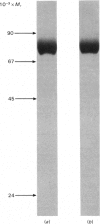
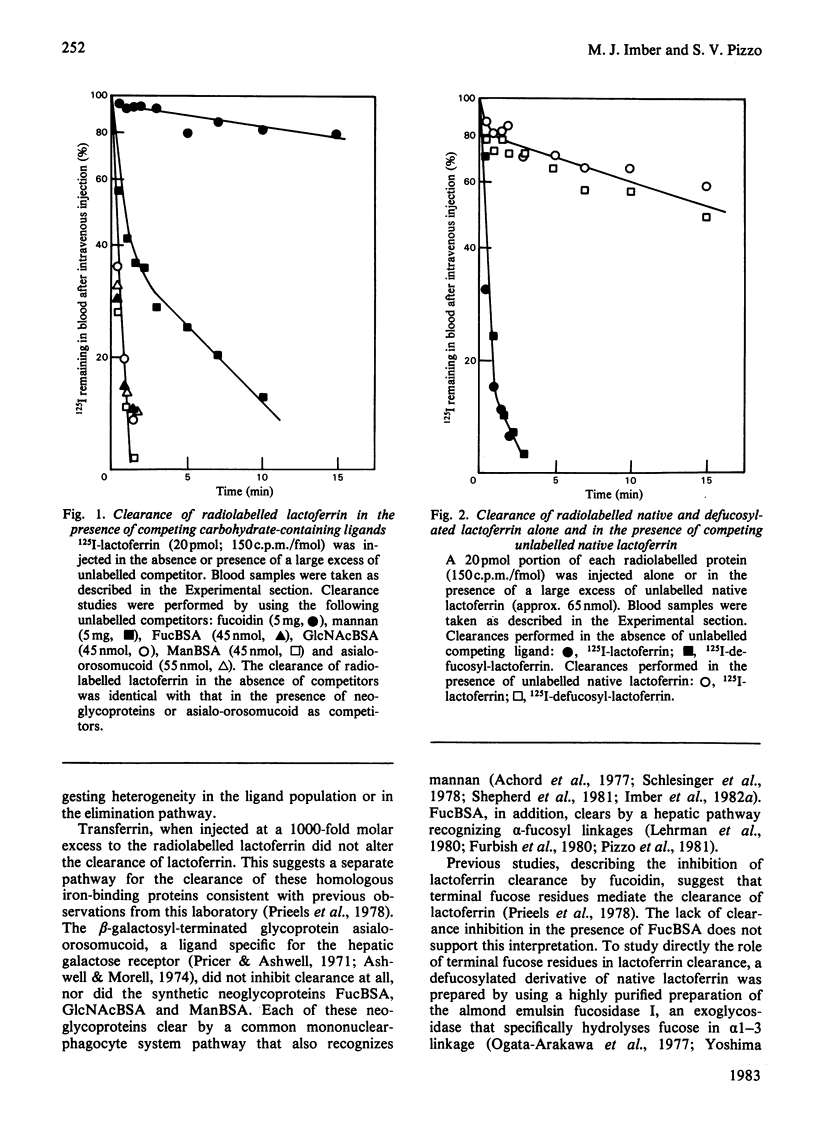
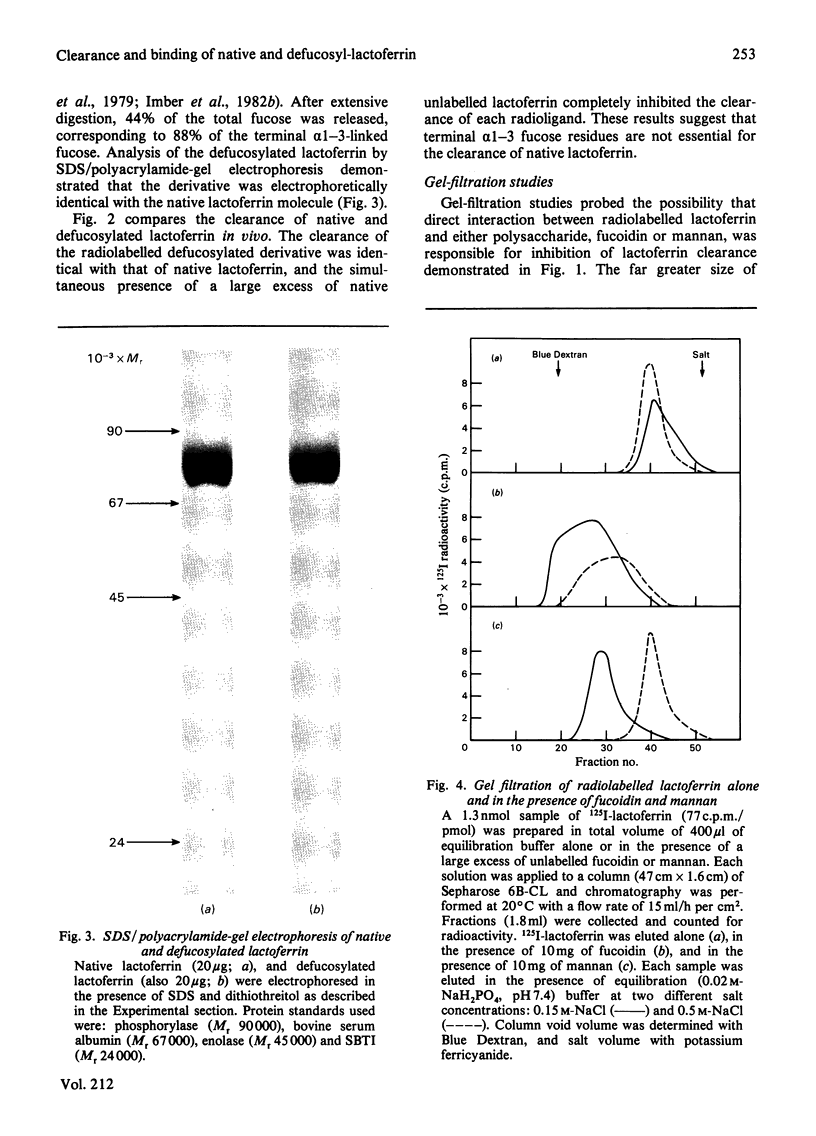
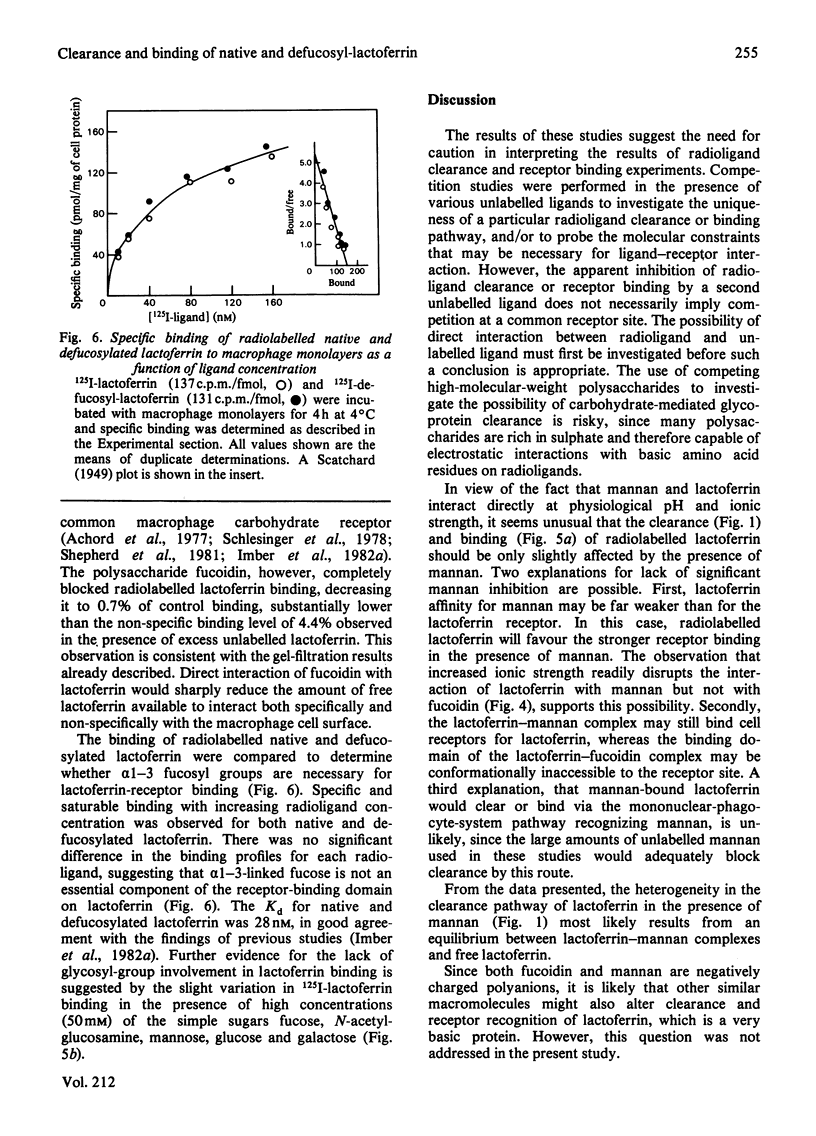
Abstract
These studies explore the role of carbohydrate recognition systems and the direct involvement of terminal alpha 1-3-linked fucose in the clearance of lactoferrin from the murine circulation and in the specific binding of lactoferrin to receptors on murine peritoneal macrophages. As previously reported, radiolabelled lactoferrin cleared very rapidly (t1/2 less than 1 min) after intravenous injection into mice. However, competing levels of ligands specific for the hepatic galactose receptor (asialo-orosomucoid), the hepatic fucose receptor (fucosyl-bovine serum albumin), and the mononuclear-phagocyte system pathway recognizing mannose, N-acetylglucosamine and fucose (mannosyl-, N-acetylglucosaminyl- and fucosyl-bovine serum albumin) did not block radiolabelled lactoferrin clearance in vivo or binding to mouse peritoneal macrophage monolayers in vitro. Almond emulsin alpha 1-3-fucosidase was used to prepare defucosylated lactoferrin in which 88% of the alpha 1-3-linked fucose was hydrolysed. No difference in clearance or receptor binding was observed between radiolabelled native and defucosylated lactoferrin. Fucoidin, a fucose-rich algal polysaccharide, completely inhibits the clearance in vivo and macrophage binding in vitro of lactoferrin. This effect, however, is probably not the result of competition for binding to the fucose receptor, since gel-filtration studies demonstrated formation of a stable complex between lactoferrin and fucoidin. The present results indicate that the lactoferrin-clearance pathway is distinct from several pathways mediating glycoprotein clearance through recognition of terminal galactose, fucose, N-acetylglucosamine or mannose. Furthermore, alpha 1-3-linked fucose on lactoferrin is not essential for lactoferrin clearance in vivo or specific binding to macrophage receptors in vitro.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D. T., Brot F. E., Sly W. S. Inhibition of the rat clearance system for agalacto-orosomucoid by yeast mannans and by mannose. Biochem Biophys Res Commun. 1977 Jul 11;77(1):409–415. doi: 10.1016/s0006-291x(77)80213-1. [DOI] [PubMed] [Google Scholar]
