Abstract
Obesity paradox refers to the clinical observation that when acute cardiovascular decompensation occurs, patients with obesity may have a survival benefit. This apparently runs counter to the epidemiology of obesity, which may increase the risk for non‐communicable diseases (NCDs). The scientific community is split on obesity paradox, with some supporting it, while others call it BMI paradox. This review: (a) defines the obesity paradox, and its proposed role in overall mortality in NCDs; (b) delineates evidence for and against obesity paradox; (c) presents the importance of using different indices of body mass to assess the risk in NCDs; (d) examines the role of metabolically healthy obesity in obesity paradox, and emerging importance of cardio‐respiratory fitness (CRF) as an independent predictor of CVD risk and all‐cause mortality in patients with/without obesity. Evidence suggests that the development of obesity and insulin resistance are influenced by genetic (or ethnic) make up and dietary habits (culture) of the individuals. Hence, this review presents lean diabetes, which has higher total CVD and non‐CVD mortality as compared to diabetics with obesity and the possibility of maternal factors programming cardiometabolic risk during fetal development, which may lead to a paradigm shift in our understanding of obesity.
Keywords: BMI paradox, cardio‐respiratory fitness, cardiovascular decompensation, lean diabetes, metabolically healthy obesity, non‐communicable diseases, obesity paradox
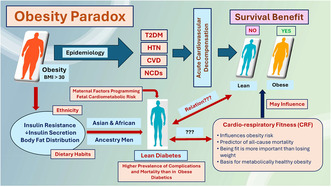
Obesity paradox is a clinical observation that when acute cardiovascular decompensation occurs, patients with obesity may have survival benefits. Development of insulin resistance, decrease in insulin secretion, and body fat distribution in obesity varies considerably based on ethnicity and dietary habits of people. Maternal factors may program fetal cardiovascular risk, which often leads to development lean diabetes, which has higher prevalence of complications and mortality than in obese diabetics. Cardio‐respiratory fitness (CRF) has emerged as an independent risk factor for death, irrespective of the obesity status of the subject. CRF may also influence mortality in obesity paradox.
1. INTRODUCTION
With over 2 billion people being overweight (Body mass index or BMI >25 Kg/m2), obesity is a global problem (Sørensen et al., 2022). Of these about 600 million people are having obesity (BMI >30 Kg/m2). If we apply the Asia‐Pacific classification of obesity recommended by the World Health Organization (n.d.) (WHO—Obesity), the actual number of subjects with obesity will be much higher (Table 1). Most of these people are living in emerging economies (BRICS countries) and in rapidly developing countries of Asia, Africa, and South America (Figure 1; Jakovljevic & Milovanovic, 2015; Koliaki et al., 2023). Incidentally, these are also the regions where there is steep rise in the prevalence of non‐communicable diseases (NCDs), such as diabetes mellitus, hypertension, cardiovascular and cerebrovascular disorders with acute events, chronic kidney disease, and other NCDs (Jakovljevic & Milovanovic, 2015; Ndubuisi, 2021; Remais et al., 2013). Thus, emerging economies and the developing world are bearing a disproportionately higher burden in dealing with obesity and its consequences. A closer examination of this disparity reveals many variables starting from genetic make up to dietary and lifestyle habits, to environmental factors (Hu, 2011; van Vliet‐Ostaptchouk et al., 2012; Bhurosy & Jeewon, 2014; Albuquerque et al., 2017; Kang et al., 2021). But most studies on obesity and its complications as well as their management are performed on people in developed countries, with different genetic makeup, dietary habits, and lifestyle. It is time to examine whether we are understanding the complex subject of obesity in its totality or in titbits which are easier to do. In this context, the controversial subject “obesity paradox” offers an opportunity to re‐examine our position on obesity and contemplate whether we are getting it right or oversimplifying a complex subject for our convenience to handle or deal with.
TABLE 1.
Cut points for overweight and obesity indicators used for South Asians versus Westerners.
| Indicators | South Asians | Westerners |
|---|---|---|
| Body mass index (BMI) kg/m2 | ||
| Underweight | <18.5 | <18.5 |
| Normal Weight | 18.8–22.9 | 18.5–24.9 |
| Overweight | 23.0–24.9 | 25.0–29.9 |
| Obese I | 25.0–29.9 | 30.0–34.9 |
| Obese II | 30.0–34.9 | 35.0–39.9 |
| Obese III | 35.0–39.9 | >40.0 |
| Waist circumference (cm) | ||
| Men | ≥90 | ≥102 |
| Women | ≥80 | ≥88 |
| Waist hip ratio | ||
| Men | ≥0.9 | ≥0.9 |
| Women | ≥0.8 | ≥0.8 |
| Body fat percentage | ||
| Men | ≥20% | ≥25% |
| Women | ≥33% | ≥35% |
Adopted from Kapoor N, Endotext [Internet] 2021. https://www.ncbi.nlm.nih.gov/books/NBK568563/ Creative Commons License. Attribution‐NonCommercial‐NoDerivs 2.0 Generic (CC BY‐NC‐ND 2.0).
FIGURE 1.
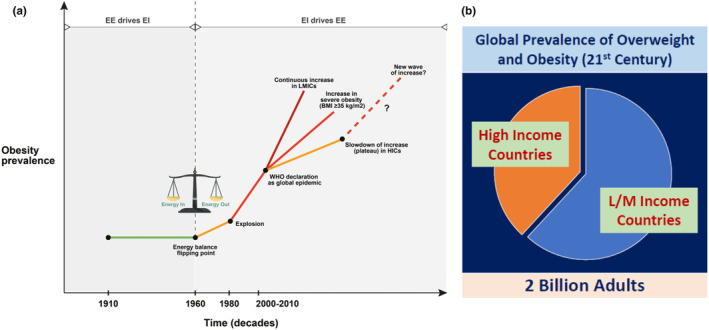
Prevalence of overweight and obesity in high income (HIC) and Low‐middle income (LMIC) countries. (a) A graphical summary of global obesity prevalence trends over decades of the 20th and 21st century. Reproduced from Koliaki et al. (2023) under creative commons CC BY license. (b) Proportion of global prevalence of overweight and obesity in twentieth century in high‐income and low‐middle income countries, based on several reports.
