Abstract
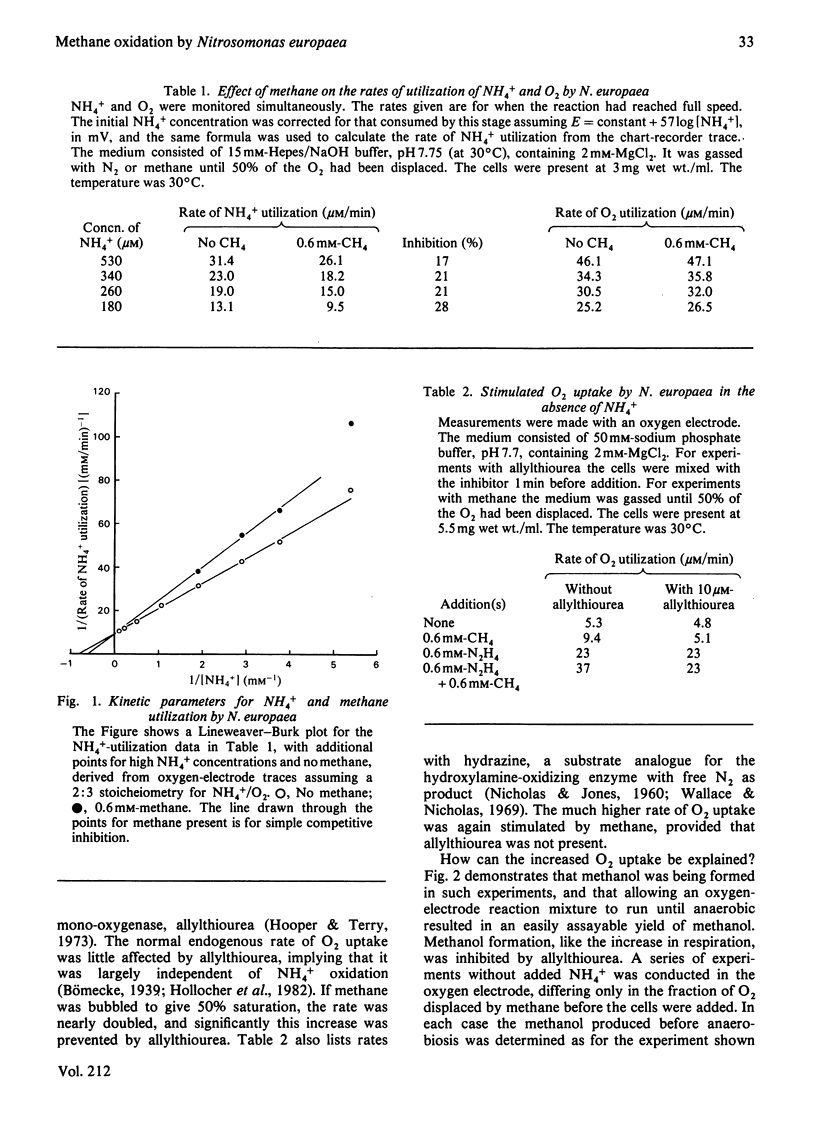
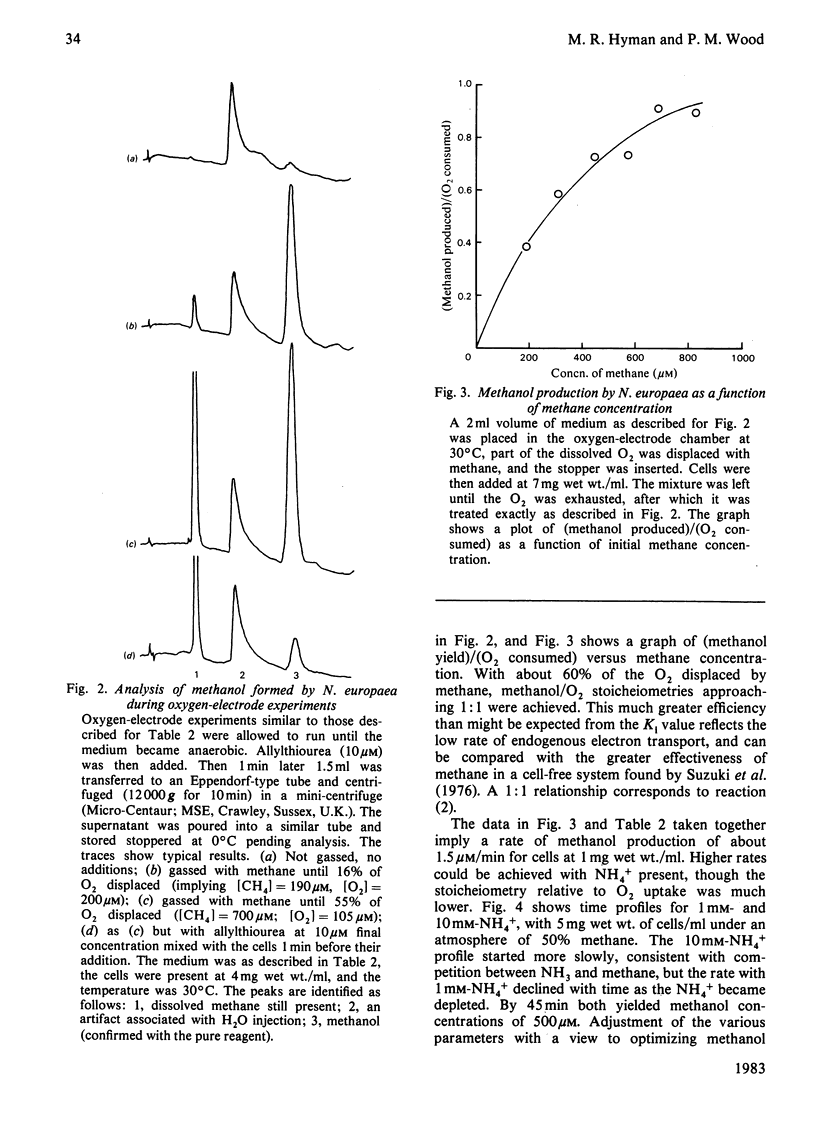
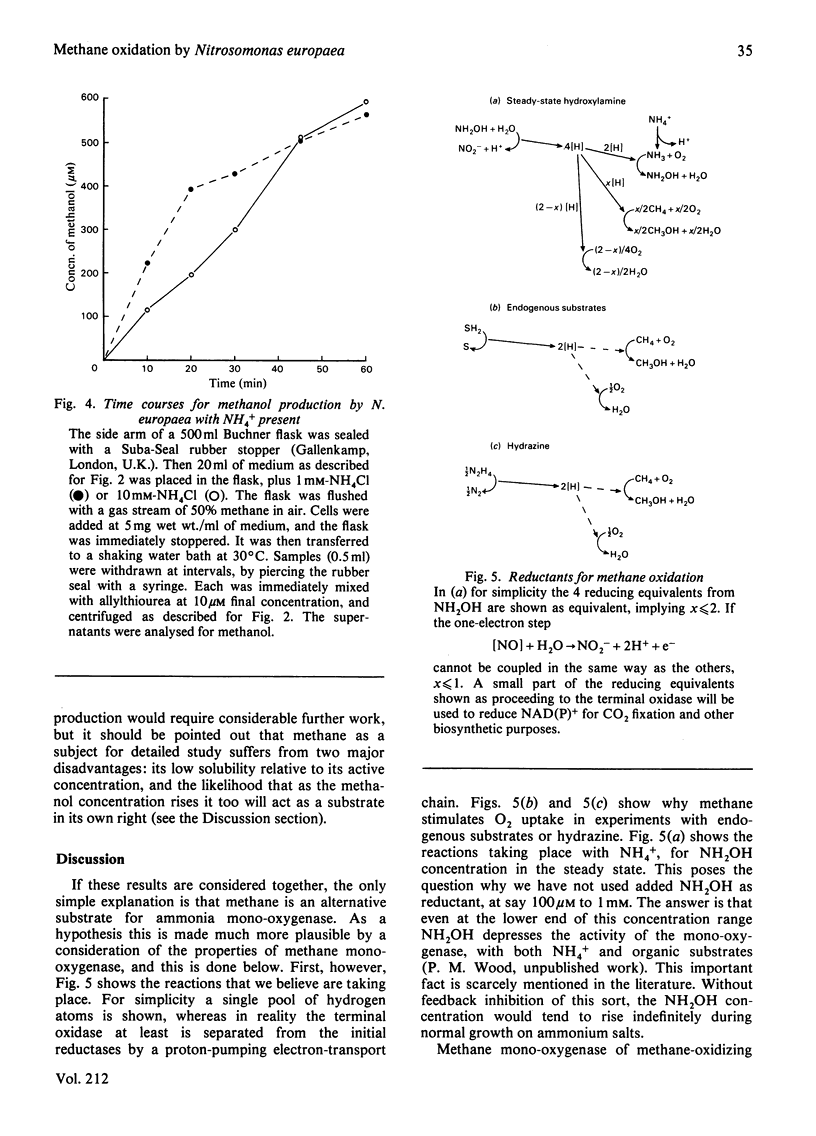
Methane inhibited NH4+ utilization by Nitrosomonas europaea with a Ki of 2mM. O2 consumption was not inhibited. In the absence of NH4+, or with hydrazine as reductant, methane caused nearly a doubling in the rate of O2 uptake. The stimulation was abolished by allylthiourea, a sensitive inhibitor of the oxidation of NH4+. Analysis revealed that methanol was being formed in these experiments, with yields approaching 1 mol of methanol per mol of O2 consumed under certain conditions. When cells were incubated with NH4+ under an atmosphere of 50% methane, 50 microM-methanol was generated in 1 h. It is concluded that methane is an alternative substrate for the NH3-oxidizing enzyme (ammonia mono-oxygenase),m albeit with a much lower affinity than for methane mono-oxygenase of methanotrophs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colby J., Dalton H. Resolution of the methane mono-oxygenase of Methylococcus capsulatus (Bath) into three components. Purification and properties of component C, a flavoprotein. Biochem J. 1978 May 1;171(2):461–468. doi: 10.1042/bj1710461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H., Whittenbury R. An improved assay for bacterial methane mono-oxygenase: some properties of the enzyme from Methylomonas methanica. Biochem J. 1975 Nov;151(2):459–462. doi: 10.1042/bj1510459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Stirling D. I., Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977 Aug 1;165(2):395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozd J. W. Energy coupling and respiration in Nitrosomonas europaea. Arch Microbiol. 