- Aisen P., Leibman A. Lactoferrin and transferrin: a comparative study. Biochim Biophys Acta. 1972 Feb 29;257(2):314–323. doi: 10.1016/0005-2795(72)90283-8. [DOI] [PubMed] [Google Scholar]
- Arnold R. R., Cole M. F., McGhee J. R. A bactericidal effect for human lactoferrin. Science. 1977 Jul 15;197(4300):263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Ballou C. E., Raschke W. C. Polymorphism of the somatic antigen of yeast. Science. 1974 Apr 12;184(4133):127–134. doi: 10.1126/science.184.4133.127. [DOI] [PubMed] [Google Scholar]
- Bennett R. M., Kokocinski T. Lactoferrin turnover in man. Clin Sci (Lond) 1979 Nov;57(5):453–460. doi: 10.1042/cs0570453. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., DeSousa M., Smithyman A., Ralph P., Hamilton J., Kurland J. I., Bognacki J. Specificity and modulation of the action of lactoferrin, a negative feedback regulator of myelopoiesis. Blood. 1980 Feb;55(2):324–333. [PubMed] [Google Scholar]
- Broxmeyer H. E. Lactoferrin acts on Ia-like antigen-positive subpopulations of human monocytes to inhibit production of colony stimulatory activity in vitro. J Clin Invest. 1979 Dec;64(6):1717–1720. doi: 10.1172/JCI109635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H. E., Smithyman A., Eger R. R., Meyers P. A., de Sousa M. Identification of lactoferrin as the granulocyte-derived inhibitor of colony-stimulating activity production. J Exp Med. 1978 Oct 1;148(4):1052–1067. doi: 10.1084/jem.148.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Armstrong J. A. The role of lactoferrin in the bactericidal function of polymorphonuclear leucocytes. Immunology. 1979 Apr;36(4):781–791. [PMC free article] [PubMed] [Google Scholar]
- Furbish F. S., Krett N. L., Barranger J. A., Brady R. O. Fucose plays a role in the clearance and uptake of glucocerebrosidase by rat liver cells. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1768–1774. doi: 10.1016/s0006-291x(80)80103-3. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Wada H. G., Sussman H. H. Identification of transferrin receptors on the surface of human cultured cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6406–6410. doi: 10.1073/pnas.76.12.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber M. J., Glasgow L. R., Pizzo S. V. Purification of an almond emulsin fucosidase on Cibacron blue-sepharose and demonstration of its activity toward fucose-containing glycoproteins. J Biol Chem. 1982 Jul 25;257(14):8205–8210. [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V. Clearance and binding of two electrophoretic "fast" forms of human alpha 2-macroglobulin. J Biol Chem. 1981 Aug 10;256(15):8134–8139. [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V., Johnson W. J., Adams D. O. Selective diminution of the binding of mannose by murine macrophages in the late stages of activation. J Biol Chem. 1982 May 10;257(9):5129–5135. [PubMed] [Google Scholar]
- Karle H., Hansen N. E., Malmquist J., Karle A. K., Larsson I. Turnover of human lactoferrin in the rabbit. Scand J Haematol. 1979 Oct;23(4):303–312. doi: 10.1111/j.1600-0609.1979.tb02865.x. [DOI] [PubMed] [Google Scholar]
- Markowetz B., Van Snick J. L., Masson P. L. Binding and ingestion of human lactoferrin by mouse alveolar macrophages. Thorax. 1979 Apr;34(2):209–212. doi: 10.1136/thx.34.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. Y., Taylor C. R. Distribution of transferrin, ferritin, and lactoferrin in human tissues. J Clin Pathol. 1978 Apr;31(4):316–327. doi: 10.1136/jcp.31.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata-Arakawa M., Muramatsu T., Kobata A. alpha-L-fucosidases from almond emulsin: characterization of the two enzymes with different specificities. Arch Biochem Biophys. 1977 May;181(1):353–358. doi: 10.1016/0003-9861(77)90514-8. [DOI] [PubMed] [Google Scholar]
- Pizzo S. V., Lehrman M. A., Imber M. J., Guthrow C. E. The clearance of glycoproteins in diabetic mice. Biochem Biophys Res Commun. 1981 Jul 30;101(2):704–708. doi: 10.1016/0006-291x(81)91315-2. [DOI] [PubMed] [Google Scholar]
- Pricer W. E., Jr, Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. J Biol Chem. 1971 Aug 10;246(15):4825–4833. [PubMed] [Google Scholar]
