Abstract
Class switching consists in the substitution of the heavy-chain constant region of immunoglobulin M (IgM) with that of IgG, IgA, or IgE. This enables antibodies to acquire new effector functions that are crucial to combat invading pathogens. Class switching usually requires engagement of CD40 on B cells by CD40 ligand (CD40L) on antigen-activated CD4+ T cells and the production of cytokines. The process must be regulated tightly because abnormal IgG and IgA production favors the onset of autoimmunity, whereas increased switching to IgE leads to atopy. These inflammatory disorders can be triggered or exacerbated by costimulatory signals. Although thoroughly investigated on T cells, the roles of the inhibitory receptors CD85j, LAIR-1, and CD152 on B-cell functions have not been fully elucidated. In this study we show that cross-linking of the B-cell inhibitory receptors by specific monoclonal antibodies inhibits IgG and IgE production, reduces the percentage of IgG- and IgE-expressing B cells, and down-regulates interleukin 8 (IL-8), IL-10, and tumor necrosis factor alpha production. These effects were demonstrated using different B-cell stimulatory pathways (recall antigens, CD40L-transfected cells plus IL-4, and lipopolysaccharide plus IL-4). It thus appears that CD85j, LAIR-1, and CD152 play a central role for the control of IL-4-driven isotype switching.
Expression and functions of inhibitory receptors have been investigated mainly in studies of T lymphocytes and NK cells. The negative role exerted by CD85j (LIR-1-ILT2), LAIR-1, and CD152 (CTLA-4) on T-cell functions has been thoroughly characterized (11, 16, 19, 26, 28). T-cell inhibitory receptor cross-linking by monoclonal antibodies (MAbs) and goat anti-mouse (GAM) antiserum or physiologically induced by their ligands expressed on antigen-presenting cells down-regulates cytokine production (e.g., interleukin 2 [IL-2], and gamma interferon [IFN-γ], IL-4), IL-2 receptor α chain expression, and cell cycle progression (4, 16, 26, 27, 28). However, inhibitory receptors are also constitutively expressed or can be induced on B lymphocytes, and their functional outcome still awaits full characterization.
CD85j is found on monocytes, B cells, NK cells, and T cells. This receptor binds major histocompatibility complex (MHC) class I or viral MHC class I homologues (8, 9) and is a transmembrane molecule with four immunoreceptor tyrosine-based inhibition motifs (ITIMs) in its cytoplasmic tail (2, 3). Tyrosine phosphorylation of ITIMs establishes docking sites for the SH2 domain-containing phosphatase SHP-1 that subsequently transduces inhibitory signals by dephosphorylating and inactivating downstream tyrosine kinases (2). Cross-linking of CD85j inhibits activation of B cells, T cells, NK cells, and macrophages (6, 7, 26).
The leukocyte-associated Ig-like receptor-1 (LAIR-1) is expressed on the majority of human peripheral blood mono-nuclear cells (PBMCs), including NK cells, T cells, B cells, monocytes, and dendritic cells, as well as on the majority of thymocytes (17). LAIR-1 is a transmembrane glycoprotein with a single extracellular Ig-like domain and a cytoplasmic tail that comprises two ITIMs. Cross-linking of LAIR-1 delivers a signal that inhibits the functions of NK cells, B cells, T cells, and dendritic cell precursors (17, 22, 27, 31). However, this inhibition is less efficient than that mediated by other receptors expressed on T lymphocytes, such as CD85j and CD152 (27).
Another inhibitory receptor, namely, CD152, can be induced on B cells by activated T lymphocytes (15) or by CD40 or lipopolysaccharide (LPS) stimulation in the presence of IL-4 (21). In addition, CD152 is constitutively expressed on B cells from non-Hodgkin's lymphomas (33). Although its role on B-cell functions has not been established completely, CD152 cross-linking down-regulates IL-4-driven Ig production and inhibits the expression of Cɛ and Cγ1 germ line mRNA as well as of activating transcription factors (21).
All of these studies have explored the regulatory role of inhibitory receptors in B-cell activation, at least for CD85j and LAIR-1, only by measuring the inhibition of Ca2+ mobilization triggered via the B-cell antigen receptor (7, 17). In fact, Ca+ mobilization is only one aspect of early B-cell activation, whereas isotype switching and Ig secretion are subsequent steps. In normal B cells, switching from IgM to IgG, IgA, or IgE requires two signals, one delivered by CD40 ligand (CD40L) and the other provided by cytokines. Of the cytokines, IL-4 induces switching to IgG and IgE. In addition, dysregulated switching to IgG and IgA is central to the pathogenesis of autoimmune disorders, such as systemic lupus erythematosus, whereas aberrant switching to IgE underlies the pathogenesis of atopic disorders, such as allergic asthma and atopic dermatitis.
Therefore, the inhibitory effects of CD85j, LAIR-1, and CD152 cross-linking on B-lymphocyte functions have been investigated. We have identified a role for these receptors in the regulation of cytokine release and in the production of specific IgG induced by recall antigen stimulation. In addition, CD85j, LAIR-1, and CD152 cross-linking does not affect CD23 (Fcɛ receptor II) expression, whereas it inhibits IgE production under the same experimental conditions. It is conceivable that CD23 expression requires less stringent conditions and that signals leading to its expression may be regulated differently and not affected by inhibitory receptor stimulation. Finally, it appears that the inhibitory effect on IgG release mainly affects the secretory machinery rather than its de novo synthesis.
MATERIALS AND METHODS
B-cell purification, culture, and activation.
