Abstract
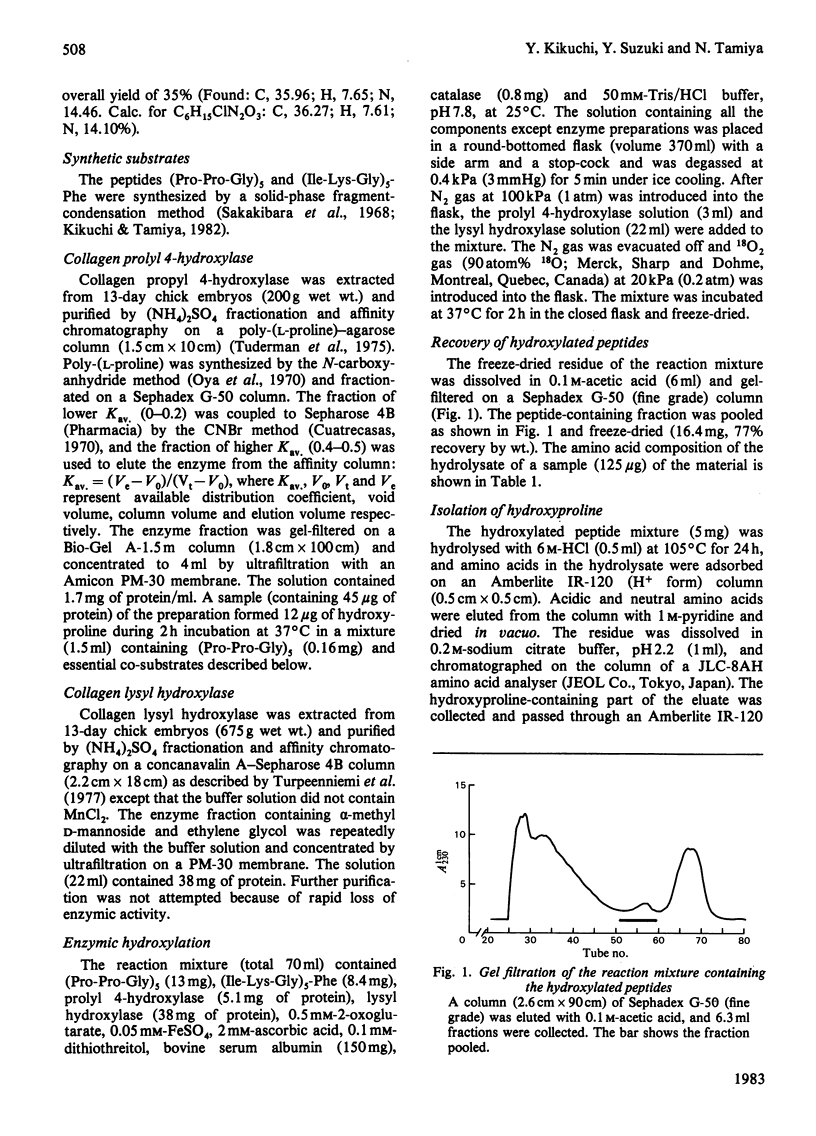
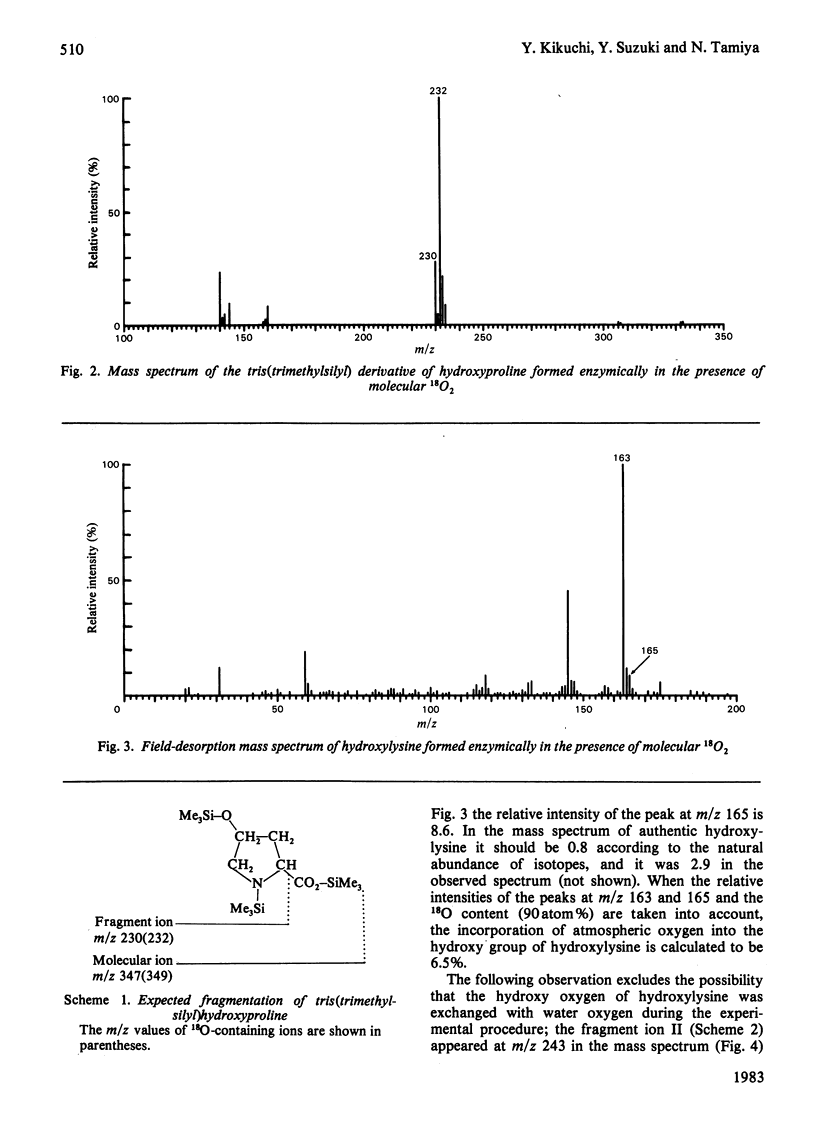
The synthetic peptides (Pro-Pro-Gly)5 and (Ile-Lys-Gly)5-Phe were hydroxylated with collagen prolyl hydroxylase and lysyl hydroxylase in an 18O2 atmosphere. The oxygen atoms in the hydroxy groups of hydroxyproline and hydroxylysine were 87% and 6.5% respectively derived from the atmospheric 18O2. The results are consistent with those reported previously for proline hydroxylation in vivo [Fujimoto & Tamiya (1962) Biochem. J. 84, 333-335; Prockop, Kaplan & Udenfriend (1962) Biochem. Biophys. Res. Commun. 9, 192-196; Fujimoto & Tamiya (1963) Biochem. Biophys. Res. Commun. 10, 498-501; Prockop, Kaplan & Udenfriend (1963) Arch. Biochem. Biophys. 101, 499-503] and in vitro [Cardinale, Rhoads & Udenfriend (1971) Biochem. Biophys. Res. Commun. 43, 537-543] and for lysine hydroxylation in vivo [Fujimoto & Tamiya (1963) Biochem. Biophys. Res. Commun. 10, 498-501]. In view of the similarities of these two oxygenase-type hydroxylation reactions the participation of intermediates is proposed, the oxygen atoms of which are exchangeable with those of water. The atmospheric oxygen atoms incorporated into the intermediate must be equilibrated with water oxygen atoms in the slower lysyl hydroxylase reaction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUTZ E. K. The role of phage specific RNA as messenger. Biochem Biophys Res Commun. 1962 Sep 25;9:192–197. doi: 10.1016/0006-291x(62)90113-4. [DOI] [PubMed] [Google Scholar]
- Cardinale G. J., Rhoads R. E., Udenfriend S. Simultaneous incorporation of 18 O into succinate and hydroxyproline catalyzed by collagen proline hydroxylase. Biochem Biophys Res Commun. 1971 May 7;43(3):537–543. doi: 10.1016/0006-291x(71)90647-4. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- FUJIMOTO D., TAMIYA N. Studies on the hydroxylation of lysine in vivo. Biochem Biophys Res Commun. 1963 Mar 25;10:498–501. doi: 10.1016/0006-291x(63)90386-3. [DOI] [PubMed] [Google Scholar]
