Abstract
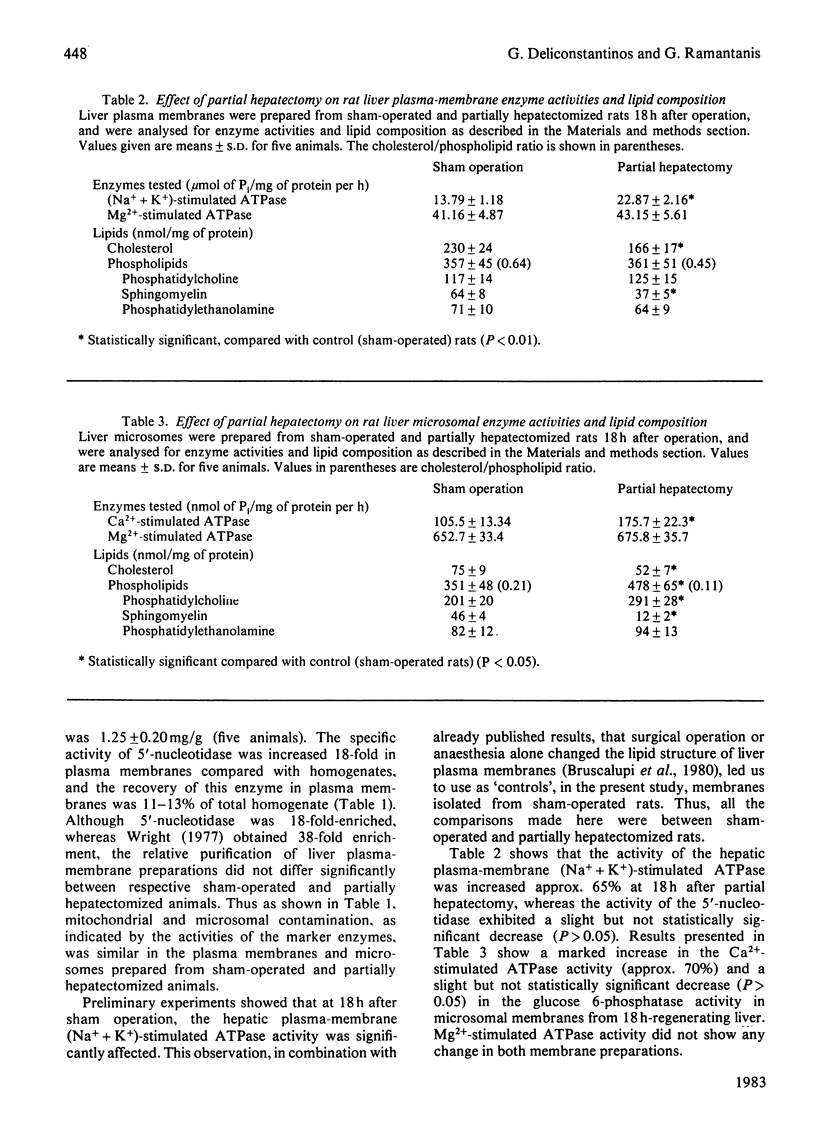
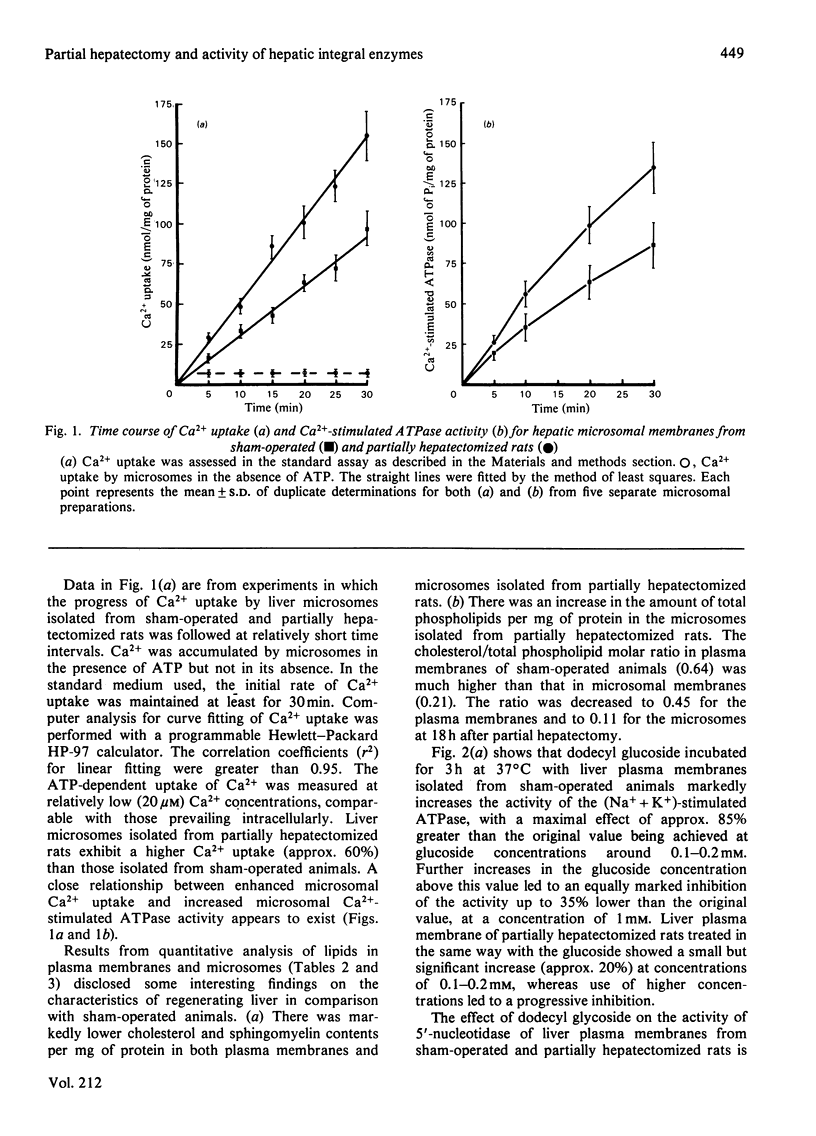
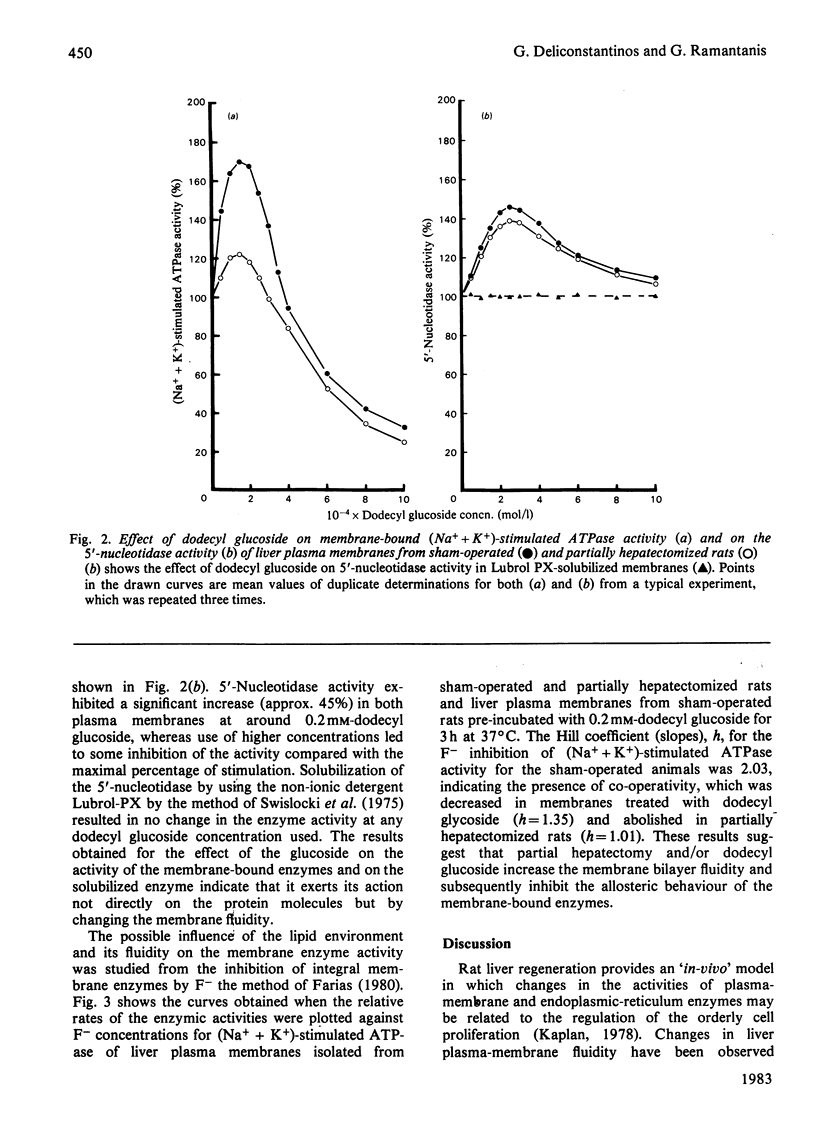
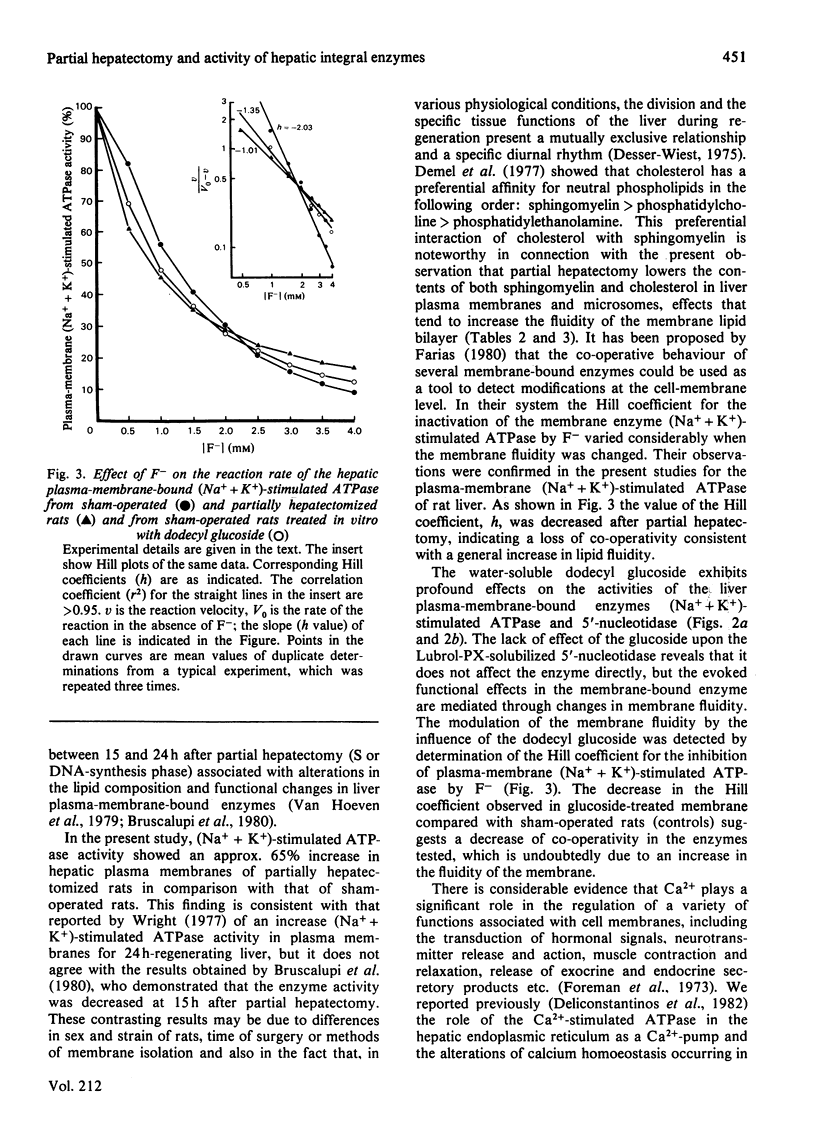
A marked increase in the activities of rat liver plasma-membrane (Na+ + K+)-stimulated ATPase and microsomal Ca2+-stimulated ATPase was observed 18h after partial hepatectomy. Lipid analyses for both membrane preparations reveal that in partially hepatectomized rats the cholesterol and sphingomyelin content are decreased with a subsequent decrease in the cholesterol/phospholipid molar ratio compared with those of sham-operated animals. Changes in the allosteric properties of plasma-membrane (Na+ + K+)-stimulated ATPase by F- (as reflected by changes in the Hill coefficient) indicated a fluidization of the lipid bilayer of both membrane preparations in 18 h-regenerating liver. The amphipathic dodecyl glucoside incorporated into the hepatic plasma membranes evoked a marked increase in the (Na+ + K+)-stimulated ATPase and 5'-nucleotidase activities. The lack of effect of the glucoside on the Lubrol-PX-solubilized 5'-nucleotidase indicates that changes in the activities of the membrane-bound enzymes caused by the glucoside are due to modulation of the membrane fluidity. Dodecyl glucoside appears to increase the membrane fluidity, evaluated through changes in the Hill coefficient for plasma-membrane (Na+ + K+)-stimulated ATPase. The biological significance of these data is discussed in terms of the differences and changes in the interaction of membrane-bound enzymes with membrane lipids during liver regeneration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alivisatos S. G., Deliconstantinos G., Papaphilis A., Theodosiadis G. P. Cooperative nature of the binding of cholesterol on to synaptosomal plasma membranes of dog brain. Biochim Biophys Acta. 1981 May 20;643(3):642–649. doi: 10.1016/0005-2736(81)90360-6. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BODANSKY O., SCHWARTZ M. K. COMPARATIVE EFFECTS OF L-HISTIDINE ON THE ACTIVITIES OF 5'-NUCLEOTIDASE AND ALKALINE PHOSPHATASE. J Biol Chem. 1963 Oct;238:3420–3427. [PubMed] [Google Scholar]
- Bruscalupi G., Curatola G., Lenaz G., Leoni S., Mangiantini M. T., Mazzanti L., Spagnuolo S., Trentalance A. Plasma membrane changes associated with rat liver regeneration. Biochim Biophys Acta. 1980 Apr 10;597(2):263–273. doi: 10.1016/0005-2736(80)90104-2. [DOI] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Deliconstantinos G. Effect of liposomes containing cholesterol on a hepatic cholesterol-7 a-hydroxylase and drug oxidation system. Experientia. 1981 Jan 15;37(1):81–83. doi: 10.1007/BF01965584. [DOI] [PubMed] [Google Scholar]