B lymphocytes were purified by negative selection from PBMCs obtained from healthy donors. Blood monocytes were removed by 1 h of adherence to plastic. Nonadherent cells were collected, and T and NK cells were removed by immunomagnetic cell sorting with specific MAb-coated microbeads (CD3 MicroBeads and CD56 MicroBeads; Miltenyi Biotec, Auburn, CA). The resulting cell population comprised <2% T-cell receptor αβ+, <0.2% CD14+, and >96% CD19+ cells, as assessed by flow cytometric analysis (Fig. 1A).
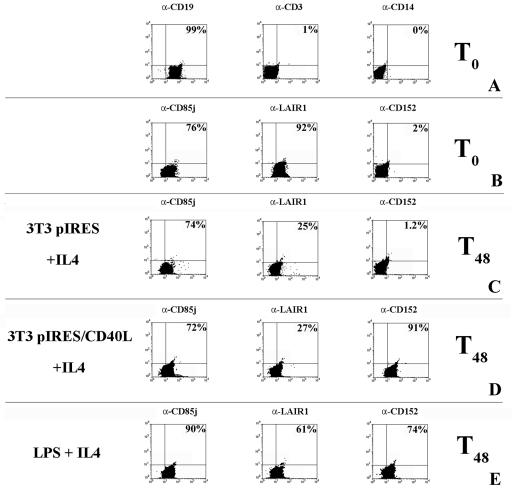
FIG. 1.
Expression of inhibitory molecules on the surface of B lymphocytes. CD85j, LAIR-1, and CD152 expression was evaluated by surface staining and flow cytometric analysis. (A) Percentages of purified B cells (CD19+), contaminant T cells (CD3+), and monocytes (CD14+). α-CD19, anti-CD19 antibody. (B) Inhibitory receptor expression in unstimulated B lymphocytes. (C) Surface expression of inhibitory molecules 48 h after stimulation with 3T3 pIRES cells plus IL-4 (a control for CD40L stimulation). (D) Surface expression of inhibitory molecules 48 h after stimulation with 3T3 pIRES/CD40L cells (CD40L-transfected cells) in the presence of IL-4. (E) Surface expression of inhibitory molecules 48 h after stimulation with LPS plus IL-4. The percentages of positive cells are shown.
B lymphocytes (5 × 105/ml) were cultured in 24-well plates with RPMI 1640 medium, 5% fetal calf serum, 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). Activation was obtained using fibroblasts transfected with CD40L (clone 3T3 pIRES/CD40L) or with LPS (1 μg/ml) and IL-4 (200 U/ml). Optimal doses of LPS and IL-4 were established in preliminary experiments.
Generation of CD40L transfectants.
The human CD40L coding region was amplified by cDNA derived from T cells activated in vitro using the following primers: CD40LFW (CGAATTCCCAGTGTGCTGGACCATGATCGAAACATACAACC) and CD40LRV (GCGGATCCCCAGTGTGATGGTCAGAGTTTGAGTAAGCC). The PCR product was digested using the EcoRI and BamHI restriction enzymes (sequences were included in the FW and RV primers) and cloned in EcoRI-BamHI-digested pIRESneo vector (BD-Clontech, Milan, Italy) carrying the antibiotic resistance gene. Plasmid DNA containing the CD40L insert was sequenced and transfected into NIH 3T3 cells using the Lipofectamine reagent (Invitrogen, Milan, Italy). Cells growing after antibiotic selection (G418, 150 μg/ml) were cloned by limiting dilutions, and clones were tested for CD40L expression by immunofluorescence and flow cytometry. A strongly expressing clone (termed 3T3 pIRES/CD40L) was selected and used for functional studies. A mock-transfected cell line (termed 3T3 pIRES) was established as well.
Antibodies.
The following antibodies were used for immunofluorescence staining and B-lymphocyte cultures: anti-CD19 (HIB19, IgG1), anti-CD20 (2H7, IgG2b), and anti-CD14 (D5E6, IgG2a) (Becton Dickinson, Milan, Italy), anti-CD3 (UCHT1, IgG1), anti-CD4 (OKT4, IgG2a), anti-CD8 (OKT8, IgG2b) (American Type Culture Collection, Rockville, MD), anti-CD85j (HP-F1 MAb, IgG1; kindly provided by Miguel Lopez-Botet, Universitat Pompeu Fabra, Barcelona, Spain), anti-LAIR-1 (clone DX26 IgG1, kindly provided by Joe Philips, DNAX, Stanford, CA), and anti-CD152 (IgG1; kindly provided by Antonio Lanzavecchia, Institute for Research in Biomedicine, Bellinzona, Switzerland).
Flow cytometric analyses.
Cells (5 ×105) were preincubated with Fc blocking MAb (KD1, IgG1, anti-CD16) to prevent nonspecific binding of labeled MAbs and then double stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD19 (clone HIB19; Becton Dickinson) and anti-CD85j, anti-LAIR-1, or anti-CD152 MAb. To evaluate membrane-bound Ig, phycoerythrin-conjugated anti-IgM (clone JDC-15), FITC-conjugated anti-IgG (clone G18-145), and FITC-conjugated anti-IgE (clone G7-26) (Becton Dickinson) MAbs were used. IgM− IgG+ and IgM− IgE+ cells were considered switched cells. FITC- or phycoerythrin-conjugated isotype-matched MAbs were used as controls. Aliquots of 104 cells were analyzed, and fluorescence signals were collected in log mode using a FACScalibur (Becton Dickinson).
Selection of antigen-specific T lymphocytes.
