Abstract
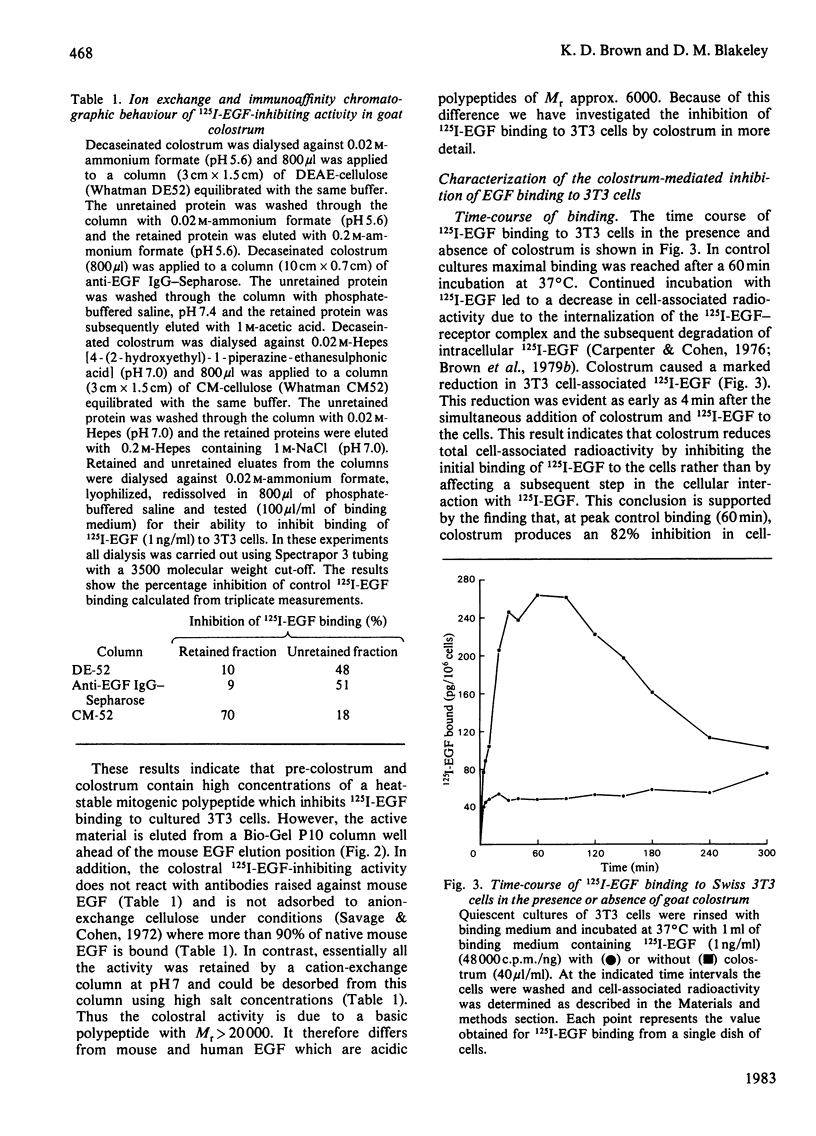
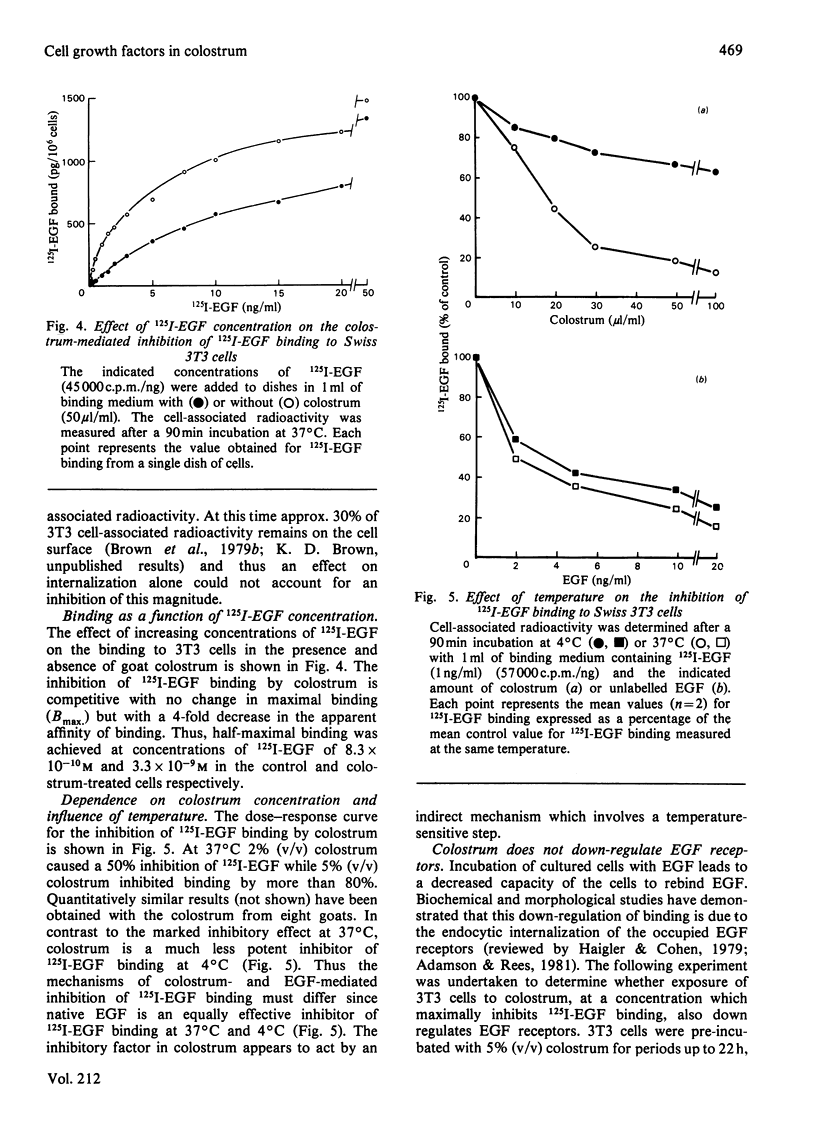
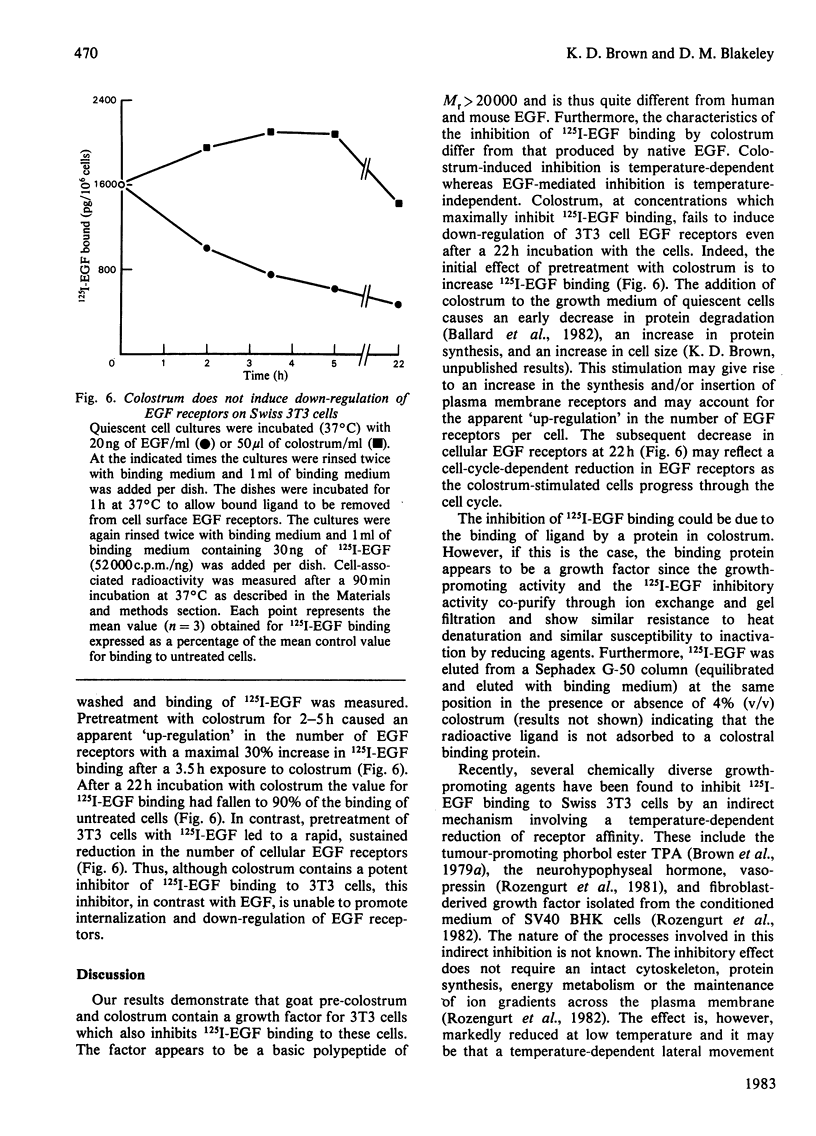
Pre-colostrum and colostrum from goats cause a marked inhibition of the binding of 125I-labelled epidermal growth factor (125I-EGF) to Swiss 3T3 cells. The ability of these secretions to inhibit 125I-EGF binding is closely correlated with the ability to stimulate DNA synthesis in quiescent 3T3 cell cultures, suggesting that goat mammary secretions may contain an EGF-related mitogen. However, the material in colostrum which inhibits 125I-EGF binding to Swiss 3T3 cells is a basic protein with Mr greater than 20000 and is thus quite different from mouse and human EGF. Furthermore, the colostral-mediated inhibition of 125I-EGF binding, although rapid and apparently competitive, differs from the inhibition of binding induced by native, unlabelled EGF. Thus, the inhibitory effect of colostrum is markedly decreased when the assay temperature is shifted from 37 degrees C to 4 degrees C whereas unlabelled EGF is an effective competitive inhibitor at both 37 degrees C and 4 degrees C. Incubation of cells with EGF causes a reduction in cell surface EGF receptors whereas exposure to colostrum does not induce down-regulation of the EGF receptor. Our results suggest that the colostral factor does not bind directly to EGF receptors but inhibits 125I-EGF binding by an indirect mechanism which involves a temperature-sensitive step.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Rees A. R. Epidermal growth factor receptors. Mol Cell Biochem. 1981 Feb 11;34(3):129–152. doi: 10.1007/BF02359619. [DOI] [PubMed] [Google Scholar]
