Abstract
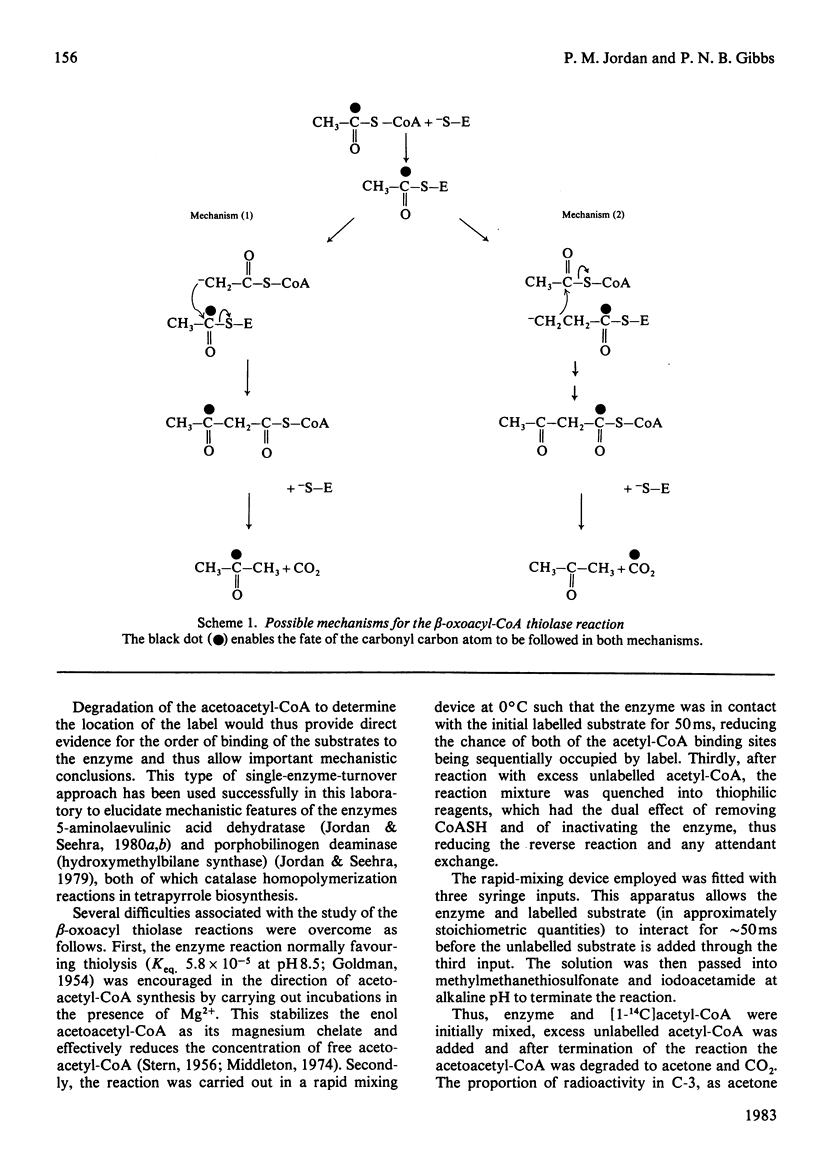
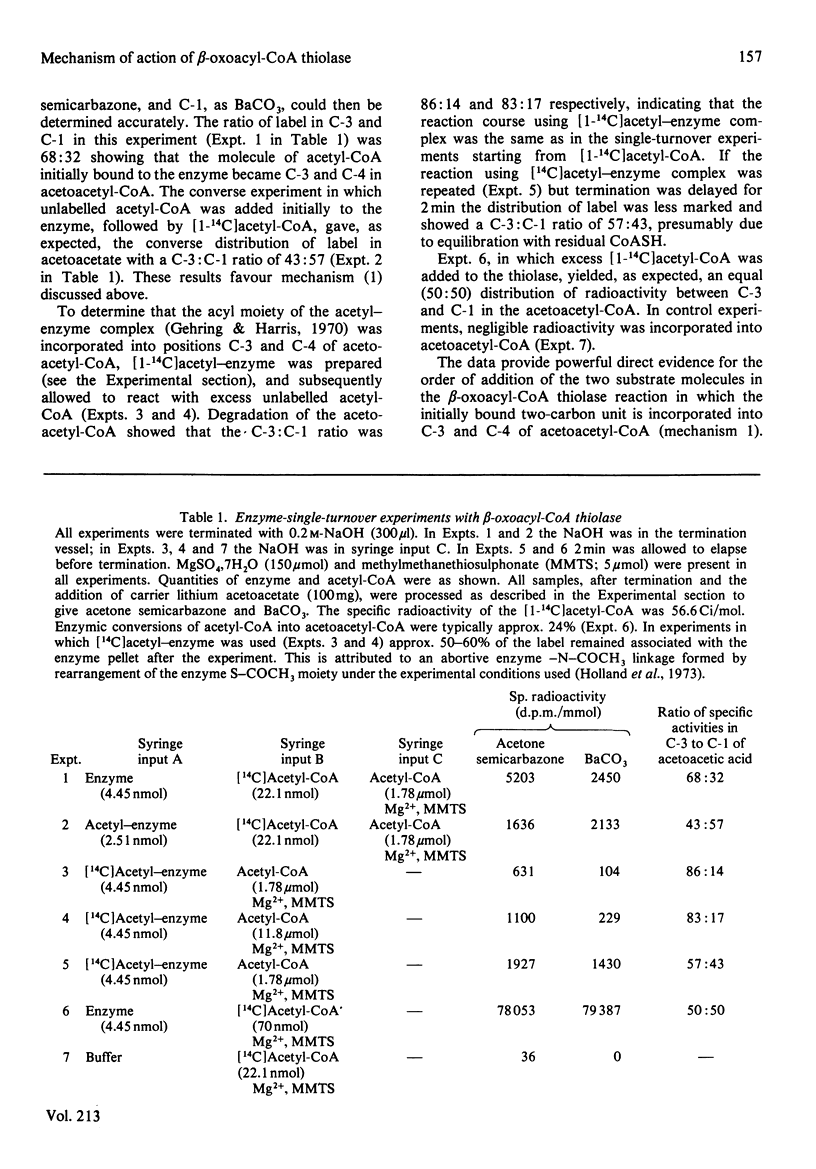
Single-turnover enzyme reactions were employed with beta-oxoacyl-CoA thiolase purified from rat liver cytosol to determine the order of binding of the two acetyl-CoA molecules to the enzyme during the formation of acetoacetyl-CoA. Equimolar quantities of [1-14C]acetyl-CoA and enzyme were mixed initially in a rapid mixing device and the reaction was quenched by addition of an excess of unlabelled acetyl-CoA. Degradation of the resulting acetoacetyl-CoA revealed that the larger proportion of the radioactivity was in C-3. In the converse experiment, in which unlabelled acetyl-CoA was mixed with enzyme and the reaction was quenched with [1-14C]acetyl-CoA, radioactivity was incorporated preferentially into C-1. Similar results were obtained when [14C]acetyl-enzyme complex isolated by gel filtration was reacted with unlabelled acetyl-CoA, the radioactivity appearing largely in C-3. These findings lead to the conclusion that of the two molecules of acetyl-CoA that are bound by the enzyme and converted into acetoacetyl-CoA, it is the one giving rise to C-3 and -4 that is bound initially to the enzyme in the form of the acetyl-enzyme intermediate complex.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloxham D. P., Chalkley R. A., Coghlin S. J., Salam W. Synthesis of chloromethyl ketone derivatives of fatty acids. Their use as specific inhibitors of acetoacetyl-coenzyme A thiolase, cholesterol biosynthesis and fatty acid synthesis. Biochem J. 1978 Dec 1;175(3):999–1011. doi: 10.1042/bj1750999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GOLDMAN D. S. Studies on the fatty acid oxidizing system of animal tissues. VII. The beta-ketoacyl coenzyme A cleavage enzyme. J Biol Chem. 1954 May;208(1):345–357. [PubMed] [Google Scholar]
- Gehring U., Harris J. I. Amino acid sequence around the reactive cysteine residues in thiolase. FEBS Lett. 1968 Aug;1(3):150–152. doi: 10.1016/0014-5793(68)80044-4. [DOI] [PubMed] [Google Scholar]
- Gehring U., Harris J. I. The active site cysteines of thiolase. Eur J Biochem. 1970 Nov;16(3):492–498. doi: 10.1111/j.1432-1033.1970.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Holland P. C., Clark M. G., Bloxham D. P. Inactivation of pig heart thiolase by 3-butynoyl coenzyme A, 3-pentynoyl coenzyme A, and 4-bromocrotonyl coenzyme A. Biochemistry. 1973 Aug 14;12(17):3309–3315. doi: 10.1021/bi00741a024. [DOI] [PubMed] [Google Scholar]
- Huth W., Jonas R., Wunderlich I., Seubert W. On the mechanism of ketogenesis and its control. Purification, kinetic mechanism and regulation of different forms of mitochondrial acetoacetyl-CoA thiolases from ox liver. Eur J Biochem. 1975 Nov 15;59(2):475–489. doi: 10.1111/j.1432-1033.1975.tb02476.x. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Seehra J. S. 13C NMR as a probe for the study of enzyme-catalysed reactions: mechanism of action of 5-aminolevulinic acid dehydratase. FEBS Lett. 1980 Jun 2;114(2):283–286. doi: 10.1016/0014-5793(80)81134-3. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Seehra J. S. The biosynthesis of uroporphyrinogen III: order of assembly of the four porphobilinogen molecules in the formation of the tetrapyrrole ring. FEBS Lett. 1979 Aug 15;104(2):364–366. doi: 10.1016/0014-5793(79)80853-4. [DOI] [PubMed] [Google Scholar]
- LYNEN F. Functional group of coenzyme A and its metabolic relations, especially in the fatty acid cycle. Fed Proc. 1953 Sep;12(3):683–691. [PubMed] [Google Scholar]
- Middleton B. The kinetic mechanism and properties of the cytoplasmic acetoacetyl-coenzyme A thiolase from rat liver. Biochem J. 1974 Apr;139(1):109–121. doi: 10.1042/bj1390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton B. The oxoacyl-coenzyme A thiolases of animal tissues. Biochem J. 1973 Apr;132(4):717–730. doi: 10.1042/bj1320717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN J. R. Optical properties of aceto-acetyl-S-coenzyme A and its metal chelates. J Biol Chem. 1956 Jul;221(1):33–44. [PubMed] [Google Scholar]
- Willadsen P., Eggerer H. Substrate stereochemistry of the acetyl-CoA acetyltransferase reaction. Eur J Biochem. 1975 May;54(1):253–258. doi: 10.1111/j.1432-1033.1975.tb04135.x. [DOI] [PubMed] [Google Scholar]