PBMCs were obtained from healthy donors using heparinized venous blood and Ficoll density gradients, cultured with tetanus toxoid (TT) (5 μg/ml) or with purified protein derivative from M. tuberculosis (PPD) (10 μg/ml) in 24-well plates; human recombinant IL-2 at a final concentration of 50 U/ml was added on culture days 2 and 4. Selection of CD4+ T-cell lines was achieved by repeated restimulation cycles with TT or PPD in the presence of autologous irradiated PBMCs.
T-cell-B-cell cooperation assay.
B lymphocytes in complete medium were pulsed with antigens for 4 h at 37°C (TT [5 μg/ml] and PPD [10 μg/ml]). Antigen-specific CD4+ T cells (103) were dispensed in flat-bottom 96-well plates with 5 × 103 autologous B cells in 200 μl medium per well. After 2 days of incubation, 150 μl of medium was replaced with fresh medium without antigen. MAbs to inhibitory receptors, irrelevant MAbs, and GAM antiserum were added as indicated. The supernatants were collected after 6 and 12 days and tested for specific IgG antibodies. In order to detect the helper activity of the T-cell lines, controls were provided by culturing B lymphocytes in the absence of T cells or antigens.
ELISA.
Total IgG concentration was assessed by sandwich enzyme-linked immunosorbent assay (ELISA) using the G18-145 MAb (2 μg/ml; Becton Dickinson) for capture. Culture supernatants and the Ig standard (IgG from human serum; Sigma-Aldrich, Milan, Italy) were serially diluted and added to the wells. To detect bound IgG, an alkaline phosphatase-conjugated goat anti-human IgG antiserum (Sigma) was utilized. Phosphatase substrate (p-nitrophenyl phosphate; Sigma) was used to develop the reaction. The reference straight line obtained by plotting the absorbance versus standard IgG concentrations was employed to evaluate the amount of IgG in the supernatant.
To test specific antibodies in the supernatants, an ELISA was performed as follows. Microtiter plates were coated with TT or PPD (5 μg/ml) in 0.05 M sodium carbonate buffer, pH 9.6. After overnight incubation at 4°C, plates were washed three times with phosphate-buffered saline (PBS) containing Tween 20 (0.01%). Wells were blocked by incubation for 1 h with PBS containing 0.5% casein. Undiluted supernatants were used at 50 μl per well. Plates were incubated for 2 h at room temperature. The secondary reagent used for detection was an alkaline phosphatase-conjugated goat anti-human IgG antiserum (Sigma) at 100 μl per well. After 1 h of incubation, wells were washed and the phosphatase substrate was added. Absorbance was read at 405 nm in a SLT 340 ATC (SLT Laboratory Instruments, Vienna, Austria), after 45 min. Results were calculated using standard IgG as described above.
Western blotting.
Western blotting was used to detect total IgG in the supernatant and IgG retained in the B cells. Proteins were separated by 10% gradient polyacrylamide gel electrophoresis in a discontinuous buffer system on a Mini-Protean system (Bio-Rad Laboratories S.r.l., Milan, Italy). The separated components were electroblotted onto polyvinylidene difluoride membranes. Blots were washed with 0.15 M NaCl, 0.05 M Tris, pH 7.5, with 0.3% Tween 20, reacted with a 1:10,000 dilution of the rabbit F(ab′)2 anti-human IgG (heavy plus light chains) horseradish peroxidase conjugate (Southern Biotechnology, Birmingham, AL) for 1 h at room temperature, and washed. Blots were then developed using a commercially available chemiluminescence detection kit (BMB, Indianapolis, IN) according to the manufacturer's instructions.
Detection of intracellular cytokines.
Intracellular cytokine staining was performed using a modification of the method described by Jung et al. (14). Briefly, cells were washed twice in PBS and stained with MAbs specific for CD20. Cells were then fixed with 2% paraformaldehyde on ice for 10 min in the dark, permeabilized with fluorescence-activated cell sorting permeabilizing solution (Becton Dickinson), and incubated with anticytokine MAbs (IL-8, IL-10, IL-12, and tumor necrosis factor alpha [TNF-α]) for 30 min at room temperature in the dark. Nonrelevant Ig isotype-matched MAbs were used as controls. After further washing with PBS, cells were analyzed by flow cytometry using a FACScalibur (Becton Dickinson).
RESULTS
Surface expression of inhibitory molecules on B lymphocytes.
To exert their modulatory effect, the inhibitory receptors need previously activated cells. For this reason, the presence of the inhibitory molecules CD85j, LAIR-1, and CD152 on the surface membrane of freshly purified B cells and following their activation with CD40L-transfected cells plus IL-4 or with LPS plus IL-4 was analyzed. B cells were >96% pure, as determined by anti-CD19 MAb staining. T-cell and monocyte contamination was consistently below 2% and 0.2%, respectively, as assessed by anti-CD3 and anti-CD14 MAbs (Fig. 1A).
CD85j is constitutively expressed on the surfaces of freshly isolated B cells and is maintained following activation for 2 days (Fig. 1B, C, D, and E) and for up to 6 days (not shown).
LAIR-1 is also detected on the surfaces of fresh B cells, but the percentage of positive cells decreases after 2 days of activation and decreases markedly when CD40L-transfected cells are used as stimulators (Fig. 1B, C, D, and E). A further decrease is observed for longer periods (4 and 6 days [not shown]).
In contrast to the two receptors discussed above, CD152 is not expressed constitutively on the surfaces of fresh B cells (Fig. 1B) but is found on B cells activated for 2 days with CD40L-transfected cells plus IL-4 and LPS plus IL-4 (Fig. 1D and E). A good control is provided by stimulation with mock-transfected cells (Fig. 1C) which do not induce CD152 expression. In follow-up studies and similar to what occurs for T cells (15, 19, 21), CD152 expression is transient, as it decreases after 2 to 3 days and disappears on day 6 of stimulation (not shown).
