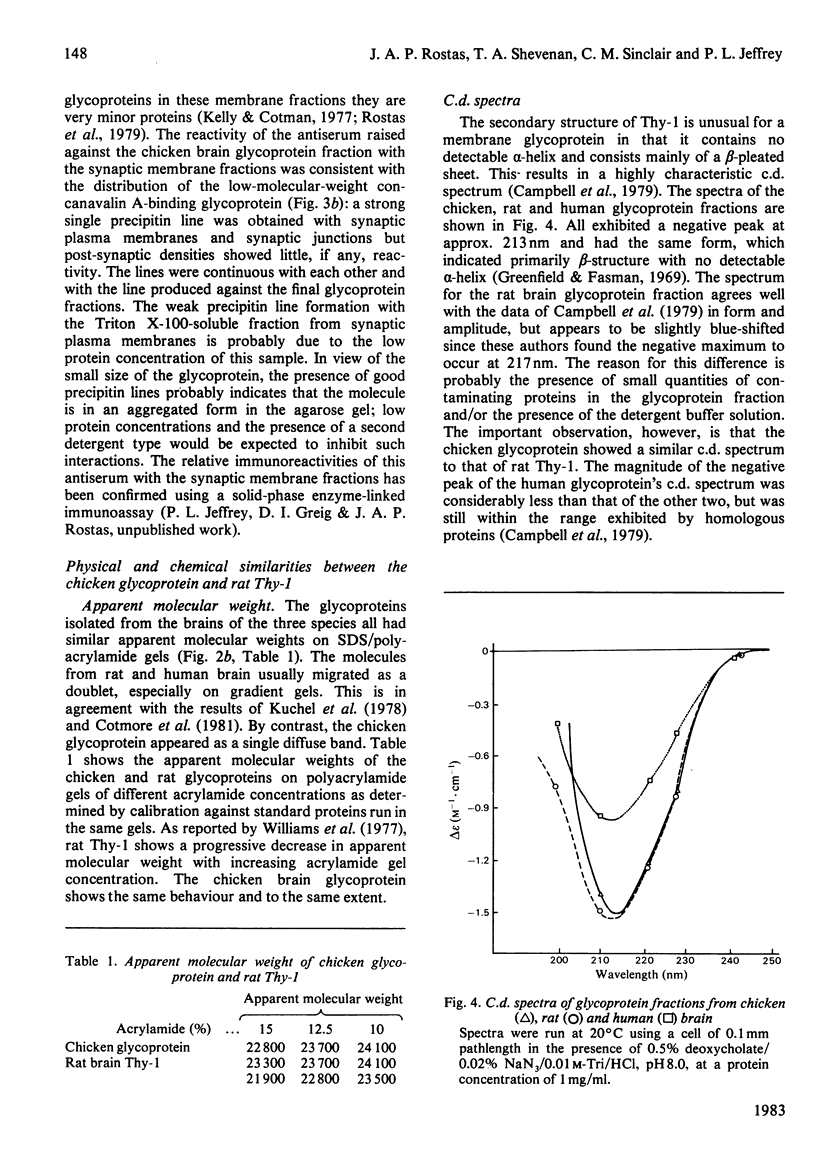
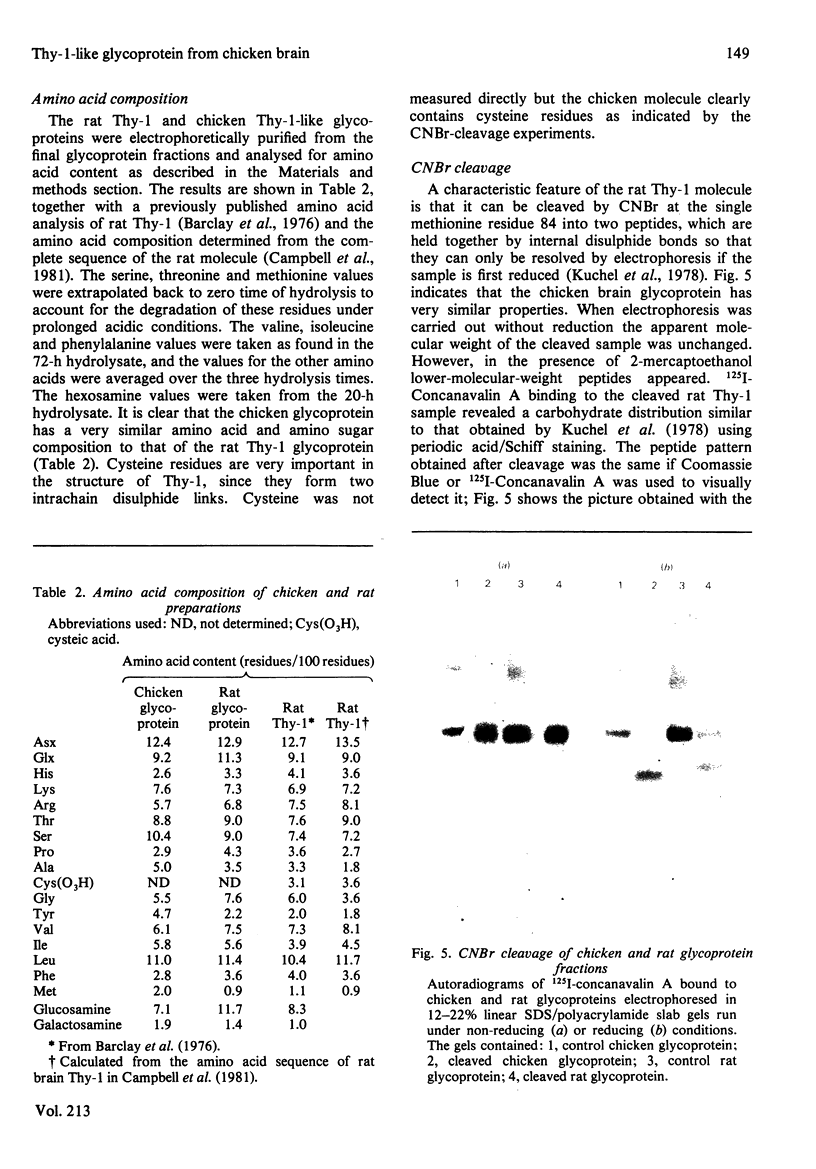
Abstract
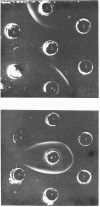
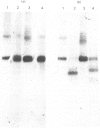
We have purified from chicken forebrain a membrane glycoprotein that is enriched in purified synaptic membranes and has an apparent mol.wt. of 22 800 in 15% sodium dodecyl sulphate/polyacrylamide gels. This molecule was compared with rat and human brain Thy-1 glycoproteins purified by the same procedure in order to determine whether it could be a homologue of Thy-1. Although polyvalent heterologous antisera raised against the rat and chicken molecules showed no immunological cross-reactivity with the other glycoprotein, a great deal of physical and chemical similarity was demonstrated between the chicken glycoprotein and rat Thy-1. Their apparent molecular weights, subcellular localization and amino acid and amino sugar compositions are very similar. C.d. spectra show that both molecules contain predominantly a beta-sheet and structure with no detectable alpha-helix. Electrophoretic analysis of the CNBr-cleaved molecules under reducing and non-reducing conditions shows that both molecules contain intramolecular disulphide bridges. Taken together these results suggest that the chicken brain glycoprotein is an immunologically distinct homologue of the mammalian Thy-1 glycoproteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton R. T., Addis J., Carl G. F., McClain L. D., Bridgers W. F. Association of Thy-1 differentiation alloantigen with synaptic complexes isolated from mouse brain. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3283–3287. doi: 10.1073/pnas.75.7.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acton R. T., Morris R. J., Williams A. F. Estimation of the amount and tissue distribution of rat Thy-1.1 antigen. Eur J Immunol. 1974 Sep;4(9):598–602. doi: 10.1002/eji.1830040904. [DOI] [PubMed] [Google Scholar]
- Barclay A. N., Letarte-Muirhead M., Williams A. F., Faulkes R. A. Chemical characterisation of the Thy-1 glycoproteins from the membranes of rat thymocytes and brain. Nature. 1976 Oct 14;263(5578):563–567. doi: 10.1038/263563a0. [DOI] [PubMed] [Google Scholar]
- Barclay A. N., Letarte-Muirhead M., Williams A. F. Purification of the Thy-1 molecule from rat brain. Biochem J. 1975 Dec;151(3):699–706. doi: 10.1042/bj1510699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. G., Gagnon J., Reid K. B., Williams A. F. Rat brain Thy-1 glycoprotein. The amino acid sequence, disulphide bonds and an unusual hydrophobic region. Biochem J. 1981 Apr 1;195(1):15–30. doi: 10.1042/bj1950015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. G., Williams A. F., Bayley P. M., Reid K. B. Structural similarities between Thy-1 antigen from rat brain and immunoglobulin. Nature. 