- Deliconstantinos G., Ramantanis G. Evoked effects of oestradiol on hepatic cholesterol 7 alpha-hydroxylase and drug oxidase in castrated rats. Int J Biochem. 1982;14(9):811–815. doi: 10.1016/0020-711x(82)90102-1. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Jansen J. W., van Dijck P. W., van Deenen L. L. The preferential interaction of cholesterol with different classes of phospholipids. Biochim Biophys Acta. 1977 Feb 14;465(1):1–10. doi: 10.1016/0005-2736(77)90350-9. [DOI] [PubMed] [Google Scholar]
- Desser-Wiest L. Autosynchronization of rat liver cells with endogenous corticosterone after partial hepatectomy. Cell Tissue Kinet. 1975 Jan;8(1):1–9. doi: 10.1111/j.1365-2184.1975.tb01201.x. [DOI] [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BENEDETTI E. L., RUEMKE P. STUDIES ON PLASMA MEMBRANES. I. CHEMICAL COMPOSITION AND ENZYME CONTENT OF PLASMA MEMBRANES ISOLATED FROM RAT LIVER. Biochim Biophys Acta. 1964 Jul 15;90:126–145. doi: 10.1016/0304-4165(64)90125-4. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Farías R. N. Membrane cooperative enzymes as a tool for the investigation of membrane structure and related phenomena. Adv Lipid Res. 1980;17:251–282. doi: 10.1016/b978-0-12-024917-6.50012-4. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Hays D. M. Surgical research aspects of hepatic regeneration. Surg Gynecol Obstet. 1974 Oct;139(4):609–619. [PubMed] [Google Scholar]