It is of note that, in the course of the activation experiments, the percentage of CD19+ B cells consistently remained above 96% (not shown).
Cross-linking of inhibitory receptors down-regulates both antigen-specific and total IgG production.
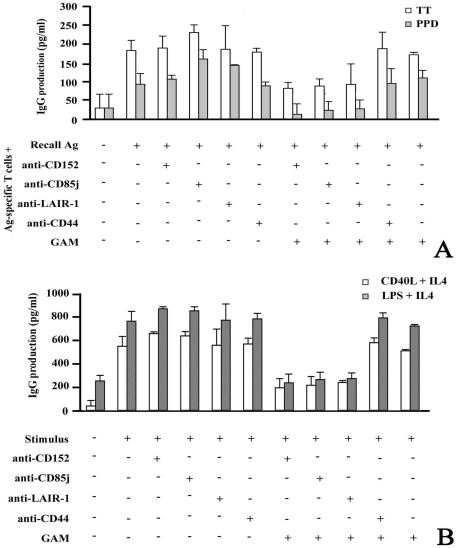
We evaluated the effect of inhibitory receptor cross-linking on antigen-specific IgG production. To this end, B cells were stimulated with recall antigens (TT and PPD) and with autologous antigen-specific T-cell clones (>98% CD3+ CD4+). Isotype-matched MAbs or anti-CD85j, anti-LAIR-1, and anti-CD152 MAbs were added alone or cross-linked with GAM antiserum. Figure 2A illustrates the results. After 6 days of culture, recall antigens in the presence of antigen-specific T cells stimulate B cells to produce detectable amounts of anti-TT or anti-PPD IgG. The addition of MAbs to the inhibitory receptors has no effect, but their cross-linking with GAM antiserum yields a strong reduction of antigen-specific IgG production (ranging from 66% to 100%, 65% to 100%, and 68% to 87% for anti-CD85j, LAIR-1, and anti-CD152, respectively). Irrelevant isotype-matched MAbs have no effect on specific IgG production (Fig. 2A).
FIG. 2.
Cross-linking of inhibitory molecules down-regulates antigen-specific and total IgG production. (A) Total IgG antibodies specific for the indicated antigens (Ag) were produced in a T-cell-B-cell cooperation test. The supernatant was collected after 6 days, and IgG specific for TT and PPD was determined by ELISA. The averages ± standard deviations (error bars) of three different experiments are shown. Similar results were obtained with two other donors. (B) B cells were stimulated with CD40L-transfected cells plus IL-4 or with LPS plus IL-4, a system mimicking the interaction between T and B lymphocytes, in the presence (+) of cross-linked MAbs to CD152, CD85j, and LAIR-1. Anti-CD44 MAb was included as an irrelevant isotype-matched control. The columns indicate the IgG concentrations in the culture supernatants after 6 days of stimulation.
The presence of inhibitory receptors on the surfaces of both B and T lymphocytes does not allow to determine whether a reduction of specific Ig production is attributable to a direct effect on B cells or to a loss of helper effect due to inhibition of T cells. To this end, the role of inhibitory receptors was investigated in two experimental models in which B cells are activated in the absence of T cells, i.e., by CD40L or LPS and IL-4.
Purified B cells can be activated via CD40L plus IL-4, a system that mimics the interaction between T and B lymphocytes. Alternatively, activation can be obtained using LPS and IL-4, resembling what occurs in some bacterial infections.
B cells activated by adding CD40L-transfected cells plus IL-4 produce larger amounts of total IgG, in comparison with those measured following recall antigen stimulation (Fig. 2A and B). Under these experimental conditions, soluble anti-inhibitory receptor MAbs have virtually no effect on IgG production (Fig. 2B). In contrast, cross-linking of CD85j, LAIR-1, and CD152 by GAM antiserum leads to a down-regulation of IgG production (Fig. 2B), which is more effective during the first 6 days of B-cell treatment. After this time, the effect of the cross-linked inhibitory receptors decreases, possibly due to a dilution of the MAbs or to their inactivation.
The same results were obtained when IgG production was measured following stimulation of B lymphocytes with LPS plus IL-4 (Fig. 2B).
An inhibitory effect on IgE production was also detected under the same conditions of B-cell stimulation (not shown).
Cross-linking of inhibitory receptors reduces the frequency of IgG+ and IgE+ B lymphocytes.
Antigen-activated B cells undergo a series of genetic events, including isotype switch of immunoglobulin heavy-chain constant region genes. For this reason, flow cytometric analyses were performed to demonstrate that the inhibitory effect of CD85j, LAIR-1, or CD152 cross-linking could result in a reduction of the frequency of IgG- and IgE-producing cells. Although it has long been appreciated that T cells are intimately involved in isotype switching, we performed experiments in a T-cell-independent system in which B-cell activation was obtained via CD40L-transfected fibroblasts plus IL-4 and/or LPS plus IL-4.
Figure 3A shows the percentages of IgM+, IgG+, and IgE+ B cells, respectively, 6 and 12 days after stimulation with CD40L-transfected cells plus IL-4. Since during culture, IgM+ nonswitched B cells might bind, via their Fc receptors, antibodies produced by other B cells and thus be detected as IgG- or IgE-expressing cells, we performed a two-color analysis, considering only IgM− cells as those expressing IgG or IgE.
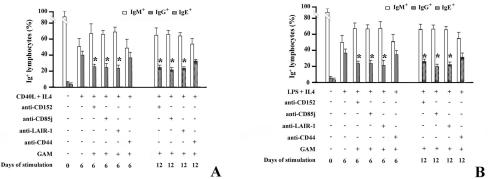
FIG. 3.
