D.A., Ramsey J.M. Geographical, landscape and host associations of Trypanosoma cruzi DTUs and lineages. Parasit Vectors. 2016;9:631. doi: 10.1186/s13071-016-1918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Monje-Rumi M.M., Brandán C.P., Ragone P.G., et al. Trypanosoma cruzi diversity in the Gran Chaco: mixed infections and differential host distribution of TcV and TcVI. Infect Genet Evol. 2015;29:53–59. doi: 10.1016/j.meegid.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 144.Gürtler R.E., Cardinal M.V. Reservoir host competence and the role of domestic and commensal hosts in the transmission of Trypanosoma cruzi. Acta Trop. 2015;151:32–50. doi: 10.1016/j.actatropica.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 145.Guhl F., Ramírez J.D. Retrospective molecular integrated epidemiology of Chagas disease in Colombia. Infect Genet Evol. 2013;20:148–154. doi: 10.1016/j.meegid.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 146.Monteiro W.M., Magalhães L.K., Santana Filho F.S., et al. Trypanosoma cruzi TcIII/Z3 genotype as agent of an outbreak of Chagas disease in the Brazilian Western Amazonia. Trop Med Int Health. 2010;15:1049–1051. doi: 10.1111/j.1365-3156.2010.02577.x. [DOI] [PubMed] [Google Scholar]
- 147.Martins K., Andrade Cde M., Barbosa-Silva A.N., et al. Trypanosoma cruzi III causing the indeterminate form of Chagas disease in a semi-arid region of Brazil. Int J Infect Dis. 2015;39:68–75. doi: 10.1016/j.ijid.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 148.Rodrigues-Dos-Santos Í., Melo M.F., de Castro L., et al. Exploring the parasite load and molecular diversity of Trypanosoma cruzi in patients with chronic Chagas disease from different regions of Brazil. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Garzón E.A., Barnabé C., Córdova X., et al. Trypanosoma cruzi isoenzyme variability in Ecuador: first observation of zymodeme III genotypes in chronic chagasic patients. Trans R Soc Trop Med Hyg. 2002;96:378–382. doi: 10.1016/s0035-9203(02)90367-6. [DOI] [PubMed] [Google Scholar]
- 150.Burgos J.M., Diez M., Vigliano C., et al. Molecular identification of Trypanosoma cruzi discrete typing units in end-stage chronic Chagas heart disease and reactivation after heart transplantation. Clin Infect Dis. 2010;51:485–495. doi: 10.1086/655680. [DOI] [PubMed] [Google Scholar]
- 151.Flores-López C.A., Esquivias-Flores E.A., Guevara-Carrizales A. Phylogenetic description of Trypanosoma cruzi isolates from Dipetalogaster maxima: occurrence of TcI, TcIV, and TcIV – USA. Infect Genet Evol. 2023;113 doi: 10.1016/j.meegid.2023.105465. [DOI] [PubMed] [Google Scholar]
- 152.Ramírez J.D., Guhl F., Rendón L.M., Rosas F., Marin-Neto J.A., Morillo C.A. Chagas cardiomyopathy manifestations and Trypanosoma cruzi genotypes circulating in chronic chagasic patients. PLoS Negl Trop Dis. 2010;4 doi: 10.1371/journal.pntd.0000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guhl F., Auderheide A., Ramírez J.D. From ancient to contemporary molecular eco-epidemiology of Chagas disease in the Americas. Int J Parasitol. 2014;44:605–612. doi: 10.1016/j.ijpara.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 154.Carrasco H.J., Segovia M., Llewellyn M.S., et al. Geographical distribution of Trypanosoma cruzi genotypes in Venezuela. PLoS Negl Trop Dis. 2012;6 doi: 10.1371/journal.pntd.0001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Perez-Molina J.A., Poveda C., Martinez-Perez A., et al. Distribution of Trypanosoma cruzi discrete typing units in Bolivian migrants in Spain. Infect Genet Evol. 2014;21:440–442. doi: 10.1016/j.meegid.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 156.Tavares de Oliveira M., Fuzo C.A., da Silva M.C., et al. Correlation of TcII discrete typing units with severe chronic Chagas cardiomyopathy in patients from various Brazilian geographic regions. PLoS Negl Trop Dis. 2022;16 doi: 10.1371/journal.pntd.0010713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ricci A.D., Bracco L., Salas-Sarduy E., et al. The Trypanosoma cruzi antigen and epitope atlas: antibody specificities in Chagas disease patients across the Americas. Nat Commun. 2023;14:1850. doi: 10.1038/s41467-023-37522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Majeau A., Dumonteil E., Herrera C. Identification of highly conserved Trypanosoma cruzi antigens for the development of a universal serological diagnostic assay. Emerg Microbes Infect. 