Using BMI as a benchmark, many experimental, clinical, and epidemiological studies linked overweight and obesity for the development of a variety of NCDs, such as type‐2 diabetes mellitus (T2DM), hypertension, and diseases of the heart, liver, lung, and the kidney among others (Akhter et al., 2021; Ejigu & Tiruneh, 2023; Felisbino‐Mendes et al., 2020; Fuentes et al., 2023; Kilpi et al., 2014; Kivimaki et al., 2022; Lobstein & Brinsden, 2014; Nyberg et al., 2018; Webber et al., 2012; Zatońska et al., 2021; Zhang et al., 2024). Obesity is also a determining factor for longevity (Donini et al., 2012; Lenz et al., 2009; Lung et al., 2019; Peeters et al., 2003; Solomon & Manson, 1997; Tam et al., 2020). Currently, NCDs account for 41 million deaths per year globally (74% of all deaths). Of these 77% of deaths occur in low‐ and middle‐income countries (WHO–NCDs). It is projected that by the year 2030, NCDs will cause 52 million deaths. According to the WHO, about 35.8 million (2.3%) of global DALYs (disability adjusted life years) are lost by overweight or obesity (WHO–NCDs). In June 2023, the American Medical Association officially recognized obesity as a disease state with multiple pathophysiological aspects requiring a range of interventions to advance obesity treatment and prevention. AMA Policy # H‐440‐842 (n.d.). According to an extensive analysis made by McKinsey Global Institute, in 2014 four preventable causes accounted for the loss of $7.6 trillion globally (9.51% of global GDP in 2014). These are smoking ($2.1 trillion), armed violence, war, and terrorism ($2.1 trillion), obesity ($2.0 trillion), and alcoholism ($1.4 trillion) (Dobbs et al., 2014). Hence, logically reducing the global burden of obesity will eventually result in lower number of deaths due to NCDs with substantial savings in healthcare costs. However, it is easier said than done. Because the accumulated knowledge on obesity and its health consequences in various racial or ethnic groups strongly suggests that one size fits all formulas may not work. For example, the primary metabolic abnormality in overweight or obesity—development of insulin resistance—is highly variable among different ethnic groups in a gender‐dependent manner (reviewed in Kishore, 2022). Storing fat is an evolutionary protective mechanism for survival (Genné‐Bacon, 2014; Speakman & Elmquist, 2022; Wells, 2012). Optimum levels of adipose tissue act like a fuel injection system of the internal combustion engine, and thus regulate whole body energy and glucose homeostasis. The “set‐point theory” postulates that human body has a predetermined weight or fat mass set to a range. Compensatory physiological mechanisms and redundant pathways maintain that set‐point (Ganipisetti & Bollimunta, 2023). By secreting bioactive molecules or hormones such as leptin and adiponectin, and other substances, adipose tissue regulates energy for growth and efficient function of the immune system.
In the above context, obesity paradox offers an opportunity to re‐evaluate and understand the complex subject of obesity with the sole intention of knowing enough to know whether we are right or wrong. Obesity paradox refers to the clinical observation that when acute cardiovascular decompensation occurs, obese patients may have a survival benefit. In recent years several review articles have been published on the obesity paradox (Assaf & Antoun, 2021; Braun et al., 2015; Donini et al., 2020; Dramé & Godaert, 2023; Liu et al., 2022; Simati et al., 2023). In addition to presenting the salient features in those review articles, this review provides additional information. The review presents unbiased facts with evidence on all aspects of obesity paradox. This review also attempts to provide a comprehensive landscape of obesity by dealing with different types of obesity vis‐à‐vis cardiometabolic risk, lean diabetes (LD), and potential maternal and infant nutritional insults in programming obese phenotype and cardiometabolic risk. Finally, this review article deals with obesity paradox and a related condition, LD on the same platform. It is the expectation of the author that this review will stimulate a new wave of thinking and approach to tackle the global problem of obesity. Although this review may not offer answers to all the questions we may have or give a final verdict on “fact or fiction” this is a small step in the right direction.
2. HISTORICAL ASPECTS
Dr. Kalantar‐Zedah first proposed the term “reverse epidemiology” in the journal Kidney International in 2003, and in the Journal of the American College of Cardiology in 2004 (Kalantar‐Zadeh et al., 2003, 2004). He reported that conventional risk factors for cardiovascular diseases (CVD) in general population, such as high body mass, serum cholesterol, and blood pressure, were often found to be protective and associated with greater survival among patients on maintenance hemodialysis (Figure 2). At that time, it was considered a contradiction to prevailing medical concepts of prevention of CVD. Although first described in dialysis patients, subsequently obesity paradox was found in those with heart failure, myocardial infarction, acute coronary syndrome, chronic obstructive pulmonary disease (COPD), rheumatoid arthritis, and older residents in nursing homes (Dramé & Godaert, 2023; Eitmann et al., 2024; Escalante et al., 2005; Kalantar‐Zadeh et al., 2017; Lainscak et al., 2012; Liu et al., 2022). It should be noted that the obesity paradox does not contradict the epidemiological data that obesity predisposes people to the development of NCDs. Obesity paradox states that once people develop NCDs, being obese offers a protective mechanism against mortality at times of cardiovascular decompensation.
FIGURE 2.
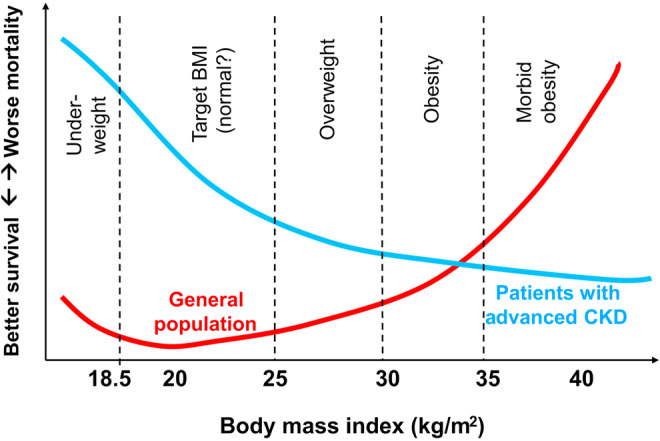
Reverse association of BMI and survival in patients with advanced chronic kidney disease (CKD) as compared to the general population. Reproduced from Kalantar‐Zadeh et al. (2017) under creative commons CC‐BY‐NC‐ND.
3. CRITIQUES OF OBESITY PARADOX
As expected, not everyone agrees about the possibility of obesity protecting patients with chronic NCDs. The critiques argue: (i) body fat might offer protection to survive during periods of low nutrition; (ii) the non‐obese population at risk are those who have lost weight because of more severe illness; (iii) people with obesity are diagnosed early with NCDs; (iv) BMI is poor indicator of body fat; (v) BMI cut‐offs are not appropriate, and (vi) the observed obesity paradox is due to Collider Stratification Bias. It is possible to verify or eliminate the first three critiques in carefully controlled studies. But the argument against BMI is counterintuitive, as the same BMI is the benchmark in epidemiological studies which revealed that obesity predisposes to NCDs. The issue of Collider Stratification Bias is explained below.
Collider for a pair of variables is often a third variable influenced by both. Unlike a confounding factor, a collider may introduce a spurious association between the cause and effect, and thus negatively affect the outcome, that is, in this case being obese may protect against mortality, instead of causing mortality. Thus, a collider differs from a confounder (Figure 3). There are good reviews on collider bias with examples for the readers who would like to know more about on this subject (Day et al., 2016; Tönnies et al., 2022). However, it has been shown that collider bias alone cannot fully explain obesity paradox. Collider bias may explain only a small discrepancy between the association and the casual effect observed (Sperrin et al., 2016). Collider bias must also be strong enough to have a consistent association reversing the casual effect (Glymour & Vittinghoff, 2014). Last, when the population is unselected, collider bias may not work (Flegal et al., 2013). We know that is not the case with obesity.
FIGURE 3.