Cross-linking of inhibitory receptors reduces the frequency of IgG+ and IgE+ B lymphocytes. (A) B lymphocytes were stimulated with CD40L-transfected cells plus IL-4 in the presence (+) of isotype-matched control MAbs or with the cross-linked MAbs specific for the inhibitory receptors. After 6 and 12 days, B lymphocytes were analyzed for the expression of membrane IgM, IgG, and IgE. Data are percentages of IgM+, IgM− IgG+ (IgG+), and IgM− IgE+ (IgE+) cells at the indicated time of culture. Samples that are significantly different (P < 0.05) from the control culture (B cells stimulated with CD40L-transfected cells plus IL-4) are indicated with an asterisk. (B) B lymphocytes were stimulated with LPS plus IL-4 (+) in the presence (+) of MAbs as indicated. After 6 and 12 days, B lymphocytes were analyzed for the expression of membrane IgM, IgG, and IgE. Data represent the percentages of IgM+, IgM− IgG+ (IgG+), and IgM− IgE+ (IgE+) cells at the indicated times of culture. Samples that are significantly different (P < 0.05) from the control culture (B cells treated with LPS plus IL-4) are indicated with an asterisk.
We found that CD85j, LAIR-1, or CD152 cross-linking does not reduce the percentage of IgM-expressing B cells. Conversely, after cross-linking of the inhibitory receptors, the percentage of IgG+ cells decreases and reaches its peak on day 6 of culture (Fig. 3A). As expected from the sequence of isotype switching (23), the frequency of IgE+ B cells reaches its highest level after 12 days of culture. Upon cross-linking of inhibitory receptors, the percentage of IgE-expressing cells is drastically reduced. We conclude that inhibitory receptors not only down-regulate IgG (and IgE) production but also reduce the percentage of IgG+ and IgE+ B lymphocytes.
Similar results were obtained when B lymphocytes were activated by LPS and IL-4 (Fig. 3B).
Cross-linking of inhibitory receptors preferentially reduces the secretion of IgG.
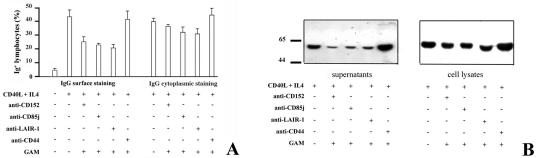
To further analyze the effects of inhibitory receptors on B-cell functions, we performed experiments aimed at defining the kinetics of IgG secretion. The results show that cross-linking of CD85j, LAIR-1, or CD152 leads to a stronger inhibitory effect on IgG secretion than that on IgG retention in the cytoplasmic compartment. As shown by cytoplasmic staining, the percentage of IgG+ B cells after 6 days of stimulation with CD40L-transfected cells and IL-4 is reduced only slightly by cross-linking of inhibitory receptors (Fig. 4A). Comparable results obtained by Western blotting of B-lymphocyte supernatants and lysates after 6 days of culture (Fig. 4B) support this contention.
FIG. 4.
Cross-linking of inhibitory receptors preferentially inhibits the secretion of IgG. (A) B cells, stimulated with CD40L-transfected cells plus IL-4 (+) in the presence (+) of the specified MAb for 6 days, were analyzed for surface and cytoplasmic expression of IgG. (B) B cells, stimulated with CD40L-transfected cells plus IL-4 in the presence of the specified MAb for 6 days, were analyzed by Western blotting of supernatants and cell lysates. The positions of molecular size markers (in kilodaltons) are indicated to the left of the gel.
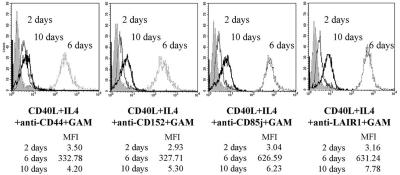
Cross-linking of inhibitory receptors sustains CD23 expression.
Since activation via CD40-CD40L interaction in the presence of IL-4 up-regulates the expression of CD23 (Fcɛ receptor II), a low-affinity IgE receptor (34), we investigated whether cross-linking of inhibitory molecules also affects the expression of this molecule. Barely expressed after 24 h, CD23 is detectable after 3 days (not shown), reaches its highest level at 6 days, and is down-regulated after 10 days of culture (Fig. 5). CD152 cross-linking does not inhibit the expression of this receptor and seems to sustain CD23 expression, as after 10 days of culture its level is higher that in the control groups (Fig. 5). In addition, CD85j or LAIR-1 cross-linking up-regulates CD23 expression after 6 days of culture, and its level remains higher than that in controls until the 10th day (Fig. 5).
FIG. 5.
Cross-linking of inhibitory receptors does not inhibit CD23 expression. B cells were stimulated with CD40L-transfected cells plus IL-4 in the presence of the specified MAbs for the indicated number of days and were analyzed for the expression of CD23. Shaded histograms represent the fluorescence of cells stained with the antiserum alone. MFI, mean fluorescence intensity.
Cytokine production and its modulation by inhibitory receptor cross-linking.