- Prieels J. P., Pizzo S. V., Glasgow L. R., Paulson J. C., Hill R. L. Hepatic receptor that specifically binds oligosaccharides containing fucosyl alpha1 leads to 3 N-acetylglucosamine linkages. Proc Natl Acad Sci U S A. 1978 May;75(5):2215–2219. doi: 10.1073/pnas.75.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger P. H., Doebber T. W., Mandell B. F., White R., DeSchryver C., Rodman J. S., Miller M. J., Stahl P. Plasma clearance of glycoproteins with terminal mannose and N-acetylglucosamine by liver non-parenchymal cells. Studies with beta-glucuronidase, N-acetyl-beta-D-glucosaminidase, ribonuclease B and agalacto-orosomucoid. Biochem J. 1978 Oct 15;176(1):103–109. doi: 10.1042/bj1760103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd V. L., Lee Y. C., Schlesinger P. H., Stahl P. D. L-Fucose-terminated glycoconjugates are recognized by pinocytosis receptors on macrophages. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1019–1022. doi: 10.1073/pnas.78.2.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell C. P., Lee Y. C. Preparation of some new neoglycoproteins by amidination of bovine serum albumin using 2-imino-2-methoxyethyl 1-thioglycosides. Biochemistry. 1980 Oct 14;19(21):4899–4904. doi: 10.1021/bi00562a030. [DOI] [PubMed] [Google Scholar]
- Tsay G. C., Dawson G. A sensitive spectrophotometric method for detection of L-fucose. Anal Biochem. 1977 Apr;78(2):423–427. doi: 10.1016/0003-2697(77)90103-8. [DOI] [PubMed] [Google Scholar]
- Wada H. G., Hass P. E., Sussman H. H. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979 Dec 25;254(24):12629–12635. [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]
- Yoshima H., Takasaki S., Ito-Mega S., Kobata A. Purification of almond emulsin alpha-L-fucosidase I by affinity chromatography. Arch Biochem Biophys. 1979 May;194(2):394–398. doi: 10.1016/0003-9861(79)90632-5. [DOI] [PubMed] [Google Scholar]
- van Snick J. L., Markowetz B., Masson P. L. The ingestion and digestion of human lactoferrin by mouse peritoneal macrophages and the transfer of its iron into ferritin. J Exp Med. 1977 Sep 1;146(3):817–827. doi: 10.1084/jem.146.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]