- Moore L. Carbon disulfide hepatoxicity and inhibition of liver microsome calcium pump. Biochem Pharmacol. 1982 Apr 1;31(7):1465–1467. doi: 10.1016/0006-2952(82)90050-8. [DOI] [PubMed] [Google Scholar]
- NEVILLE D. M., Jr The isolation of a cell membrane fraction from rat liver. J Biophys Biochem Cytol. 1960 Oct;8:413–422. doi: 10.1083/jcb.8.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaphilis A., Deliconstantinos G. Modulation of serotonergic receptors by exogenous cholesterol in the dog synaptosomal plasma membrane. Biochem Pharmacol. 1980 Dec;29(24):3325–3327. doi: 10.1016/0006-2952(80)90311-1. [DOI] [PubMed] [Google Scholar]
- Swislocki N. I., Magnuson T., Tierney J. Properties of rat liver plasma membrane adenylate cyclase after chromatography on O-diethylaminoethyl-cellulose and agarose-hexane-GTP. Arch Biochem Biophys. 1977 Feb;179(1):157–165. doi: 10.1016/0003-9861(77)90099-6. [DOI] [PubMed] [Google Scholar]
- Wright G. H. Changes in plasma membrane enzyme activities during liver regeneration in the rat. Biochim Biophys Acta. 1977 Nov 1;470(3):368–381. doi: 10.1016/0005-2736(77)90128-6. [DOI] [PubMed] [Google Scholar]
- van Hoeven R. P., van Blitterswijk W. J., Emmelot P. Fluorescence polarization measurements on normal and tumour cells and their corresponding plasma membranes. Biochim Biophys Acta. 1979 Feb 20;551(1):44–54. doi: 10.1016/0005-2736(79)90351-1. [DOI] [PubMed] [Google Scholar]