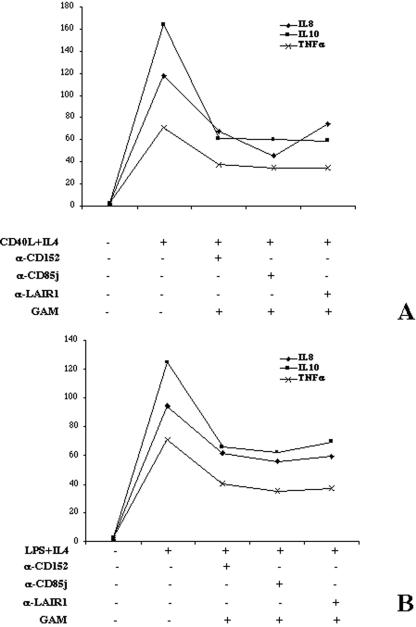
Finally, B cells were stimulated with CD40L-transfected cells plus IL-4 or with LPS plus IL-4, with or without cross-linking of CD85j, LAIR-1, and CD152, and cytokine production was evaluated. In fact, several cytokines are relevant not only for the proliferation and activation of T lymphocytes but also for synergistic effects on the proliferation and differentiation of B cells.
Reverse transcription-PCR revealed that cytokines were produced by B cells in both stimulation conditions. However, the technique did not allow us to measure the effect of inhibitory receptor cross-linking (data not shown). Therefore, the cytoplasmic accumulation of cytokines was determined evaluating the mean fluorescence intensity of cells stained with labeled anticytokine MAbs. As shown in Fig. 6, the production of IL-8, IL-10, and TNF-α was increased in activated B cells, whereas IL-12 production was too low for proper evaluation. Of note, when the inhibitory receptors were cross-linked, a sharp reduction of IL-8, IL-10, and TNF-α was observed, independently of the type of B-cell-activating signal (Fig. 6A and B).
FIG. 6.
Characterization of cytokine production and its modulation induced by inhibitory receptor cross-linking. B lymphocytes were stimulated with CD40L-transfected cells plus IL-4 (A) or with LPS plus IL-4 (B) in the presence (+) of the indicated cross-linked MAbs. The mean fluorescence intensity after 2 days of stimulation is reported. α-CD152, anti-CD152 antibody.
DISCUSSION
Expression of inhibitory receptors CD85j, LAIR-1, and CD152 on B cells has been previously described (6, 15, 21, 30, 31, 33, 34), but their functions have not been fully characterized. CD152 expression on B cells was reported to need direct cell-to-cell contact between B cells and activated T cells (15) or anti-CD40 or LPS stimulation in the presence of IL-4 (21). In our study we confirm this last observation using CD40L stable transfectants as stimulators. The finding that CD152 expression is induced only when both CD40L transfectants and IL-4 or LPS and IL-4 are present suggests that a strong B-cell-activating signal is required.
Cross-linking of inhibitory receptors down-regulates IgG production. These results are not related to the activation pathway tested, i.e., recall antigens, CD40L-transfected cells plus IL-4 or LPS plus IL-4. In addition, this cross-linking decreases the percentage of both IgG+ and IgE+ cells. The results suggest that inhibitory receptors may inhibit IL-4-driven isotype switching.
It is noteworthy that CD85j, LAIR-1, and CD152 cross-linking does not affect CD23 expression, whereas under the same experimental conditions it inhibits IgE production. Despite the high degree of sequence homology between CD23 and ɛ gene promoters (34), CD23 expression, unlike IgE production, can be induced by stimulation with IL-4, LPS, or anti-CD40 alone (13, 34). Thus, CD23 seems to require less-stringent conditions, and the signals leading to its expression could be regulated differently and not be affected by inhibitory receptors.
The finding that inhibitory receptors counteract the effect of CD40 stimulation on Ig production suggests that they might play a role in the regulation of B-cell activities in germinal centers. Maturation of the humoral response occurs in germinal centers, where CD40 can be engaged by its ligand CD154 (CD40L), leading B cells to isotype switching under the control of IFN-γ and IL-4 produced by Th1 and Th2 cells, respectively. Interestingly, it has been reported that CD85j, LAIR-1, and CD152 play a role in down-regulating cytokine production by T cells (16). Thus, inhibitory receptors could block B-cell differentiation to IgE-secreting cells not only indirectly by dampening the IFN-γ- and IL-4-secreting T cell populations but also, as indicated by our results, by directly counteracting the effects of IL-4 on CD40L- or LPS-stimulated B cells.
Interestingly, cross-linking of inhibitory receptors leads to a down-regulation of cytokines produced by activated B cells. This effect is evident on IL-10, IL-8, and TNF-α. These cytokines are relevant not only for the proliferation and activation of T lymphocytes but also for synergistic effects on the proliferation and differentiation of B cells.
In detail, IL-10 is a homodimeric cytokine that strongly inhibits the activation of myeloid cells, including monocytes, dendritic cells, and macrophages, resulting in a reduced production of proinflammatory mediators, such as cytokines, chemokines, and adhesion and accessory molecules, and leads to a decreased T-cell stimulation (5, 10, 20). This cytokine inhibits monocyte and dendritic cell function but can also stimulate human B-cell activation, proliferation, and differentiation (12). IL-10 is a potent cofactor for the proliferation of human B cells activated by anti-IgM, Staphylococcus aureus Cowan 1, or CD40cross-linking (24, 25). In addition, IL-10 enhances immunoglobulin production by naïve and committed B cells and acts as a switch factor for IgG1, IgG3, and IgA production.
IL-8 is a member of the CXC chemokine family and plays an important role as an activator and chemoattractant for B cells and other cell types, including neutrophils (1, 29). B-lymphocyte trafficking and accumulation may occur under the direct control of IL-8 (29).
TNF-α is a cytokine that plays an important role in innate immunity and has many effects, ranging from inflammation to apoptosis. TNF-α enhances the production of proinflammatory molecules and adhesion molecules (e.g., intercellular adhesion molecule-1, P-selectin, and E-selectin) (32). Moreover, this cytokine can induce a significant chemotactic response of human B lymphocytes (18).