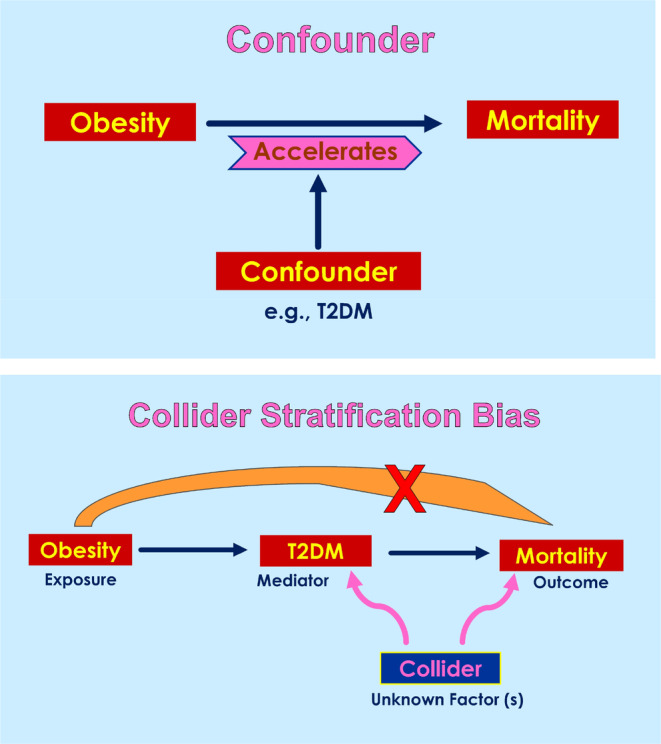
Confounder versus Collider Stratification Bias. Unlike a confounding factor, a collider may introduce a spurious association between the cause and effect, and thus negatively affect the outcome, that is, in this case obesity may protect against mortality, instead of causing mortality.
4. OBESITY PARADOX VERSUS BMI PARADOX
If we use BMI as the benchmark, then we will observe a J‐shaped relation between BMI and mortality risk in subjects with no CVD, with optimum BMI being between 20 and 25 Kg/m2. But in subjects with established CVD, this relation between BMI and mortality risk becomes U‐shaped with the optimum range of BMI shifting to the right, 25–30 Kg/m2. However, this phenomenon could not be seen if we plot waist circumference (WC in cm) instead of BMI in the same population (Figure 4) (Antonopoulos & Tousoulis, 2017). Based on this observation critiques say what we see is BMI paradox, not obesity paradox. However, we knew that abdominal or visceral adiposity leads to deleterious metabolic disturbances, and subcutaneous fat accumulation has a benign effect on cardiometabolic risk (Blüher, 2020; Liu et al., 2011; Neeland et al., 2013; Sam, 2018). Several anthropometric indices independent of obesity paradox have been proposed (Table 2; Sommer et al., 2020; Wu et al., 2021; Gažarová et al., 2022; Li et al., 2024). These can be used in routine clinical practice. Sophisticated imaging analysis gives better information about distribution of fat depots in the body (Table 2) but are not usable routinely in the clinics.
FIGURE 4.
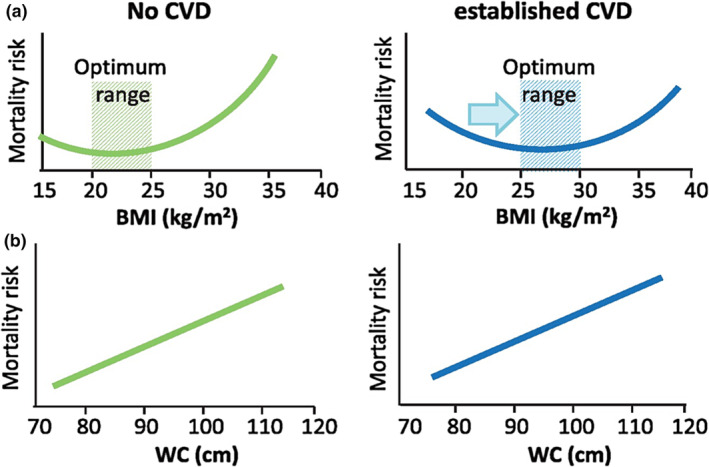
Association among BMI, waist circumference, and mortality risk. When BMI is used as the benchmark of obesity, the mortality risk takes a J‐ or U‐shaped curve in patients with CVD or without CVD, respectively. The optimum range of BMI also shifts from 20–25 to 35–30 Kg/m2. However, when the waist circumference (WC) is taken as the parameter, the association with mortality risk is linear in both groups of subjects. Reproduced with permission from Antonopoulos & Tousoulis. (2017). Oxford University Press License No. 5812090316656 dated June 18, 2024.
TABLE 2.
Assessment of overweight and obesity independent of BMI.
| Clinically usable anthropometric indices independent of BMI |
| Waist circumference (WC) |
| Waist‐to‐hip ratio (WHR) |
| Waist‐to‐height ratio (WHtR) |
| Waist‐to‐hip‐to‐height ratio (WHHR) |
| Increased Body Fat Percent |
| Imaging indices for information on fat distribution (Not usable routinely) |
| Volumetric analysis of body fat depots (CT or MRI) |
| Intramuscular fat accumulation (Independent risk factor for CVD) |
| Ectopic fat accumulation (Bone marrow or liver) |
5. CRF INFLUENCES OBESITY RISK
Cardio‐respiratory fitness (CRF) is the measure of the ability of circulatory and respiratory systems to supply oxygen to skeletal muscle mitochondria for energy production needed during sustained physical activity (Carrad et al., 2022; Franklin et al., 2023; Raghuveer et al., 2020). In 2016 the American Heart Association published an official document advocating that CRF, quantified as VO2 max/peak is a clinical vital sign, and advised that it should be routinely assessed as part of clinical practice (Ross et al., 2016). Meta‐analysis showed that CRF is a quantitative predictor of all‐cause mortality and cardiovascular events in healthy men and women (Ezzatvar et al., 2021; Fosstveit et al., 2024; Jiménez‐Pavón et al., 2019; Kodama et al., 2009; Lang et al., 2024). A cross‐sectional study from Germany showed that higher CRF is strongly associated with lower cardiovascular risk factors in firefighters, who suffer cardiovascular events on duty (Strauss et al., 2021). Recent studies also confirmed the relationship between CRF, cardiovascular risk factors, atherosclerosis, and morbidity and mortality (Chu et al., 2020; Lang et al., 2024).
Studies aimed at understanding the relationship between CRF, obesity, body composition, cardiometabolic risk and all‐cause mortality, and obesity paradox brought out interesting observations. For instance, examination of leanness and the hazards of obesity vis‐à‐vis CRF revealed that the health benefits of leanness are limited to fit subjects, and being fit may reduce the hazards of obesity and improve the quality of life (Blair et al., 1996; Flesaker et al., 2021; Lee et al., 1999). A cohort study involving 1 million men provided evidence for association between low levels of CRF and obesity with later risk of chronic disability due to CVD. The study suggested that preventive action may begin at young ages and include promotion of CRF and healthy body weight (Henriksson et al., 2020 Eur Heart J). A prospective observational study examined the relationship between low CRF, and mortality in subjects with normal weight, overweight and obesity, and reported that low CRF was a strong and independent predictor of CVD and all‐cause mortality, and of comparable importance with diabetes mellitus and other CVD risk factors (Wei et al., 1999 JAMA). A prospective study in women revealed that low CRF and higher BMI were independently associated with incident T2DM, with the protective effect of CRF seen in individuals who were overweight or obese (Sui et al., 2008). Even a moderate intensity exercise training improved CRF in women (Branch et al., 2000).