2024;13 doi: 10.1080/22221751.2024.2315964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Couto-Silva C.M., Nunes K., Venturini G., et al. Indigenous people from Amazon show genetic signatures of pathogen-driven selection. Sci Adv. 2023;9 doi: 10.1126/sciadv.abo0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stanaway J.D., Roth G. The burden of Chagas disease: estimates and challenges. Glob Heart. 2015;10:139–144. doi: 10.1016/j.gheart.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 161.Franco-Paredes C., Villamil-Gómez W.E., Schultz J., et al. A deadly feast: elucidating the burden of orally acquired acute Chagas disease in Latin America - public health and travel medicine importance. Travel Med Infect Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101565. [DOI] [PubMed] [Google Scholar]
- 162.Nunes M.C.P., Beaton A., Acquatella H., et al. Chagas cardiomyopathy: an update of current clinical knowledge and management: a scientific statement from the American Heart Association. Circulation. 2018;138:e169–e209. doi: 10.1161/CIR.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 163.de Oliveira M.T., Schmidt A., da Silva M.C., Donadi E.A., da Silva J.S., Marin-Neto J.A. Parasitic load correlates with left ventricular dysfunction in patients with chronic Chagas cardiomyopathy. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.741347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Coura J.R. Chagas disease: what is known and what is needed-a background article. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):113–122. doi: 10.1590/s0074-02762007000900018. [DOI] [PubMed] [Google Scholar]
- 165.Ramos Nascimento B., Dias Nassar Naback A., Marino Pena Santos B., et al. Prevalence of clinical forms of Chagas disease: a systematic review and meta-analysis - data from the RAISE study. Lancet Reg Health Am. 2024;30 doi: 10.1016/j.lana.2024.100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cucunubá Z.M., Okuwoga O., Basáñez M.-G., Nouvellet P. Increased mortality attributed to Chagas disease: a systematic review and meta-analysis. Parasit Vectors. 2016;9:42. doi: 10.1186/s13071-016-1315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Keegan R., Yeung C., Baranchuk A. Sudden cardiac death risk stratification and prevention in Chagas disease: a non-systematic review of the literature. Arrhythm Electrophysiol Rev. 2020;9:175–181. doi: 10.15420/aer.2020.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chadalawada S., Rassi A., Jr., Samara O., et al. Mortality risk in chronic Chagas cardiomyopathy: a systematic review and meta-analysis. ESC Heart Fail. 2021;8:5466–5481. doi: 10.1002/ehf2.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Maron B.J. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379:655–668. doi: 10.1056/NEJMra1710575. [DOI] [PubMed] [Google Scholar]
- 170.Martins-Melo F.R., Castro M.C., Werneck G.L. Levels and trends in Chagas disease-related mortality in Brazil, 2000-2019. Acta Trop. 2021;220 doi: 10.1016/j.actatropica.2021.105948. [DOI] [PubMed] [Google Scholar]
- 171.Sartori A.M.C., Ibrahim K.Y., Nunes Westphalen E.V., et al. Manifestations of Chagas disease (American trypanosomiasis) in patients with HIV/AIDS. Ann Trop Med Parasitol. 2007;101:31–50. doi: 10.1179/136485907X154629. [DOI] [PubMed] [Google Scholar]
- 172.Reimer-McAtee M.J., Mejia C., Clark T., et al. HIV and Chagas Disease: an evaluation of the use of real-time quantitative polymerase chain reaction to measure levels of Trypanosoma cruzi parasitemia in HIV patients in Cochabamba, Bolivia. Am J Trop Med Hyg. 2021;105:643–650. doi: 10.4269/ajtmh.20-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shikanai-Yasuda M.A., Mediano M.F.F., Novaes C.T.G., et al. Clinical profile and mortality in patients with T. cruzi/HIV co-infection from the multicenter data base of the ‘Network for healthcare and study of Trypanosoma cruzi/HIV co-infection and other immunosuppression conditions’. PLoS Negl Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0009809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bern C. Chagas disease in the immunosuppressed host. Curr Opin Infect Dis. 2012;25:450–457. doi: 10.1097/QCO.0b013e328354f179. [DOI] [PubMed] [Google Scholar]