IL-10, IL-8, and TNF-α down-regulation mediated by cross-linking of inhibitory receptors reduce proinflammatory and chemoattractant factors. Inhibitory molecules could act at the end of the immune response, when the factors causing it have disappeared.
In conclusion, our results demonstrate that CD85j, LAIR-1, and CD152 receptors expressed on B cells inhibit IgG and IgE production. This down-regulation is not related to the pathway of stimulation. In addition, we have also observed this inhibition in an antigen-specific T-cell-B-cell cooperation test, using two different systems that do not need the presence of T lymphocytes. Activation via CD40L plus IL-4 mimics the functional interaction between T and B lymphocytes, and LPS plus IL-4 reproduces the conditions of certain bacterial infections.
These mechanisms induced by cross-linking of the B-cell inhibitory receptors could be relevant for the regulation of immediate-type hypersensitive responses.
Acknowledgments
This work was supported by grants from Fondazione CARIGE, Compagnia di S. Paolo, and Ministero per l'Istruzione, l'Università e la Ricerca Scientifica.
We thank Caterina Valetti and Laura Mattioli (Department of Experimental Medicine, Section of Human Anatomy, University of Genova, Genova, Italy) for technical support during Western blot analyses and helpful discussions of the data.
REFERENCES
- 1.Alter, A., M. Duddy, S. Hebert, K. Biernacki, A. Prat, J. P. Antel, V. W. Yong, R. K. Nuttall, C. J. Pennington, D. R. Edwards, and A. Bar-Or. 2003. Determinants of human B cell migration across brain endothelial cells. J. Immunol. 170:4497-4505. [DOI] [PubMed] [Google Scholar]
- 2.Binstadt, B. A., K. M. Brumbaugh, C. J. Dick, A. M. Scharenberg, B. L. Williams, M. Colonna, L. L. Lanier, J. P. Kinet, R. T. Abraham, and P. J. Leibson. 1996. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity 6:629-638. [DOI] [PubMed] [Google Scholar]
- 3.Borges, L., M. L. Hsu, N. Fanger, M. Kubin, and D. Cosman. 1997. A family of human lymphoid and myeloid Ig-like receptors, some of which bind to MHC class I molecules. J. Immunol. 159:5192-5196. [PubMed] [Google Scholar]
- 4.Brunner, M. C., C. A. Chambers, F. K. Chan, J. Hanke, A. Winoto, and J. P. Allison. 1999. CTLA-4-mediated inhibition of early events of T cell proliferation. J. Immunol. 162:5813-5820. [PubMed] [Google Scholar]
- 5.Caux, C., C. Massacrier, B. Vanbervliet, C. Barthelemy, Y. J. Liu, and J. Banchereau. 1994. Interleukin 10 inhibits T cell alloreaction induced by human dendritic cells. Int. Immunol. 6:1177-1185. [DOI] [PubMed] [Google Scholar]
- 6.Colonna, M., F. Navarro, T. Bellon, M. Llano, P. Garcia, J. Samaridis, L. Angman, M. Cella, and M. Lopez-Botet. 1997. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 186:1809-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colonna, M., J. Samaridis, M. Cella, L. Angman, R. L. Allen, C. A. O'Callaghan, R. Dunbar, G. S. Ogg, V. Cerundolo, and A. Rolink. 1998. Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J. Immunol. 160:3096-3100. [PubMed] [Google Scholar]
- 8.Cosman, D., N. Fanger, and L. Borges. 1999. Human cytomegalovirus, MHC class I and inhibitory signalling receptors: more questions than answers. Immunol. Rev. 168:177-185. [DOI] [PubMed] [Google Scholar]
- 9.Cosman, D., N. Fanger, L. Borges, M. Kubin, W. Chin, L. Peterson, and M. L. Hsu. 1997. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity 7:273-282. [DOI] [PubMed] [Google Scholar]
- 10.de Waal Malefyt, R., J. Abrams, B. Bennett, C. G. Figdor, and J. E. de Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietrich, J., M. Cella, and M. Colonna. 2001. Ig-like transcript 2 (ILT2)/leukocyte Ig-like receptor 1 (LIR1) inhibits TCR signaling and actin cytoskeleton reorganization. J. Immunol. 166:2514-2521. [DOI] [PubMed] [Google Scholar]
- 12.Go, N. F., B. E. Castle, R. Barrett, R. Kastelein, W. Dang, T. R. Mosmann, K. W. Moore, and M. Howard. 1990. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J. Exp. Med. 172:1625-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeppson, J. D., H. R. Patel, N. Sakata, J. Domenico, N. Terada, and E. W. Gelfand. 1998. Requirement for dual signals by anti-CD40 and IL-4 for the induction of nuclear factor-κB, IL-6, and IgE in human B lymphocytes. J. Immunol. 161:1738-1742. [PubMed] [Google Scholar]
- 14.Jung, T., U. Schauer, C. Heusser, C. Neumann, and C. Rieger. 1993. Detection of intracellular cytokines by flow cytometry. J. Immunol. Methods 159:197-207. [DOI] [PubMed] [Google Scholar]
- 15.Kuiper, H. M., M. Brouwer, P. S. Linsley, and R. A. van Lier. 1995. Activated T cells can induce high levels of CTLA-4 expression on B cells. J. Immunol. 155:1776-1783. [PubMed] [Google Scholar]