Thus, being fit is more important than losing weight in terms of lowering CVD mortality risk. Unfit subjects with obesity have almost two‐fold higher CVD risk compared to obese, but fit individuals. Subjects who are fit, but obese (fat but fit) have lower CVD risk compared to normal weight, but unfit individuals, as assessed by relative risk of all‐cause mortality by CRF levels (Hainer et al., 2009). So, BMI alone used as benchmark of obesity cannot identify the CVD risk.
6. METABOLICALLY HEALTHY OBESITY
Recently, the concept that metabolically healthy obesity (MHO) phenotype exists has gained ground. MHO is defined as subjects with BMI of 30.0 devoid of signs of metabolic disorders, such as high blood pressure, high fasting blood glucose, low high‐density lipoprotein cholesterol, or high triglycerides. Wang et al. (2023) reported that among the 20,430 participants in 10 National Health and Nutrition Examination Survey (NHANES) cycles between 1999–2018 and 2017–2018, about 10.6%–15.0% US adults exhibited MHO phenotype. Figure 5 shows the physical, physiological and molecular characteristics of MHO versus metabolically unhealthy obesity (MUHO) (Chait & den Hartigh, 2020). Although MHO appears to be in a transient state, the fact it can be promoted by exercise is gaining ground (Ortega et al., 2018; Su et al., 2022; Tsatsoulis & Paschou, 2020). There is evidence that conversion between metabolically healthy and unhealthy obesity is possible from midlife to late life in all BMI categories (Ler et al., 2024), thus making this phenomenon worth probing further. Several studies documented the beneficial effects of regular exercise on regulation of the function of white and brown fat depots (Dewal & Stanford, 2019; Garriston & Boudina, 2021; Honkala et al., 2020; Jin et al., 2024; Lee et al., 2019; Stroh & Stanford, 2023; Vissers et al., 2013). Figure 6 shows how exercise remodels white and brown adipose tissue in individuals with diet‐induced obesity (Garriston & Boudina, 2021). Excellent review articles are available on the remodeling of white and brown adipose tissue and its role in energy metabolism, pathophysiology of metabolic disorders, and therapeutic drug development (Auger & Kajimura, 2023; Choe et al., 2016; Magro & Dias, 2024; Sakaguchi, 2024).
FIGURE 5.
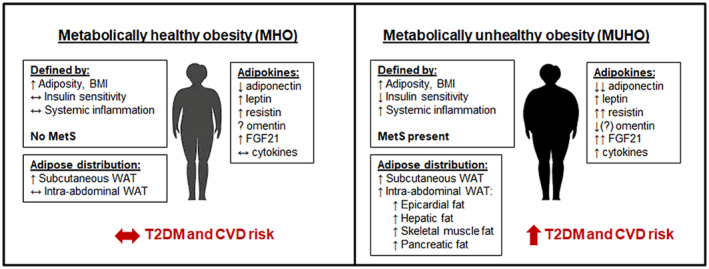
Metabolically healthy obesity (MHO) versus metabolically unhealthy obesity (MUHO). Note the differences between the two groups in insulin sensitivity, systemic inflammation, distribution of adipose tissue, and levels of adipokines. Figure reproduced from Chait & den Hartigh. (2020), under Creative Commons CC BY 4.0 Attribution 4.0 International.
FIGURE 6.
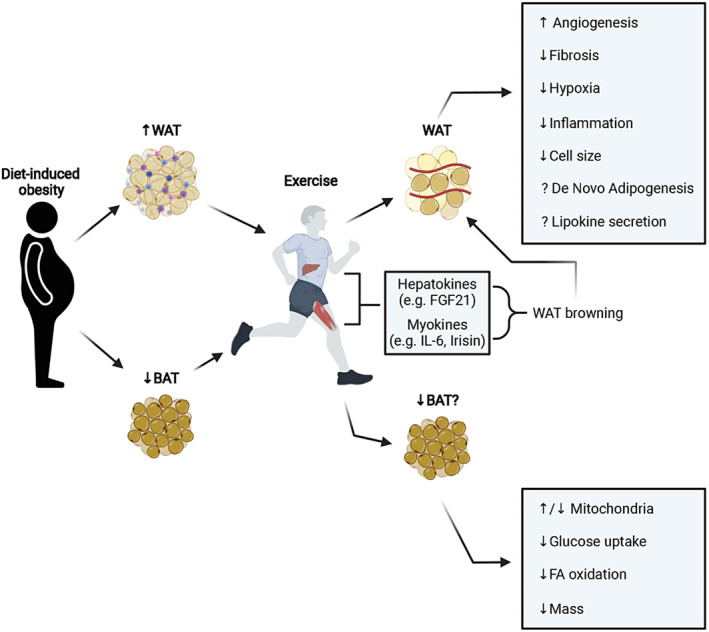
Remodeling of white and brown adipose tissues by exercise in subjects with diet‐induced obesity. As shown, in both types of adipose tissue, exercise has significant effect in modulating structure and function at the cellular and molecular levels. BAT, brown adipose tissue. WAT, white adipose tissue; FA, fatty acid; FGF21, fibroblast growth factor 21; IL‐6, interleukin‐6. Reproduced from Garriston & Boudina, (2021), under the creative commons attribution license (CC‐BY).
Conversely, there are subjects with metabolic obesity but with normal weight, the Metabolically obese, normal weight (MONW). The prevalence of MONW varies from 5%–45% depending on social and demographic factors as well as in defining the parameters (Conus et al., 2007). The MONW phenotype is associated with high prevalence of cardiometabolic dysregulation, metabolic syndrome, and cardiovascular risk factors (Table 3). In women, MONW is independently associated with increased risk of cardiometabolic mortality (Romero‐Corral & Somers, 2010). Table 4 lists the cardiometabolic abnormalities to be evaluated in all types of subjects with obesity to know which category they belong to (Wildman et al., 2008). To be considered as MONW phenotype, the subject should have BMI <25 kg/m2 and two or more cardiometabolic abnormalities.
TABLE 3.
Metabolic characteristics of MONW and MHNW individuals.
| Parameter | MONW | MHNW |
|---|---|---|
| Body mass index | Low | Low |
| Visceral fat | High | Low |
| Fat mass (as % of body mass) | High | Low |
| Lean body mass | Low | High |
| Insulin sensitivity | Low | High |
| Liver fat | High | Low |
| Serum triglycerides | High | Low |
Adopted from Karelis et al. (2004).
TABLE 4.
Cardiometabolic abnormalities to be considered in patients for risk assessment.
| Cardiometabolic abnormalities to be considered in patients | |
|---|---|
| 1 | High blood pressure ≥130/85 mm Hg or use of antihypertensive medications |
| 2 | High fasting serum triglyceride levels ≥150 mg/dL |
| 3 | Low HDL cholesterol level <40 mg/dL (men) or <50 mg/dL (women) or use of statins |
| 4 | High blood glucose levels ≥100 mg/dL (fasting) or use of anti‐diabetic medications |
| 5 | Insulin resistance: HOMA‐IR >5.13 (90th percentile) |
| 6 | Systemic inflammation: hsCRP level >0.1 mg/L (90th percentile) |
Adopted from Wildman et al. (2008).