- 175.Lee B.Y., Bacon K.M., Bottazzi M.E., Hotez P.J. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect Dis. 2013;13:342–348. doi: 10.1016/S1473-3099(13)70002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gómez-Ochoa S.A., Rojas L.Z., Echeverría L.E., Muka T., Franco O.H. Global, regional, and national trends of Chagas disease from 1990 to 2019: comprehensive analysis of the Global Burden of Disease Study. Glob Heart. 2022;17:59. doi: 10.5334/gh.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mejía-Jaramillo A.M., Agudelo-Uribe L.A., Dib J.C., Ortiz S., Solari A., Triana-Chávez O. Genotyping of Trypanosoma cruzi in a hyper-endemic area of Colombia reveals an overlap among domestic and sylvatic cycles of Chagas disease. Parasit Vectors. 2014;7:108. doi: 10.1186/1756-3305-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Montgomery S.P., Parise M.E., Dotson E.M., Bialek S.R. What do we know about Chagas disease in the United States? Am J Trop Med Hyg. 2016;95:1225–1227. doi: 10.4269/ajtmh.16-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Beatty N.L., Klotz S.A. Autochthonous Chagas disease in the United States: how are people getting infected? Am J Trop Med Hyg. 2020;103:967–969. doi: 10.4269/ajtmh.19-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ibáñez-Cervantes G., León-García G., Castro-Escarpulli G., et al. Evolution of incidence and geographical distribution of Chagas disease in Mexico during a decade (2007-2016) Epidemiol Infect. 2018;147:e41. doi: 10.1017/S0950268818002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.González-Guzmán S., González-Cano P., Bagu E.T., et al. Seroprevalence of Trypanosoma cruzi in eight blood banks in Mexico. Arch Med Res. 2022;53:625–633. doi: 10.1016/j.arcmed.2022.08.007. [DOI] [PubMed] [Google Scholar]
- 183.Rivas N., González-Guzmán S., Alejandre-Aguilar R. First record of Triatoma barberi Usinger, 1939 (Hemiptera: Reduviidae) in northern State of Mexico, Mexico. J Vector Ecol. 2018;43:337–339. doi: 10.1111/jvec.12319. [DOI] [PubMed] [Google Scholar]
- 184.Ponce C. Current situation of Chagas disease in Central America. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):41–44. doi: 10.1590/s0074-02762007005000082. [DOI] [PubMed] [Google Scholar]
- 185.Flores-Ferrer A., Marcou O., Waleckx E., Dumonteil E., Gourbière S. Evolutionary ecology of Chagas disease; what do we know and what do we need? Evol Appl. 2018;11:470–487. doi: 10.1111/eva.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.International Organization for Migration Americas and the Caribbean. https://www.iom.int/americas-and-caribbean-0
- 187.Conners E.E., Vinetz J.M., Weeks J.R., Brouwer K.C. A global systematic review of Chagas disease prevalence among migrants. Acta Trop. 2016;156:68–78. doi: 10.1016/j.actatropica.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Requena-Méndez A., Aldasoro E., de Lazzari E., et al. Prevalence of Chagas disease in Latin-American migrants living in Europe: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.El Ghouzzi M.-H., Boiret E., Wind F., et al. Testing blood donors for Chagas disease in the Paris area, France: first results after 18 months of screening. Transfusion. 2010;50:575–583. doi: 10.1111/j.1537-2995.2009.02476.x. [DOI] [PubMed] [Google Scholar]
- 190.Jackson Y., Gétaz L., Wolff H., et al. Prevalence, clinical staging and risk for blood-borne transmission of Chagas disease among Latin American migrants in Geneva, Switzerland. PLoS Negl Trop Dis. 2010;4:e592. doi: 10.1371/journal.pntd.0000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Requena-Méndez A., Moore D.A.J., Subirà C., Muñoz J. Addressing the neglect: Chagas disease in London, UK. Lancet Glob Health. 2016;4:e231–e233. doi: 10.1016/S2214-109X(16)00047-4. [DOI] [PubMed] [Google Scholar]
- 192.Navarro M., Reguero L., Subirà C., Blázquez-Pérez A., Requena-Méndez A. Estimating chagas disease prevalence and number of underdiagnosed, and undertreated individuals in Spain. Travel Med Infect Dis. 2022;47 doi: 10.1016/j.tmaid.2022.102284. [DOI] [PubMed] [Google Scholar]
- 193.Jackson Y., Myers C., Diana A., et al. Congenital transmission of Chagas disease in Latin American immigrants in Switzerland. Emerg Infect Dis. 2009;15:601–603. doi: 10.3201/eid1504.080438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Martinez de Tejada B., Jackson Y., Paccolat C. Irion O. Dépistage et prise en charge de la maladie de Chagas congénitale à Genève. Rev Med Suisse. 2009;5(2091–2):2094–2096. [PubMed] [Google Scholar]