- Fujimoto D., Tamiya N. Incorporation of O from air into hydroxyproline by chick embryo. Biochem J. 1962 Aug;84(2):333–335. doi: 10.1042/bj0840333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke C. W., Leimer K. Trimethylsilylation of amino acids. Effect of solvents on derivatization using bis(trimethylsilyl)trifluoroacetamide. J Chromatogr. 1970 Dec;53(2):201–208. doi: 10.1016/s0021-9673(01)98459-6. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Fujimoto D., Tamiya N. The enzymic hydroxylation of protocollagen models. Biochem J. 1969 Nov;115(3):569–574. doi: 10.1042/bj1150569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivirikko K. I., Prockop D. J. Partial purification and characterization of protocollagen lysine hydroxylase from chick embryos. Biochim Biophys Acta. 1972 Feb 28;258(2):366–379. doi: 10.1016/0005-2744(72)90228-8. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Shudo K., Sakakibara S., Prockop D. J. Studies on protocollagen lysine hydroxylase. Hydroxylation of synthetic peptides and the stoichiometric decarboxylation of -ketoglutarate. Biochemistry. 1972 Jan 4;11(1):122–129. doi: 10.1021/bi00751a021. [DOI] [PubMed] [Google Scholar]
- Lindblad B., Lindstedt G., Lindstedt S. The mechanism of enzymic formation of homogentisate from p-hydroxyphenylpyruvate. J Am Chem Soc. 1970 Dec 16;92(25):7446–7449. doi: 10.1021/ja00728a032. [DOI] [PubMed] [Google Scholar]
- Morris H. R., Williams D. H., Ambler R. P. Determination of the sequences of protein-derived peptides and peptide mixtures by mass spectrometry. Biochem J. 1971 Nov;125(1):189–201. doi: 10.1042/bj1250189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllylä R., Tuderman L., Kivirikko K. I. Mechanism of the prolyl hydroxylase reaction. 2. Kinetic analysis of the reaction sequence. Eur J Biochem. 1977 Nov 1;80(2):349–357. doi: 10.1111/j.1432-1033.1977.tb11889.x. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A., LIKINS R. C. The conversion of lysine to hydroxylysine and its relation to the biosynthesis of collagen in several tissues of the rat. J Biol Chem. 1957 Nov;229(1):101–109. [PubMed] [Google Scholar]
- PROCKOP D., KAPLAN A., UDENFRIEND S. Oxygen-18 studies on the conversion of proline to collagen hydroxyproline. Arch Biochem Biophys. 1963 Jun;101:499–503. doi: 10.1016/0003-9861(63)90509-5. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Weinstein E., Mulveny T. Hydroxylation of lysine in a polypeptide precursor of collagen. Biochem Biophys Res Commun. 1966 Jan 4;22(1):124–128. doi: 10.1016/0006-291x(66)90613-9. [DOI] [PubMed] [Google Scholar]