7. LEAN DIABETES
7.1. Epidemiology and pathophysiology of lean diabetes
Although obesity is considered as the driver of T2DM, it is now recognized that a significant proportion of patients with diabetes are not obese, leading to the term Lean Diabetes (LD). LD is also known as Atypical Diabetes, Malnutrition‐related Diabetes, Tropical Diabetes, and by other names. LD does not meet the classification of T2DM given by the American Diabetes Association/World Health Organization (n.d.). In fact, as described below, LD may be a hybrid of T1DM and T2DM. Epidemiologically, LD is prevalent in men of Asian or African ancestry, often with history of childhood nutritional insults (Faraz et al., 2021; Kibirige et al., 2022). Nutritional insults in general refer to undernutrition or overnutrition or deficiency of certain components of the diet, such as micronutrients (Hsu & Tain, 2019). The prevalence of LD is also rising rapidly in the United States. Over a five‐year period (2015–2020), there was a 17.8% increase in LD in adults as compared to 2.1% increase in diabetes among people with overweight or obesity. The increase in the prevalence of LD in the United States is attributed to larger increases among women and people of color (Adesoba & Brown, 2023).
Typically, LD has an early age of onset. LD patients do not develop ketosis on withdrawal of insulin, but they might have higher total CVD and non‐CVD mortality vs. obese diabetics (reviewed in: George et al., 2015; Olaogun et al., 2020; Kishore, 2022; Salvatore et al., 2023). In addition, risk of hypoglycemia, and death is higher in LD patients. Morphologically, large adipocytes can be found in Asian LD patients associated with low levels of adiponectin and fatty acid breakdown, that age faster (cellular senescence). Thus, LD adipocytes switch from “fat storage” to “fat spillage”, negatively affecting CV system. LD patients also have higher HbA1c, fasting and post‐prandial blood glucose levels as compared to obese diabetics (reviewed in: Faraz et al., 2021; Kishore, 2022; Kibirige et al., 2022). Furthermore, microvascular complications of diabetes (e.g., retinopathy), nephropathy, and neuropathy are more common among male LD patients. The prevalence of LD is steadily increasing among Asians, especially in South Asia. In a prospective study sponsored by the Indian Council of Medical Research (ICMR), involving nine centers in India, the prevalence of LD varied from 11% to 25% (Das, 1993, 1994). The leanness in patients of this ICMR study persisted even after 5 years of follow up period. Thus, it seems leanness was the inherent characteristic of these individuals.
7.2. Ethnic differences in insulin sensitivity, insulin secretion, and body fat distribution
Several studies documented significant and clear ethnic differences in insulin sensitivity, insulin secretion, and body fat distribution, which incidentally correlates with the manifestation of LD with increased risk for developing CVD in certain ethnic populations. For instance, healthy Asian Indians have significantly greater abdominal and visceral fat, and insulin resistance compared to age and BMI matched Caucasians (Chandalia et al., 2007; Raji et al., 2001). Similarly, genetic background of Africans and East Asians makes them more and differentially susceptible to diabetes than Caucasians (Kodama et al., 2013). Interestingly, lean, non‐diabetic Asian Indians have decreased insulin sensitivity and insulin clearance and raised leptin levels compared to Caucasians and Chinese subjects (Liew et al., 2003). In fact, among lean Asians, Chinese are the most insulin sensitive whereas Asian Indians are the least insulin sensitive (Tan et al., 2015). These basic ethnic differences are directly reflected in the high prevalence of diabetes in South Asians or Asian Indians, as compared to Chinese, Caucasians and Blacks, irrespective of where they are living (Gujral et al., 2013; Kanaya et al., 2014; Ma & Chan, 2013; Narayan & Kanaya, 2020; Narayan et al., 2021). These studies underscore the importance of ethnicity while studying obesity and diabetes, as well as while treating these conditions in the clinics. In this respect, perhaps, obesity and insulin sensitivity, or resistance stand out as compared to other NCDs.
7.3. Evolutionary basis for low lean mass in Asian Indians
As illustrated in Figures 7 and 8, modern‐day South Asians have lower lean mass (organ and muscle mass) relative to those in Europe or Americas (Rush et al., 2009), which makes them prone to develop metabolic disorders at a lower BMI than other populations. People living in the Indian subcontinent were among first few cultures that adopted agriculture in early Holocene (about 9000 years ago; Pomeroy et al., 2019). This led to transition of lifestyle from hunter‐gatherer to farming, resulting in gradually decreasing physical stature and a lack of significant increase in lean mass. This in turn effectively decreased the metabolic capacity of modern‐day Asian Indians. In line with this, anthropometric evaluation of skeletons of Asian Indians spanning over the past 11,000 years showed gradually decreased stature‐adjusted lean mass. Thus, the modern Indians are the result of genotypic and phenotypic conditioning that occurred over thousands of years, which made them susceptible for conditions such as LD, irrespective of where they live now (Wells et al., 2016). This phenotype of Asian Indians has been well documented in recent studies (Bild et al., 2002; Kanaya et al., 2014; Bancks et al., 2021).
FIGURE 7.
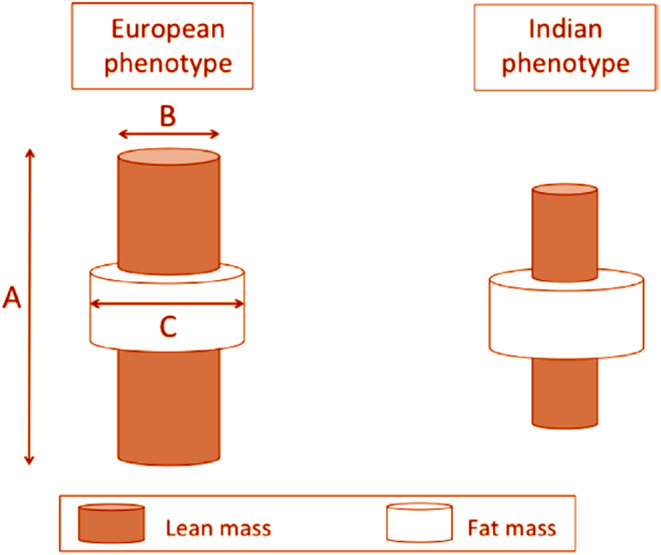
For a given Level of BMI, Asian Indians have higher proportion of fat mass in their body as compared to Westerners. The length (a) and cross‐sectional area (b) of an internal cylinder of lean mass is considered a marker for metabolic capacity, whereas the volume of the external cylinder of fat mass (c) is considered as a marker of metabolic load. Reproduced from Wells et al. (2016), under creative commons attribution 4.0 International CC‐BY 4.0.
FIGURE 8.