- 195.World Health Organization . Integrating neglected tropical diseases into global health and development: fourth WHO report on neglected tropical diseases. World Health Organization; 2018. https://www.who.int/publications/i/item/9789241565448 [Google Scholar]
- 196.Gürtler R.E., Kitron U., Cecere M.C., Segura E.L., Cohen J.E. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc Natl Acad Sci U S A. 2007;104:16194–16199. doi: 10.1073/pnas.0700863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pan American Health Organization . OPS; Washington DC: 1992. Iniciativa del Cono Sur : Agosto 1991-Diciembre 1992: Estado actual de las acciones para la eliminación de la transmisión vectorial e interrupción de la transmisión transfusional del Tripanosoma Cruzi.https://iris.paho.org/handle/10665.2/38214 [Google Scholar]
- 198.Organización Panamericana de la Salud . Organización Panamericana de la Salud; 2019. Control, interrupción de la transmisión y eliminación de la enfermedad de Chagas como problema de salud pública. Guía de evaluación, verificación y validación. [DOI] [Google Scholar]
- 199.Organización Mundial de Ia Salud − Organización Mundial de la Salud −División de Control de Enfermedades. Procesos de control de triatomíneos y control de la transmisión transfusional de Trypanosoma cruzi. Rev Patol Trop. 2002;31:269–282. [Google Scholar]
- 200.Organización Panamericana de la Salud Enfermedad de Chagas − Transmisión por el principal vector. 2019. https://www.paho.org/en/documents/map-chagas-vectorial-transmission-2019-spanish-only
- 201.Organización Panamericana de la Salud . OPS; Whashington DC: 2023. Guía metodológica para evaluaciones externas de la interrupción de la transmisión y la eliminación de la enfermedad de Chagas como problema de salud pública. [DOI] [Google Scholar]
- 202.Antonio-Campos A., Nicolás-Cruz A., Girón-Arias J.I., Rivas N., Alejandre-Aguilar R. Presence of Rhodnius prolixus Stål, 1859 (Hemiptera: Reduviidae) in Oaxaca, Mexico, ten years after the certification of its elimination. J Vector Ecol. 2019;44:293–295. doi: 10.1111/jvec.12363. [DOI] [PubMed] [Google Scholar]
- 203.Martínez-Hernández F., Villalobos G., Montañez-Valdez O.D., Martínez-Ibarra J.A. A new record of the introduced species Triatoma infestans (Hemiptera: Reduviidae) in Mexico. J Med Entomol. 2022;59:2150–2157. doi: 10.1093/jme/tjac078. [DOI] [PubMed] [Google Scholar]
- 204.Fitzpatrick S., Feliciangeli M.D., Sanchez-Martin M.J., Monteiro F.A., Miles M.A. Molecular genetics reveal that silvatic Rhodnius prolixus do colonise rural houses. PLoS Negl Trop Dis. 2008;2 doi: 10.1371/journal.pntd.0000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Guhl F., Pinto N., Aguilera G. Sylvatic triatominae: a new challenge in vector control transmission. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):71–75. doi: 10.1590/s0074-02762009000900012. [DOI] [PubMed] [Google Scholar]
- 206.Bargues M.D., Klisiowicz D.R., Gonzalez-Candelas F., et al. Phylogeography and genetic variation of Triatoma dimidiata, the main Chagas disease vector in Central America, and its position within the genus Triatoma. PLoS Negl Trop Dis. 2008;2 doi: 10.1371/journal.pntd.0000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Monteiro F.A., Peretolchina T., Lazoski C., et al. Phylogeographic pattern and extensive mitochondrial DNA divergence disclose a species complex within the Chagas disease vector Triatoma dimidiata. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Aguilar H.M., Abad-Franch F., Dias J.C.P., Junqueira A.C.V., Coura J.R. Chagas disease in the Amazon region. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):47–56. doi: 10.1590/s0074-02762007005000098. [DOI] [PubMed] [Google Scholar]
- 209.Delgado S., Ernst K.C., Pumahuanca M.L.H., et al. A country bug in the city: urban infestation by the Chagas disease vector Triatoma infestans in Arequipa, Peru. Int J Health Geogr. 2013;12:48. doi: 10.1186/1476-072X-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Provecho Y.M., Fernández M.D.P., Salvá L., et al. Urban infestation by Triatoma infestans (Hemiptera: Reduviidae), an overlooked phenomena for Chagas disease in Argentina. Mem Inst Oswaldo Cruz. 2021;116 doi: 10.1590/0074-02760210056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Medrano-Mercado N., Ugarte-Fernandez R., Butrón V., et al. Urban transmission of Chagas disease in Cochabamba, Bolivia. Mem Inst Oswaldo Cruz. 2008;103:423–430. doi: 10.1590/s0074-02762008000500003. [DOI] [PubMed] [Google Scholar]