1976 Nov 2;110(23):257–262. doi: 10.1007/BF00690236. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Strom T., Quayle J. R. Oxidation of carbon monoxide and methane by Pseudomonas methanica. J Gen Microbiol. 1975 Nov;91(1):79–91. doi: 10.1099/00221287-91-1-79. [DOI] [PubMed] [Google Scholar]
- HOFMAN T., LEES H. The biochemistry of the nitrifying organisms. IV. The respiration and intermediary metabolism of Nitrosomonas. Biochem J. 1953 Jul;54(4):579–583. doi: 10.1042/bj0540579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins I. J., Best D. J., Hammond R. C. New findings in methane-utilizing bacteria highlight their importance in the biosphere and their commercial potential. Nature. 1980 Aug 7;286(5773):561–564. doi: 10.1038/286561a0. [DOI] [PubMed] [Google Scholar]
- Hollocher T. C., Kumar S., Nicholas D. J. Respiration-dependent proton translocation in Nitrosomonas europaea and its apparent absence in Nitrobacter agilis during inorganic oxidations. J Bacteriol. 1982 Mar;149(3):1013–1020. doi: 10.1128/jb.149.3.1013-1020.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocher T. C., Tate M. E., Nicholas D. J. Oxidation of ammonia by Nitrosomonas europaea. Definite 18O-tracer evidence that hydroxylamine formation involves a monooxygenase. J Biol Chem. 1981 Nov 10;256(21):10834–10836. [PubMed] [Google Scholar]