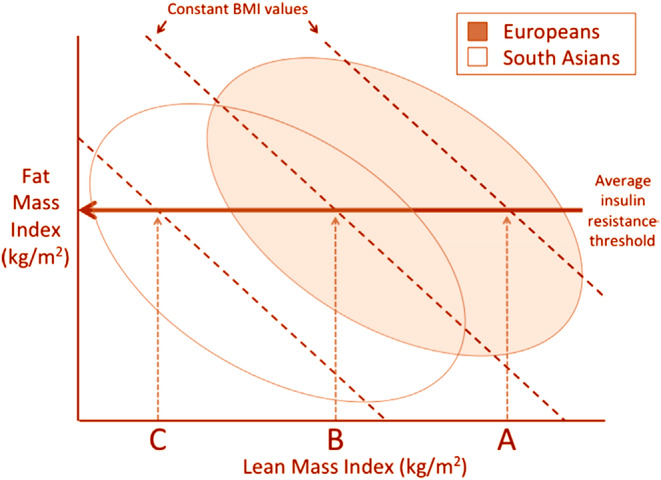
Plot of fat mass index (Fat mass divided by height squared) and lean mass index (Lean mass divided by height squared) in Europeans versus South Asians. Above a certain threshold of fat mass index, insulin resistance develops. Since Asians have lower lean mass, they develop insulin resistance at lower levels of BMI compared to Europeans. Reproduced from Wells et al. (2016) under creative commons attribution 4.0 International CC‐BY 4.0.
7.4. Diagnosis and management of lean diabetes
Unlike T1DM or T2DM, there are no specific guidelines for the diagnosis and clinical management of LD (Faraz et al., 2021; Kishore, 2022). Ironically, despite the rising prevalence of LD, a search of literature does not provide insights into the clinical management of LD. Diagnosis of LD is based on determination of BMI, and assessment of overweight and obesity independent of BMI as shown in Table 2, along with the determination of fasting and postprandial blood glucose levels. If possible, precise assessment of lean body mass vs. fat percentage using image technologies can be instituted (Table 2; Brunetti, 2007). Once the provisional diagnosis of LD is established, it is advisable to assess the insulin secreting capacity of the patients by biochemical assay for C‐peptide levels in plasma (Jones & Hattersley, 2013). Additional tests include determination of insulin sensitivity and/or insulin resistance with the technologies available in the clinics (Muniyappa et al., 2021).
Treatment of LD consists of two components, namely, to induce insulin secretion and to reduce insulin resistance. A South Asian Task Force comprising endocrinologists from India, Pakistan, Bangladesh, Nepal, Sri Lanka, Afghanistan, and the Maldives evaluated the use of GLP‐1 receptor agonists in the management of T2DM in South Asia, where LD is prevalent (Karla et al., 2019). In 2020 a clinical study was registered by researchers at the University of Leeds, United Kingdom to evaluate the combination therapy with Liraglutide (injectable GLP‐1 receptor agonist) and Pioglitazone (orally administered glitazone) in LD. The basis for this clinical trial is Liraglutide increases insulin secreting power of the pancreas, while Pioglitazone reduces resistance to insulin action Clinical Trials, NCT04657939 (n.d.).
7.5. Limitation of BMI as a measure of adiposity across populations
The data and studies presented above convey that BMI is not a reliable measure of adiposity and cardiometabolic risk, especially when considering ethnic populations, such as Asian Indians. Chittaranjan S. Yajnik, M.D. of King Edward Memorial Hospital Research Center, Pune, India, and John S. Yudkin, FRCP of International Health and Medical Education Center, University College of London, United Kingdom, who did extensive research work on low birth weight and insulin resistance and diabetes in later life illustrated the limitation of BMI as a measure of adiposity by comparing the BMI and adiposity of their own bodies (Figure 9; Yajnik & Yudkin, 2004). This marked difference between the Asian Indians and Caucasians in body composition has been attributed to evolutionary factors as discussed above.
FIGURE 9.
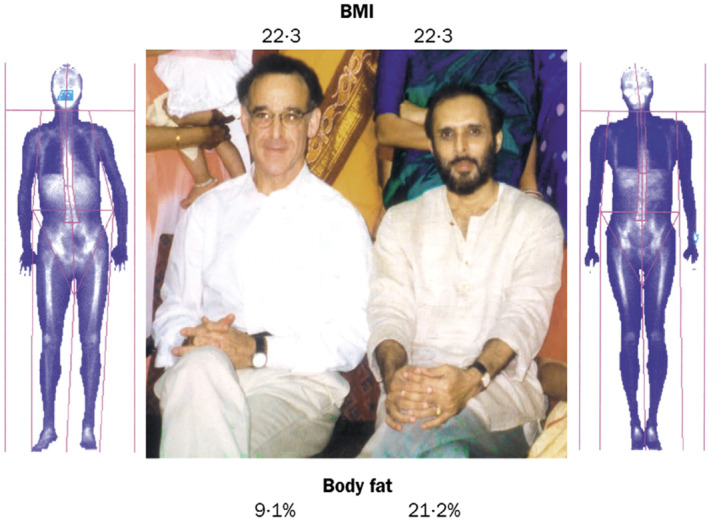
Limitations of BMI as a measure of adiposity in South Asians versus Caucasians. Two physician‐scientists, Dr. John S. Yudkin (left) and Dr. Chittaranjan S. Yajnik (right) share a near identical BMI (22.3), but as dual x‐ray absorptiometry imagery shows where the similarity ends. Dr. Yajnik has substantially more body fat (21.2%) than Dr. Yudkin (9.1%). Lifestyle may also be relevant. Dr. Yudkin runs marathons whereas Dr. Yajnik's main exercise is running to beat the closing doors of the elevator in the hospital every morning. The contribution of genes to such adiposity is yet to be determined, although the possible relevance of intrauterine undernutrition is supported by Dr. Yajnik's low birthweight. Thus, this image illustrates the role of both birthweight and lifestyle practices in body composition. Image and legend are reproduced with permission from: Yajnik CS, Yudkin JS, Lancet, 2004. Elsevier License No. 5812360591103 dated June 19, 2024.
7.6. Thin‐obese paradox babies of India
Because of the above evolutionary adaptation Asian Indian babies are born with a “thin‐fat phenotype,” comprising of thin muscles and relatively more adipose tissue (Deshmukh et al., 2011). This is not the case with Caucasian babies. A comparative study between the Indian versus British newborn babies confirmed the differences in phenotypes (Yajnik et al., 2003). As shown in Figure 10, the Indian newborns have low muscle mass and small abdominal viscera but preserved subscapular skinfold. This composition may persist postnatally thus predisposing to insulin resistance state. Because of this unique phenotype, Indian babies are metabolically programmed to develop T2DM early in adulthood, or even LD.
FIGURE 10.
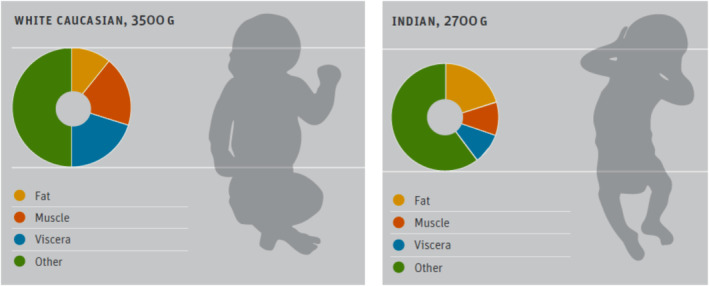
Thin‐fat Indian baby. A schematic diagram to compare the body composition of Indian and white caucasian babies. Indian babies were ~ 800 g lighter; muscle thin but more adipose tissue compared to the white babies. Reproduced from: Deshmukh et al., (2011) Sight Life Mag under Creative Commons Attribution 4.0 International License.