- 212.Levy M.Z., Malaga Chavez F.S., Cornejo Del Carpio J.G., Vilhena D.A., McKenzie F.E., Plotkin J.B. Rational spatio-temporal strategies for controlling a Chagas disease vector in urban environments. J R Soc Interface. 2010;7:1061–1070. doi: 10.1098/rsif.2009.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Levy M.Z., Barbu C.M., Castillo-Neyra R., et al. Urbanization, land tenure security and vector-borne Chagas disease. Proc Biol Sci. 2014;281 doi: 10.1098/rspb.2014.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Espinoza N., Borrás R., Abad-Franch F. Chagas disease vector control in a hyperendemic setting: the first 11 years of intervention in Cochabamba, Bolivia. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Human rights. https://www.who.int/news-room/fact-sheets/detail/human-rights-and-health
- 216.Marcus R., Henao-Martínez A.F., Nolan M., et al. Recognition and screening for Chagas disease in the USA. Ther Adv Infect Dis. 2021;8 doi: 10.1177/20499361211046086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Abad-Franch F. Chagas disease diagnosis and cure assessment: getting formally hierarchical about a naturally hierarchical problem. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Morillo C.A., Marin-Neto J.A., Avezum A., et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med. 2015;373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 219.Abad-Franch F. Trypanosoma cruzi parasitemia in chronic Chagas disease: insights from hierarchical modeling. PLoS Negl Trop Dis. 2022;16 doi: 10.1371/journal.pntd.0010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Verani J.R., Seitz A., Gilman R.H., et al. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am J Trop Med Hyg. 2009;80:410–415. [PubMed] [Google Scholar]
- 221.Barfield C.A., Barney R.S., Crudder C.H., et al. A highly sensitive rapid diagnostic test for Chagas disease that utilizes a recombinant Trypanosoma cruzi antigen. IEEE Trans Biomed Eng. 2011;58:814–817. doi: 10.1109/TBME.2010.2087334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gabaldón-Figueira J.C., Skjefte M., Longhi S., et al. Practical diagnostic algorithms for Chagas disease: a focus on low resource settings. Expert Rev Anti Infect Ther. 2023;21:1287–1299. doi: 10.1080/14787210.2023.2279110. [DOI] [PubMed] [Google Scholar]
- 223.Miranda-Arboleda A.F., Zaidel E.J., Marcus R., et al. Roadblocks in Chagas disease care in endemic and nonendemic countries: Argentina, Colombia, Spain, and the United States. The NET-Heart project. PLoS Negl Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Echeverría L.E., Marcus R., Novick G., et al. WHF IASC roadmap on Chagas disease. Glob Heart. 2020;15:26. doi: 10.5334/gh.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Olivo Freites C., Sy H., Gharamti A., et al. Chronic chagas disease-the potential role of reinfections in cardiomyopathy pathogenesis. Curr Heart Fail Rep. 2022;19:279–289. doi: 10.1007/s11897-022-00568-9. [DOI] [PubMed] [Google Scholar]
- 226.Bosch-Nicolau P., Fernández M.L., Sulleiro E., et al. Efficacy of three benznidazole dosing strategies for adults living with chronic Chagas disease (MULTIBENZ): an international, randomised, double-blind, phase 2b trial. Lancet Infect Dis. 2024;24:386–394. doi: 10.1016/S1473-3099(23)00629-1. [DOI] [PubMed] [Google Scholar]
- 227.Padilla A.M., Wang W., Akama T., et al. Discovery of an orally active benzoxaborole prodrug effective in the treatment of Chagas disease in non-human primates. Nat Microbiol. 2022;7:1536–1546. doi: 10.1038/s41564-022-01211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tarleton R.L. Effective drug discovery in Chagas disease. Trends Parasitol. 2023;39:423–431. doi: 10.1016/j.pt.2023.03.015. [DOI] [PubMed] [Google Scholar]
- 229.Sánchez-Valdéz F.J., Padilla A., Wang W., Orr D., Tarleton R.L. Spontaneous dormancy protects Trypanosoma cruzi during extended drug exposure. Elife. 2018;7 doi: 10.7554/eLife.34039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bustamante J.M., Sanchez-Valdez F., Padilla A.M., White B., Wang W., Tarleton R.L. A modified drug regimen clears active and dormant trypanosomes in mouse models of Chagas disease. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abb7656. [DOI] [PMC free article] [PubMed] [Google Scholar]