- Ballard F. J., Nield M. K., Francis G. L., Dahlenburg G. W., Wallace J. C. The relationship between the insulin content and inhibitory effects of bovine colostrum on protein breakdown in cultured cells. J Cell Physiol. 1982 Mar;110(3):249–254. doi: 10.1002/jcp.1041100305. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M. Cell growth-promoting activity in mammary secretions of the goat, cow and sheep. Br Vet J. 1983 Jan-Feb;139(1):68–78. doi: 10.1016/s0007-1935(17)30594-8. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Dicker P., Rozengurt E. Inhibition of epidermal growth factor binding to surface receptors by tumor promotors. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1037–1043. doi: 10.1016/0006-291x(79)90221-3. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Holley R. W. Epidermal growth factor and the control of proliferation of Balb 3T3 and benzo[a]pyrene-transformed Balb 3T3 cells. J Cell Physiol. 1979 Jul;100(1):139–146. doi: 10.1002/jcp.1041000114. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Yeh Y. C., Holley R. W. Binding, internalization, and degradation of epidermal growth factor by balb 3T3 and BP3T3 cells: relationship to cell density and the stimulation of cell proliferation. J Cell Physiol. 1979 Aug;100(2):227–238. doi: 10.1002/jcp.1041000204. [DOI] [PubMed] [Google Scholar]
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. Epidermal growth factor is a major growth-promoting agent in human milk. Science. 1980 Oct 10;210(4466):198–199. doi: 10.1126/science.6968093. [DOI] [PubMed] [Google Scholar]