7.7. Maternal factors programming fetal cardiometabolic development
Although the existence of cross talk between adipose tissue and placenta in obese and gestational diabetes mellitus via exosomes has been known for a while (Jayabalan et al., 2017), recently, a novel possibility that an adiposity‐related maternal factor crossing the placenta to reprogram fetal cardiometabolic development pathways has been jointly reported by Robert J. Freishtat and his team at the Children's Research Institute, Washington DC and Chittaranjan Yajnik and his group at the King Edward Memorial Hospital, Pune, India (Kunte et al., 2024). They identified adipocyte‐derived exosomes that can cross the placenta, and their microRNA contents are predicted to alter developmental pathways of gene expression. Thus, their results suggest that adipocyte‐derived small extracellular vesicular (ADsEV) microRNAs in mothers are potential regulators of fetal adiposity. This joint project was funded by the US National Institutes of Health. Furthermore, the Pune Maternal Nutrition Study conducted by Dr. Chittaranjan S. Yajnik brought out the critical role played by the maternal diet and micronutrient status, and physical workload, during the pregnancy on the size of the newborn, development of insulin resistance, and cardiometabolic risk of the offspring in adulthood (Chittranjan, 2020; Fall, 2009; Tomar et al., 2015; Yajnik et al., 2003, 2008). This novel concept, which is a work in progress, has the potential to provide insights into programming fetal cardiometabolic development or risk by maternal factors. This in turn may prompt a paradigm shift in the global war against obesity from nutritional and lifestyle changes in adulthood to prenatal, perinatal, postnatal maternal and fetal/infant nutrition and care.
8. SUMMARY
Adiposity per se is not unhealthy, but its regional distribution, the type of fat expansion, and adaptation to excess caloric intake do matter for the ultimate pathophysiological roles. The existence of metabolically benign adipose tissue can largely explain the obesity paradox. The role of CRF in influencing obesity paradox is becoming obvious by allowing excess adiposity without risk. Further research into deciphering these potential possibilities is needed. Such studies may pave the way to develop novel molecular and/or imaging technologies for accurate phenotyping of patients to capture properly the trajectories of mortality in several disease conditions. Obviously, more clinical data with evaluation of current therapeutic methods are needed to manage LD.
9. CONCLUSION
The obesity paradox may appear as an artifact. But there is substantial evidence for the existence of it. We can feel its presence in the clinic, especially in relations to certain NCDs. Based on our current knowledge, we may not be able to provide an accurate mechanistic explanation for obesity paradox. There are several missing pieces of the jigsaw puzzle. Obviously, more controlled clinical studies supported by molecular and image technologies are needed to understand the phenomenon of obesity paradox at the cellular level. The clinical characteristics, pathophysiology, management, and mortality in LD pose a new challenge for practicing physicians. Understanding the emerging concept of role of maternal factors in programming cardiometabolic risk in fetal stage will ultimately open the doors for an effective control of prevalence of global obesity and NCDs. Thus, it appears obesity is a puzzle that fits into Rumsfeld Theorem (Figure 11). Application of AI may enhance our ability to understand obesity in its true sense and how elusive it can be. In this context, obesity paradox is like the Schrödinger's Cat—it is neither fact nor fiction. It is the sum of both until we analyze and understand it more thoroughly.
FIGURE 11.
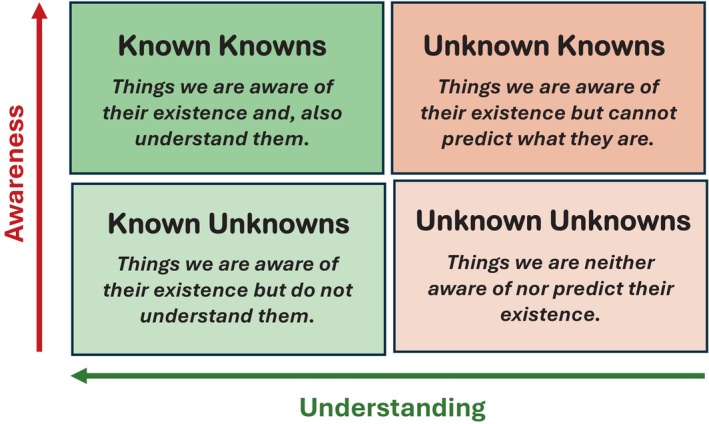
Modified rumsfeld knowledge and awareness matrix to obesity. The Rumsfeld knowledge matrix spans from “known knowns,” “known unknowns,” and “known unknowns” to “unknown unknowns.” Thus, it provides a matrix for awareness and Understanding. This model and process is very applicable to the science of obesity. The model presented here is modified by the author to suit the subject of obesity paradox.
FUNDING INFORMATION
No federal or industry support in preparing this review article.
CONFLICT OF INTEREST STATEMENT
Part of this article was based on a CME lecture given by the author at the 39th Annual Convention of the American Association of Physicians of Indian Origin (AAPI) in 2021 and appeared as non‐peer reviewed synopsis of the lecture in the publication of AAPI. In addition to his academic appointment, the author is the Co‐Founder, President, Chief Executive Officer, and Chief Scientific Officer of ePurines, Inc., a therapeutic drug development startup in the University of Utah Research Park, Salt Lake City, Utah. ePurines is a spin out of the academic research with patents conducted by the author and his colleagues and is focusing on developing anti‐obesity drugs among others. However, there are no competing interests or industrial support in preparing or publishing this article.
ETHICS STATEMENT
Not applicable.
DISCLAIMER
No artificial intelligence (AI) or AI tools have been used in the preparation of this review article.
Kishore, B. K. (2024). Reverse epidemiology of obesity paradox: Fact or fiction? Physiological Reports, 12, e70107. 10.14814/phy2.70107
DATA AVAILABILITY STATEMENT
Not applicable—no new data generated.