1979 Nov 15;282(5736):341–342. doi: 10.1038/282341a0. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Novotný J., Sternberg M. J., Campbell D. G., Williams A. F. Analysis of structural similarities between brain Thy-1 antigen and immunoglobulin domains. Evidence for an evolutionary relationship and a hypothesis for its functional significance. Biochem J. 1981 Apr 1;195(1):31–40. doi: 10.1042/bj1950031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Banker G., Churchill L., Taylor D. Isolation of postsynaptic densities from rat brain. J Cell Biol. 1974 Nov;63(2 Pt 1):441–455. doi: 10.1083/jcb.63.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Taylor D. Isolation and structural studies on synaptic complexes from rat brain. J Cell Biol. 1972 Dec;55(3):696–711. doi: 10.1083/jcb.55.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification and unusual tissue distribution of the canine and human homologues of Thy-1 (theta). J Exp Med. 1979 Mar 1;149(3):576–591. doi: 10.1084/jem.149.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Bologna M., Unger M. Role of Thy-1 antigen in the in vitro differentiation of a rat mammary cell line. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1848–1852. doi: 10.1073/pnas.76.4.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W. Identification of glycoproteins and proteins at synapses in the central nervous system. J Biol Chem. 1977 Jan 25;252(2):786–793. [PubMed] [Google Scholar]
- Kelly P. T., Luttges M. W. Electrophoretic separation of nervous system proteins on exponential gradient polyacrylamide gels. J Neurochem. 1975 May;24(5):1077–1079. doi: 10.1111/j.1471-4159.1975.tb03680.x. [DOI] [PubMed] [Google Scholar]
- Kemshead J. T., Ritter M. A., Cotmore S. F., Greaves M. F. Human Thy-1: expression on the cell surface of neuronal and glial cells. Brain Res. 1982 Mar 25;236(2):451–461. doi: 10.1016/0006-8993(82)90727-2. [DOI] [PubMed] [Google Scholar]
- Kornfeld K., Reitman M. L., Kornfeld R. The carbohydrate-binding specificity of pea and lentil lectins. Fucose is an important determinant. J Biol Chem. 1981 Jul 10;256(13):6633–6640. [PubMed] [Google Scholar]
- Kuchel P. W., Campbell D. G., Barclay A. N., Williams A. F. Molecular weights of the Thy-1 glycoproteins from rat thymus and brain in the presence and absence of deoxycholate. Biochem J. 1978 Feb 1;169(2):411–417. doi: 10.1042/bj1690411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letarte-Muirhead M., Barclay A. N., Williams A. F. Purification of the Thy-1 molecule, a major cell-surface glycoprotein of rat thymocytes. Biochem J. 1975 Dec;151(3):685–697. doi: 10.1042/bj1510685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain L. D., Tomana M., Acton R. T. Purification and characterization of mouse brain Thy-1.2 differentiation alloantigen. Brain Res. 1978 Dec 22;159(1):161–171. doi: 10.1016/0006-8993(78)90117-8. [DOI] [PubMed] [Google Scholar]
- McKenzie J. L., Fabre J. W. Distribution of Thy-1 in human brain: immunofluorescence and absorption analyses with a monoclonal antibody. Brain Res. 1981 Dec 28;230(1-2):307–316. doi: 10.1016/0006-8993(81)90409-1. [DOI] [PubMed] [Google Scholar]
- Morris R. J., Williams A. F. Antigens on mouse and rat lymphocytes recognized by rabbit antiserum against rat brain: the quantitative analysis of a xenogeneic antiserum. Eur J Immunol. 1975 Apr;5(4):274–281. doi: 10.1002/eji.1830050412. [DOI] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Rostas J. A., Kelly P. T., Cotman C. W. The identification of membrane glycocomponents in polyacrylamide gels: a rapid method using 125I-labeled lectins. Anal Biochem. 1977 Jun;80(2):366–372. doi: 10.1016/0003-2697(77)90657-1. [DOI] [PubMed] [Google Scholar]
- Rostas J. A., Kelly P. T., Pesin R. H., Cotman C. W. Protein and glycoprotein composition of synaptic junctions prepared from discrete synaptic regions and different species. Brain Res. 1979 May 18;168(1):151–167. doi: 10.1016/0006-8993(79)90133-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Mazauskas C. Immunological properties of murine thymus-dependent lymphocyte surface glycoproteins. Eur J Immunol. 1976 Aug;6(8):557–562. doi: 10.1002/eji.1830060806. [DOI] [PubMed] [Google Scholar]
- Wick G., Schauenstein K. Letter: Lack of antigenic correlation between chicken brain and thymus. Lancet. 1974 Jul 27;2(7874):214–215. doi: 10.1016/s0140-6736(74)91507-4. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N., Letarte-Muirhead M., Morris R. J. Rat thy-1 antigens from thymus and brain: their tissue distribution, purification, and chemical composition. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):51–61. doi: 10.1101/sqb.1977.041.01.009. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982 May 14;216(4547):696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]
- Williams A. F. Many cells in rat bone marrow have cell-surface Thy-1 antigen. Eur J Immunol. 1976 Jul;6(7):526–528. doi: 10.1002/eji.1830060716. [DOI] [PubMed] [Google Scholar]