- Puistola U., Turpeenniemi-Hujanen T. M., Myllylä R., Kivirikko K. I. Studies on the lysyl hydroxylase reaction. I. Initial velocity kinetics and related aspects. Biochim Biophys Acta. 1980 Jan 11;611(1):40–50. doi: 10.1016/0005-2744(80)90040-6. [DOI] [PubMed] [Google Scholar]
- Puistola U., Turpeenniemi-Hujanen T. M., Myllylä R., Kivirikko K. I. Studies on the lysyl hydroxylase reaction. II. Inhibition kinetics and the reaction mechanism. Biochim Biophys Acta. 1980 Jan 11;611(1):51–60. doi: 10.1016/0005-2744(80)90041-8. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., Udenfriend S. Decarboxylation of alpha-ketoglutarate coupled to collagen proline hydroxylase. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1473–1478. doi: 10.1073/pnas.60.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINEX F. M., VAN SLYKE D. D. The source and state of the hydroxylysine of collagen. J Biol Chem. 1955 Sep;216(1):245–250. [PubMed] [Google Scholar]
- STETTEN M. R. Some aspects of the metabolism of hydroxyproline, studied with the aid of isotopic nitrogen. J Biol Chem. 1949 Nov;181(1):31–37. [PubMed] [Google Scholar]
- Sabourin P. J., Bieber L. L. The mechanism of alpha-ketoisocaproate oxygenase. Formation of beta-hydroxyisovalerate from alpha-ketoisocaproate. J Biol Chem. 1982 Jul 10;257(13):7468–7471. [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. An affinity-column procedure using poly(L-proline) for the purification of prolyl hydroxylase. Purification of the enzyme from chick embryos. Eur J Biochem. 1975 Mar 3;52(1):9–16. doi: 10.1111/j.1432-1033.1975.tb03967.x. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Myllylä R., Kivirikko K. I. Mechanism of the prolyl hydroxylase reaction. 1. Role of co-substrates. Eur J Biochem. 1977 Nov 1;80(2):341–348. doi: 10.1111/j.1432-1033.1977.tb11888.x. [DOI] [PubMed] [Google Scholar]
- Turpeenniemi T. M., Puistola U., Anttinen H., Kivirikko K. I. Affinity chromatography of lysyl hydroxylase on concanavalin A-agarose. Biochim Biophys Acta. 1977 Jul 8;483(1):215–219. doi: 10.1016/0005-2744(77)90024-9. [DOI] [PubMed] [Google Scholar]
- Udenfriend S. Formation of hydroxyproline in collagen. Science. 1966 Jun 3;152(3727):1335–1340. doi: 10.1126/science.152.3727.1335. [DOI] [PubMed] [Google Scholar]