- 16.Merlo, A., D. Saverino, C. Tenca, C. E. Grossi, S. Bruno, and E. Ciccone. 2001. CD85/LIR-1/ILT2 and CD152 (cytotoxic T lymphocyte antigen 4) inhibitory molecules down-regulate the cytolytic activity of human CD4+ T-cell clones specific for Mycobacterium tuberculosis. Infect. Immun. 69:6022-6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyaard, L., G. J. Adema, C. Chang, E. Woollatt, G. R. Sutherland, L. L. Lanier, and J. H. Phillips. 1997. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 7:283-290. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen, L. S., J. Frydenberg, M. Lind, M. Deleuran, K. Stengaard-Pedersen, and B. Deleuran. 1997. CD19-selected B lymphocytes synthesize, secrete and migrate in the presence of IL-8. TNF-α and γIP-10 are also B lymphocyte migratory factors. Cytokine 9:747-753. [DOI] [PubMed] [Google Scholar]
- 19.Oosterwegel, M. A., R. J. Greenwald, D. A. Mandelbrot, R. B. Lorsbach, and A. H. Sharpe. 1999. CTLA-4 and T cell activation. Curr. Opin. Immunol. 11:294-300. [DOI] [PubMed] [Google Scholar]
- 20.Peguet-Navarro, J., C. Moulon, C. Caux, C. Dalbiez-Gauthier, J. Banchereau, and D. Schmitt. 1994. Interleukin-10 inhibits the primary allogeneic T cell response to human epidermal Langerhans cells. Eur. J. Immunol. 24:884-891. [DOI] [PubMed] [Google Scholar]
- 21.Pioli, C., L. Gatta, V. Ubaldi, and G. Doria. 2000. Inhibition of IgG1 and IgE production by stimulation of the B cell CTLA-4 receptor. J. Immunol. 165:5530-5536. [DOI] [PubMed] [Google Scholar]
- 22.Poggi, A., E. Tomasello, E. Ferrero, M. R. Zocchi, and L. Moretta. 1998. p40/LAIR-1 regulates the differentiation of peripheral blood precursors to dendritic cells induced by granulocyte-monocyte colony-stimulating factor. Eur. J. Immunol. 28:2086-2091. [DOI] [PubMed] [Google Scholar]
- 23.Raiter, A., A. Novogrodsky, and B. Hardy. 1999. Activation of lymphocytes by BAT and anti CTLA-4: comparison of binding to T and B cells. Immunol. Lett. 69:247-251. [DOI] [PubMed] [Google Scholar]
- 24.Rousset, F., E. Garcia, T. Defrance, C. Peronne, N. Vezzio, D. H. Hsu, R. Kastelein, K. W. Moore, and J. Banchereau. 1992. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA 89:1890-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saeland, S., V. Duvert, I. Moreau, and J. Banchereau. 1993. Human B cell precursors proliferate and express CD23 after CD40 ligation. J. Exp. Med. 178:113-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saverino, D., M. Fabbi, F. Ghiotto, A. Merlo, S. Bruno, D. Zarcone, C. Tenca, M. Tiso, G. Santoro, G. Anastasi, D. Cosman, C. E. Grossi, and E. Ciccone. 2000. The CD85/LIR-1/ILT2 inhibitory receptor is expressed by all human T lymphocytes and down-regulates their functions. J. Immunol. 165:3742-3755. [DOI] [PubMed] [Google Scholar]
- 27.Saverino, D., M. Fabbi, A. Merlo, G. Ravera, C. E. Grossi, and E. Ciccone. 2002. Surface density expression of the leukocyte-associated Ig-like receptor-1 is directly related to inhibition of human T-cell functions. Hum. Immunol. 63:534-546. [DOI] [PubMed] [Google Scholar]
- 28.Saverino, D., A. Merlo, S. Bruno, V. Pistoia, C. E. Grossi, and E. Ciccone. 2002. Dual effect of CD85/leukocyte Ig-like receptor-1/Ig-like transcript 2 and CD152 (CTLA-4) on cytokine production by antigen-stimulated human T cells. J. Immunol. 168:207-215. [DOI] [PubMed] [Google Scholar]
- 29.Schratzberger, P., S. Dunzendorfer, N. Reinisch, C. M. Kahler, and C. J. Wiedermann. 1997. Interleukin-8-induced human peripheral blood B-lymphocyte chemotaxis in vitro. Immunol. Lett. 58:167-170. [DOI] [PubMed] [Google Scholar]
- 30.Tinnell, S. B., S. M. Jacobs-Helber, E. Sterneck, S. T. Sawyer, and D. H. Conrad. 1998. STAT6, NF-κB and C/EBP in CD23 expression and IgE production. Int. Immunol. 10:1529-1538. [DOI] [PubMed] [Google Scholar]
- 31.van der Vuurst de Vries, A. R., H. Clevers, T. Logtenberg, and L. Meyaard. 1999. Leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) is differentially expressed during human B cell differentiation and inhibits B cell receptor-mediated signaling. Eur. J. Immunol. 29:3160-3167. [DOI] [PubMed] [Google Scholar]
- 32.Victor, F. C., and A. B. Gottlieb. 2002. TNF-alpha and apoptosis: implications for the pathogenesis and treatment of psoriasis. J. Drugs Dermatol. 1:264-275. [PubMed] [Google Scholar]
- 33.Xerri, L., E. Devilard, J. Hassoun, D. Olive, and F. Birg. 1997. In vivo expression of the CTLA4 inhibitory receptor in malignant and reactive cells from human lymphomas. J. Pathol. 183:182-187. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida, K., M. Matsuoka, S. Usuda, A. Mori, K. Ishizaka, and H. Sakano. 1990. Immunoglobulin switch circular DNA in the mouse infected with Nippostrongylus brasiliensis: evidence for successive class switching from μ to ɛ via γ1. Proc. Natl. Acad. Sci. USA 87:7829-7833. [DOI] [PMC free article] [PubMed] [Google Scholar]