REFERENCES
- Adesoba, T. P. , & Brown, C. C. (2023). Trends in the prevalence of lean diabetes among U.S. adults, 2015‐2020. Diabetes Care, 46(4), 885–889. [DOI] [PubMed] [Google Scholar]
- Akhter, N. , Begum, K. , Nahar, P. , Cooper, G. , Vallis, D. , Kasim, A. , & Bentley, G. R. (2021). Risk factors for non‐communicable diseases related to obesity among first‐ and second‐generation Bangladeshi migrants living in north‐east or south‐east England. International Journal of Obesity, 45(7), 1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque, D. , Nóbrega, C. , Manco, L. , & Padez, C. (2017). The contribution of genetics and environment to obesity. British Medical Bulletin, 123, 159–173. [DOI] [PubMed] [Google Scholar]
- AMA Policy # H‐440‐842 Recognition of Obesity as a Disease. American Medical Association Council on Science and Public Health. https://policysearch.ama‐assn.org/policyfinder/detail/H‐440‐842?uri=%2FAMADoc%2FHOD.xml‐0‐3858.xml
- Antonopoulos, A. S. , & Tousoulis, D. (2017). The molecular mechanisms of obesity paradox. Cardiovascular Research, 113, 1074–1086. [DOI] [PubMed] [Google Scholar]
- Assaf, R. , & Antoun, J. (2021). Review of obesity paradox. American Journal of Hospital Medicine, 5(3), 1–13. [Google Scholar]
- Auger, C. , & Kajimura, S. (2023). Adipose tissue remodeling in pathophysiology. Annual Review of Pathology, 18, 71–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancks, M. P. , Bertoni, A. G. , Carnethon, M. , Chen, H. , Cotch, M. F. , Gujral, U. P. , Herrington, D. , Kanaya, A. M. , Szko, M. , Vaidya, D. , & Kandula, N. R. (2021). Association of diabetes subgroups with race/ethnicity, risk factor burden and complications. The MASALA and MESA studies. The Journal of Clinical Endocrinology and Metabolism, 106, e2106–e2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhurosy, T. , & Jeewon, R. (2014). Overweight and obesity epidemic in developing countries: A problem with diet, physical activity, or socioeconomic status. Scientific World Journal, 2014, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bild, D. E. , Bluemke, D. A. , & Burke, G. L. (2002). Multi‐ethnic study of atherosclerosis: Objectives and design. American Journal of Epidemiology, 156, 871–881. [DOI] [PubMed] [Google Scholar]
- Blair, S. N. , Kampert, J. B. , Kohl, H. W., 3rd , Barlow, C. E. , Macera, C. A. , Paffenbarger, R. S. , & Gibbons, L. W. (1996). Influence of cardiorespiratory fitness and other precursors on cardiovascular disease and all‐cause mortality in men and women. JAMA, 276(3), 205–210. [PubMed] [Google Scholar]
- Blüher, M. (2020). Metabolically healthy obesity. Endocrine Reviews, 41(3), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch, J. D. , Pate, R. R. , & Bourque, S. P. (2000). Moderate intensity exercise training improves cardiorespiratory fitness in women. Journal of Women's Health & Gender‐Based Medicine, 9(1), 65–73. [DOI] [PubMed] [Google Scholar]
- Braun, N. , Gomes, F. , & Schuetz, P. (2015). “The obesity paradox” in disease—Is the protective effect of obesity true? Swiss Medical Weekly, 145, w14265. [DOI] [PubMed] [Google Scholar]
- Brunetti, P. (2007). The lean patient with type 2 diabetes: Characteristics and therapy challenges. International Journal of Clinical Practice, 61, 3–9. [DOI] [PubMed] [Google Scholar]
- Carrad, J. , Geurini, C. , Appenzeller‐Herzog, C. , Infanger, D. , Königstein, K. , Streese, L. , Hinrichs, T. , Hanssen, H. , Gallart‐Ayala, H. , Vanisevic, J. , & Schmidt‐Trucksäss, A. S. (2022). The metabolic signature of cardiorespiratory fitness: A systematic review. Sports Medicine, 52(3), 527–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait, A. , & den Hartigh, L. J. (2020). Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Frontiers in Cardiovascular Medicine, 7, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandalia, M. , Lin, P. , Seenivasan, T. , Livingston, E. H. , Snell, P. G. , Grundy, S. M. , & Abate, N. (2007). Insulin resistance and body fat distribution in south Asian men compared to Caucasian men. PLoS One, 2(8), e812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittranjan, Y. (2020). Vitamin B12: An intergenerational story. Nestle Nutrition Institute Workshop Series, 93, 91–102. [DOI] [PubMed] [Google Scholar]
- Choe, S. S. , Huh, J. Y. , Hwang, I. J. , Kim, J. I. , & Kim, J. B. (2016). Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Frontiers in Endocrinology, 7, 30. 10.3389/fendo.2016.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, D. J. , Rifai, M. A. , Virani, S. S. , Brawner, C. A. , Nasir, K. , & Al‐Mallah, M. A. (2020). The relationship between cardiorespiratory fitness, cardiovascular risk factors and atherosclerosis. Atherosclerosis, 304, 44–52. [DOI] [PubMed] [Google Scholar]
- Clinical Trials.gov ID: NCT04657939 Targeting beta‐cell failure in lean patients with type 2 diabetes (Lean‐DM). Sponsor: University of Leads. Last updated 2023‐02‐27. https://clinicaltrials.gov/study/NCT04657939
- Conus, F. , Rabasa‐Lhoret, R. , & Péronnet, F. (2007). Characteristics of metabolically obese normal‐weight (MONW) subjects. Applied Physiology, Nutrition, and Metabolism, 32(1), 4–12. [DOI] [PubMed] [Google Scholar]
- Das, S. (1993). Lean type 2 diabetes mellitus: Profile, peculiarities, and paradox. In Medicine Update vol 18, chapter 12 (pp. 94–104). Semantic Scholar. [Google Scholar]
- Das, S. (1994). Identity of lean‐NIDDM: Clinical, metabolic, and hormonal status. In Kochupillai N. (Ed.), Advan Endocrin Metab Diab vol 2, McMillan. Delhi. [Google Scholar]
- Day, F. R. , Loh, P.‐R. , Schott, R. A. , Ong, K. K. , & Perry, J. R. B. (2016). A robust example of collider bias in a genetic association study. American Journal of Human Genetics, 98(2), 392–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh, U. S. , Lubree, H. , & Yajnik, C. S. (2011). Role of maternal micronutrients. Intrauterine programming of non‐communicable diseases. Sight and Life Magazine, 25, 16–22. [Google Scholar]
- Dewal, R. S. , & Stanford, K. I. (2019). Effects of exercise on brown and beige adipocytes. Biochimica et Biophysica Acta, Molecular and Cell Biology of Lipids, 1864, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs, R. , Sawers, C. , Thompson, F. , Manyika, J. , Woetzel, L. , Child, P. , McKenna, S. , & Spathorou, A. (2014). Overcoming obesity: An initial economic analysis. Discussion Paper. McKinsey Global Institute . https://www.mckinsey.com/~/media/mckinsey/business%20functions/economic%20studies%20temp/our%20insights/how%20the%20world%20could%20better%20fight%20obesity/mgi_overcoming_obesity_executive_summary.pdf
- Donini, L. M. , Gennaro, C. S. , De Felice, M. R. , Rosano, A. , Pandolfo, R. , Balzo, V. D. , Cannella, C. , Ritz, P. , & Chumlea, W. (2012). A systematic review of the literature concerning the relationship between obesity and mortality in the elderly. The Journal of Nutrition, Health & Aging, 16(1), 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donini, L. M. , Pinto, A. , Giusti, A. M. , Lenzi, A. , & Poggiogalie, E. (2020). Obesity or BMI paradox? Beneath the tip of the iceberg. Frontiers in Nutrition, 7, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dramé, M. , & Godaert, L. (2023). The obesity paradox and mortality in older adults: A systematic review. Nutrients, 15, 1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitmann, S. , Matrai, P. , Hegyi, P. , