Abstract
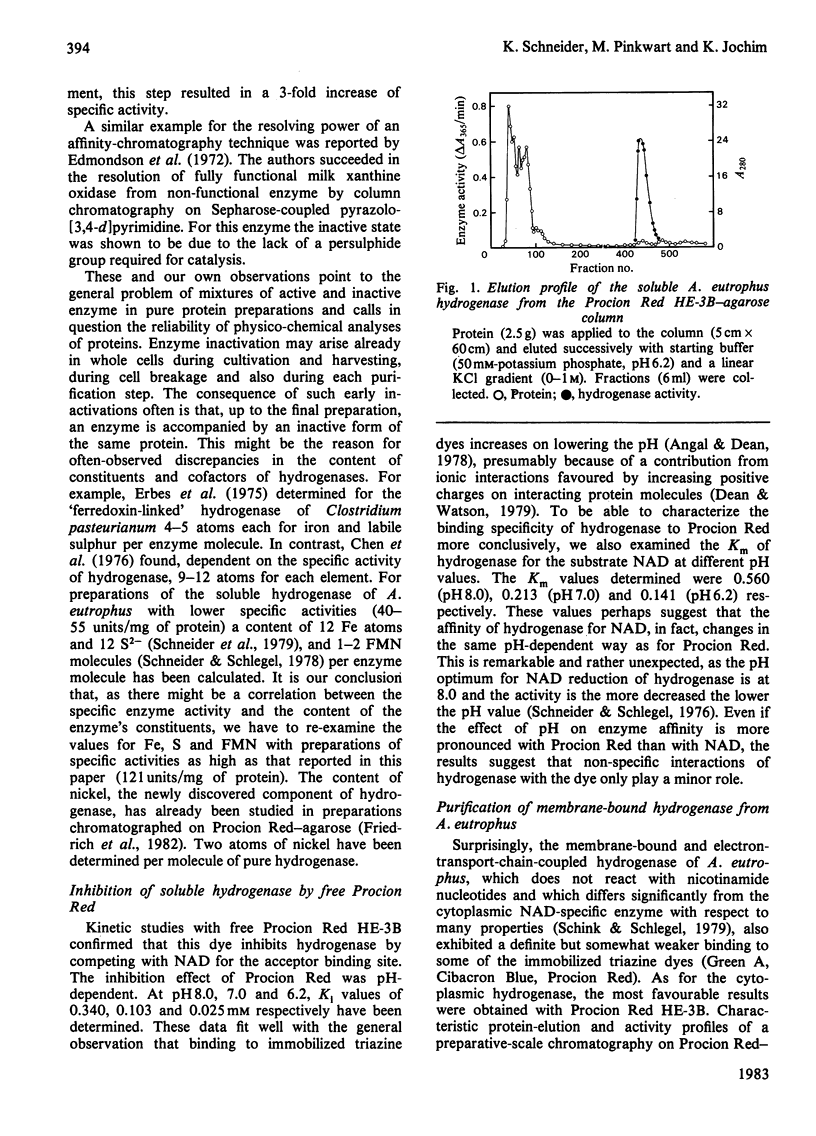
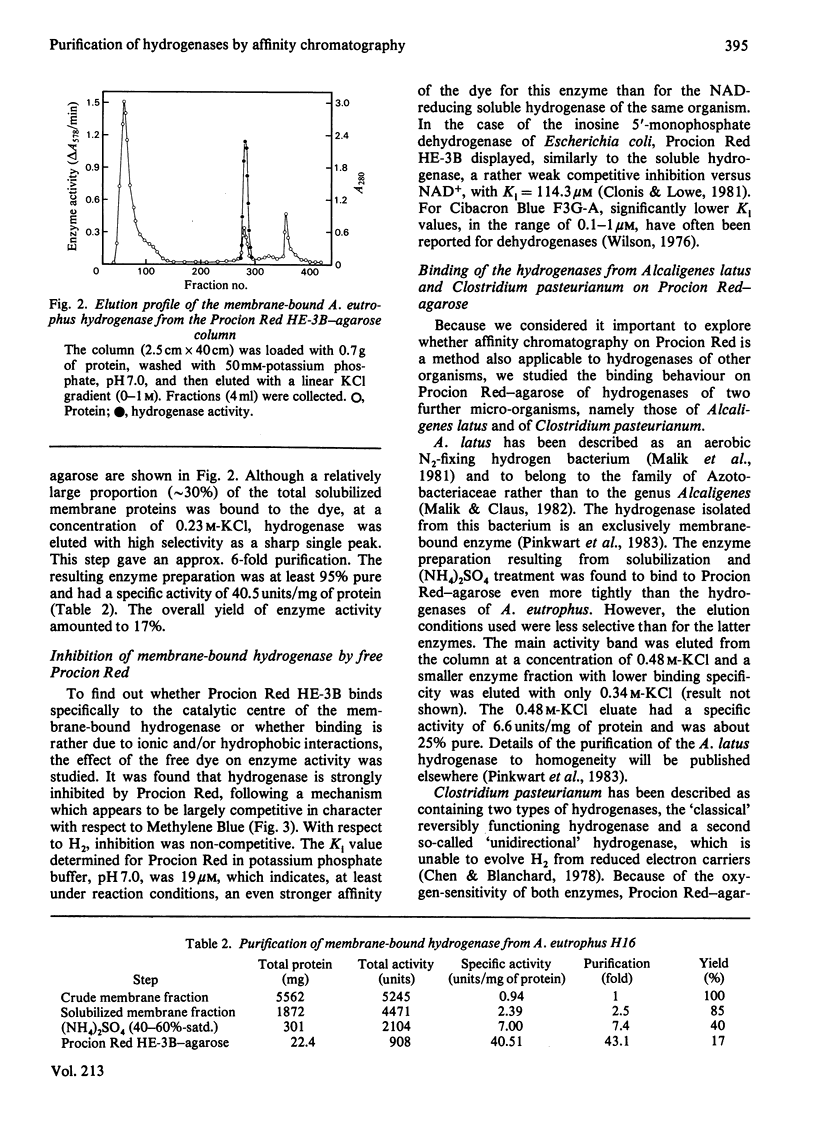
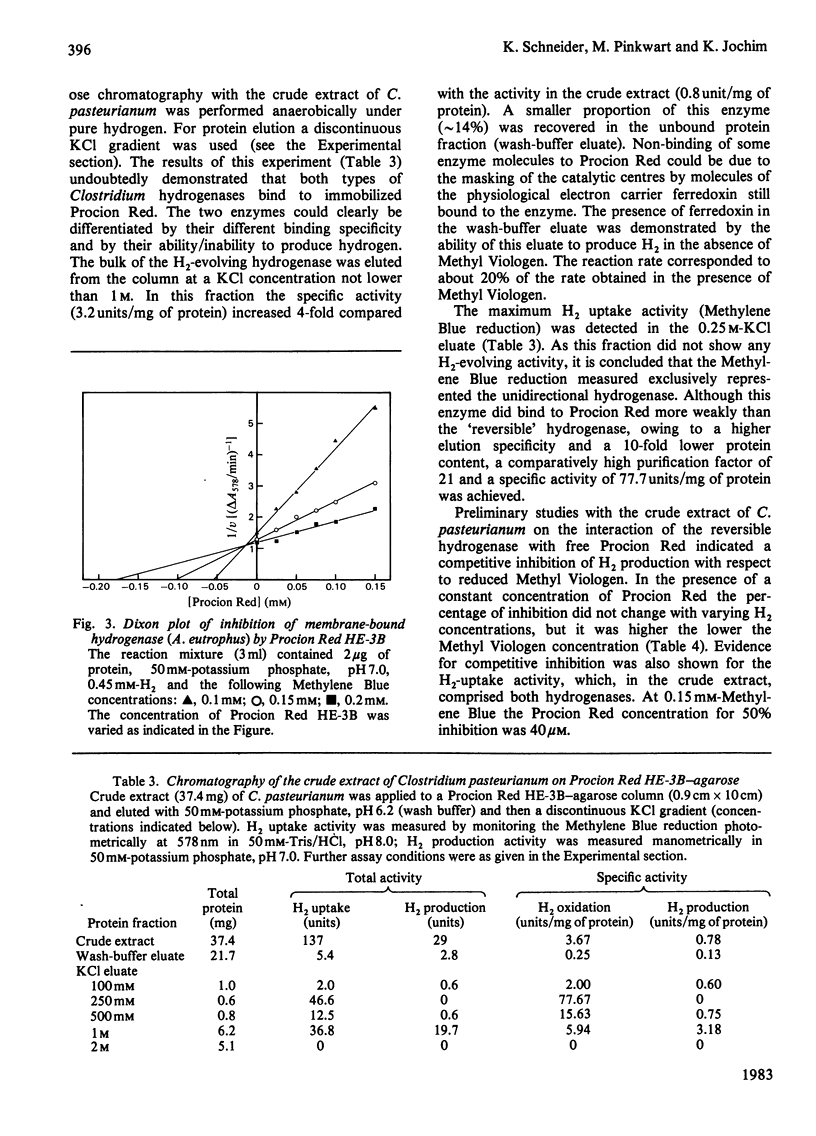
The agarose-coupled triazine dye Procion Red HE-3B has been demonstrated to be applicable as an affinity gel for the purification of five diverse hydrogenases, namely the soluble, NAD-specific and the membrane-bound hydrogenase of Alcaligenes eutrophus, the membrane-bound hydrogenase of the N2-fixing Alcaligenes latus, the reversible H2-evolving and the unidirectional H2-oxidizing hydrogenase of Clostridium pasteurianum. In the case of the soluble hydrogenase of A. eutrophus, chromatography on Procion Red-agarose even permitted the separation of inactive from active enzyme, thus yielding a 2-3-fold increase in specific activity. For the homogeneous enzyme preparation obtained after two column steps (Procion Red-agarose, DEAE-Sephacel), a specific activity of 121 mumol of H2 oxidized/min per mg of protein was determined. Kinetic studies with free Procion Red provided evidence that the diverse hydrogenases are competitively inhibited by the dye, each with respect to the electron carrier (NAD, Methylene Blue, Methyl Viologen), indicating a specific interaction between Procion Red and the catalytic centres of the enzymes. For the highly purified preparations of the soluble and the membrane-bound hydrogenase of A. eutrophus, in 50 mM-potassium phosphate, pH 7.0, Ki values for Procion Red of 103 and 19 microM have been determined.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Hall D. O. Properties of the solubilized membrane-bound hydrogenase from the photosynthetic bacterium Rhodospirillum rubrum. Arch Biochem Biophys. 1979 Jul;195(2):288–299. doi: 10.1016/0003-9861(79)90355-2. [DOI] [PubMed] [Google Scholar]
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Angal S., Dean P. D. The use of immobilized cibacron blue in plasma fractionation. FEBS Lett. 1978 Dec 15;96(2):346–348. doi: 10.1016/0014-5793(78)80433-5. [DOI] [PubMed] [Google Scholar]
- Ashton A. R., Polya G. M. The specific interaction of cibacron and related dyes with cyclic nucleotide phosphodiesterase and lactate dehydrogenase. Biochem J. 1978 Nov 1;175(2):501–506. doi: 10.1042/bj1750501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird J. K., Sherwood R. F., Carr R. J., Atkinson A. Enzyme purification by substrate elution chromatography from procion dye-polysaccharide matrices. FEBS Lett. 1976 Nov;70(1):61–66. doi: 10.1016/0014-5793(76)80726-0. [DOI] [PubMed] [Google Scholar]
- Biellmann J. F., Samama J. P., Bränden C. I., Eklund H. X-ray studies of the binding of Cibacron blue F3GA to liver alcohol dehydrogenase. Eur J Biochem. 1979 Dec;102(1):107–110. doi: 10.1111/j.1432-1033.1979.tb06268.x. [DOI] [PubMed] [Google Scholar]
- Chen J. S., Blanchard D. K. Isolation and properties of a unidirectional H2-oxidizing hydrogenase from the strictly anaerobic N2-fixing bacterium Clostridium pasteurianum W5. Biochem Biophys Res Commun. 1978 Oct 30;84(4):1144–1150. doi: 10.1016/0006-291x(78)91703-5. [DOI] [PubMed] [Google Scholar]
- Chen J. S., Mortenson L. E. Purification and properties of hydrogenase from Clostridium pasteurianum W5. Biochim Biophys Acta. 1974 Dec 18;371(2):283–298. doi: 10.1016/0005-2795(74)90025-7. [DOI] [PubMed] [Google Scholar]
- Clonis Y. D., Lowe C. R. Affinity chromatography on immobilised triazine dyes. Studies on the interaction with multinucleotide-dependent enzymes. Biochim Biophys Acta. 1981 May 14;659(1):86–98. doi: 10.1016/0005-2744(81)90273-4. [DOI] [PubMed] [Google Scholar]
- Dean P. D., Watson D. H. Protein purification using immobilised triazine dyes. J Chromatogr. 1979 Oct 1;165(3):301–319. doi: 10.1016/s0021-9673(00)88187-x. [DOI] [PubMed] [Google Scholar]
- Edmondson D., Massey V., Palmer G., Beacham L. M., 3rd, Elion G. B. The resolution of active and inactive xanthine oxidase by affinity chromatography. J Biol Chem. 1972 Mar 10;247(5):1597–1604. [PubMed] [Google Scholar]
- Edwards R. A., Woody R. W. Spectroscopic studies of Cibacron Blue and Congo Red bound to dehydrogenases and kinases. Evaluation of dyes as probes of the dinucleotide fold. Biochemistry. 1979 Nov 13;18(23):5197–5204. doi: 10.1021/bi00590a026. [DOI] [PubMed] [Google Scholar]
- Erbes D. L., Burris R. H., Orme-Johnson W. H. On the iron-sulfur cluster in hydrogenase from Clostridium pasteurianum W5. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4795–4799. doi: 10.1073/pnas.72.12.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Heine E., Finck A., Friedrich C. G. Nickel requirement for active hydrogenase formation in Alcaligenes eutrophus. J Bacteriol. 1981 Mar;145(3):1144–1149. doi: 10.1128/jb.145.3.1144-1149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C. G., Schneider K., Friedrich B. Nickel in the catalytically active hydrogenase of Alcaligenes eutrophus. J Bacteriol. 1982 Oct;152(1):42–48. doi: 10.1128/jb.152.1.42-48.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschke R. H., Campbell L. L. Purification and properties of a hydrogenase from Desulfovibrio vulgaris. J Bacteriol. 1971 Jan;105(1):249–258. doi: 10.1128/jb.105.1.249-258.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. M., Klibanov A. M., Kaplan N. O., Kamen M. D. The effect of electron carriers and other ligands on oxygen stability of clostridial hydrogenase. Biochim Biophys Acta. 1981 Jun 15;659(2):457–465. doi: 10.1016/0005-2744(81)90071-1. [DOI] [PubMed] [Google Scholar]
- Legall J., DerVartanian D. V., Spilker E., Lee J. P., Peck H. D., Jr Evidence for the involvement of non-heme iron in the active site of hydrogenase from Desulfovibrio vulgaris. Biochim Biophys Acta. 1971 Jun 15;234(3):526–530. [PubMed] [Google Scholar]
- Lowe C. R., Small D. A., Atkinson A. Some preparative and analytical applications of triazine dyes. Int J Biochem. 1981;13(1):33–40. doi: 10.1016/0020-711x(81)90133-6. [DOI] [PubMed] [Google Scholar]
- Nakos G., Mortenson L. Purification and properties of hydrogenase, an iron sulfur protein, from Clostridium pasteurianum W5. Biochim Biophys Acta. 1971 Mar 10;227(3):576–583. doi: 10.1016/0005-2744(71)90008-8. [DOI] [PubMed] [Google Scholar]
- Robson R. L., Robson R. M., Morris J. G. The biosynthesis of granulose by Clostridium pasteurianum. Biochem J. 1974 Dec;144(3):503–511. doi: 10.1042/bj1440503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- Schlesier M., Friedrich B. In vivo inactivation of soluble hydrogenase of Alcaligenes eutrophus. Arch Microbiol. 1981 Apr;129(2):150–153. doi: 10.1007/BF00455352. [DOI] [PubMed] [Google Scholar]
- Schneider K., Cammack R., Schlegel H. G., Hall D. O. The iron-sulphur centres of soluble hydrogenase from Alcaligenes eutrophus. Biochim Biophys Acta. 1979 Jun 19;578(2):445–461. doi: 10.1016/0005-2795(79)90175-2. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Identification and quantitative determination of the flavin component of soluble hydrogenase from Alcaligenes eutrophus. Biochem Biophys Res Commun. 1978 Oct 16;84(3):564–571. doi: 10.1016/0006-291x(78)90743-x. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Purification and properties of soluble hydrogenase from Alcaligenes eutrophus H 16. Biochim Biophys Acta. 1976 Nov 8;452(1):66–80. doi: 10.1016/0005-2744(76)90058-9. [DOI] [PubMed] [Google Scholar]
- Schoenmaker G. S., Oltmann L. F., Stouthamer A. H. Purification and properties of the membrane-bound hydrogenase from Proteus mirabilis. Biochim Biophys Acta. 1979 Apr 12;567(2):511–521. doi: 10.1016/0005-2744(79)90137-2. [DOI] [PubMed] [Google Scholar]
- Thompson S. T., Cass K. H., Stellwagen E. Blue dextran-sepharose: an affinity column for the dinucleotide fold in proteins. Proc Natl Acad Sci U S A. 1975 Feb;72(2):669–672. doi: 10.1073/pnas.72.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. H., Harvey M. J., Dean P. D. The selective retardation of NADP+-dependent dehydrogenases by immobilized procion red HE-3B. Biochem J. 1978 Aug 1;173(2):591–596. doi: 10.1042/bj1730591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. E. Applications of blue dextran and Cibacron Blue F3GA in purification and structural studies of nucleotide-requiring enzymes. Biochem Biophys Res Commun. 1976 Oct 4;72(3):816–823. doi: 10.1016/s0006-291x(76)80206-9. [DOI] [PubMed] [Google Scholar]
- van Heerikhuizen H., Albracht S. P., Slater E. C., van Rheenen P. S. Purification and some properties of the soluble hydrogenase from Chromatium vinosum. Biochim Biophys Acta. 1981 Jan 15;657(1):26–39. doi: 10.1016/0005-2744(81)90127-3. [DOI] [PubMed] [Google Scholar]