- Hooper A. B., Terry K. R. Specific inhibitors of ammonia oxidation in Nitrosomonas. J Bacteriol. 1973 Aug;115(2):480–485. doi: 10.1128/jb.115.2.480-485.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Laskin A. I., Felix A., Derelanko P. Microbial Oxidation of Gaseous Hydrocarbons: Production of Secondary Alcohols from Corresponding n-Alkanes by Methane-Utilizing Bacteria. Appl Environ Microbiol. 1980 Apr;39(4):720–726. doi: 10.1128/aem.39.4.720-726.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Properties of the methane mono-oxygenase from extracts of Methylosinus trichosporium OB3b and evidence for its similarity to the enzyme from Methylococcus capsulatus (Bath). Eur J Biochem. 1979 May 2;96(1):205–212. doi: 10.1111/j.1432-1033.1979.tb13030.x. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Dular U., Kwok S. C. Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J Bacteriol. 1974 Oct;120(1):556–558. doi: 10.1128/jb.120.1.556-558.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I., Kwok S. C., Dular U. Competitive inhibition of ammonia oxidation in Nitrosomonas europaea by methane, carbon monoxide or methanol. FEBS Lett. 1976 Dec 15;72(1):117–120. doi: 10.1016/0014-5793(76)80825-3. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang D. C., Suzuki I. Cytochrome c554 as a possible electron donor in the hydroxylation of ammonia and carbon monoxide in Nitrosomonas europaea. Can J Biochem. 1982 Nov;60(11):1018–1024. doi: 10.1139/o82-131. [DOI] [PubMed] [Google Scholar]
- Wallace W., Nicholas D. J. The biochemistry of nitrifying microorganisms. Biol Rev Camb Philos Soc. 1969 Jul;44(3):359–391. doi: 10.1111/j.1469-185x.1969.tb01216.x. [DOI] [PubMed] [Google Scholar]
- Whittenbury R., Phillips K. C., Wilkinson J. F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]