- Cohen S., Carpenter G. Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1317–1321. doi: 10.1073/pnas.72.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Bialecki H., Zetter B. R. Factors involved in the modulation of cell proliferation in vivo and in vitro: the role of fibroblast and epidermal growth factors in the proliferative response of mammalian cells. In Vitro. 1978 Jan;14(1):85–118. doi: 10.1007/BF02618177. [DOI] [PubMed] [Google Scholar]
- Gregory H. Isolation and structure of urogastrone and its relationship to epidermal growth factor. Nature. 1975 Sep 25;257(5524):325–327. doi: 10.1038/257325a0. [DOI] [PubMed] [Google Scholar]
- Healy D. L., Rattigan S., Hartmann P. E., Herington A. C., Burger H. G. Prolactin in human milk: correlation with lactose, total protein, and alpha-lactalbumin levels. Am J Physiol. 1980 Jan;238(1):E83–E86. doi: 10.1152/ajpendo.1980.238.1.E83. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Orth D. N. Concentrations of epidermal growth factor, nerve growth factor, and submandibular gland renin in male and female mouse tissue and fluids. Endocrinology. 1979 Dec;105(6):1382–1387. doi: 10.1210/endo-105-6-1382. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Orth D. N. Epidermal growth factor (urogastrone) in human fluids: size heterogeneity. J Clin Endocrinol Metab. 1979 Apr;48(4):673–679. doi: 10.1210/jcem-48-4-673. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. Human milk stimulates DNA synthesis and cellular proliferation in cultured fibroblasts. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5057–5061. doi: 10.1073/pnas.75.10.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Neumann J. The serum-free growth of Balb/c 3T3 cells in medium supplemented with bovine colostrum. J Supramol Struct. 1979;11(3):349–359. doi: 10.1002/jss.400110310. [DOI] [PubMed] [Google Scholar]
- Knight C. H., Peaker M. Development of the mammary gland. J Reprod Fertil. 1982 Jul;65(2):521–536. doi: 10.1530/jrf.0.0650521. [DOI] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Changes in colostrum composition and in the permeability of the mammary epithelium at about the time of parturition in the goat. J Physiol. 1974 Nov;243(1):129–151. doi: 10.1113/jphysiol.1974.sp010746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland D. B., McGrath J., Samson R. R. Antimicrobial factors in human milk. Studies of concentration and transfer to the infant during the early stages of lactation. Acta Paediatr Scand Suppl. 1978;(271):1–20. [PubMed] [Google Scholar]
- Rozengurt E., Brown K. D., Pettican P. Vasopressin inhibition of epidermal growth factor binding to cultured mouse cells. J Biol Chem. 1981 Jan 25;256(2):716–722. [PubMed] [Google Scholar]
- Rozengurt E., Collins M., Brown K. D., Pettican P. Inhibition of epidermal growth factor binding to mouse cultured cells by fibroblast-derived growth factor. Evidence for an indirect mechanism. J Biol Chem. 1982 Apr 10;257(7):3680–3686. [PubMed] [Google Scholar]
- Salomon D. S., Liotta L. A., Kidwell W. R. Differential response to growth factor by rat mammary epithelium plated on different collagen substrata in serum-free medium. Proc Natl Acad Sci U S A. 1981 Jan;78(1):382–386. doi: 10.1073/pnas.78.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Topper Y. J., Freeman C. S. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980 Oct;60(4):1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]


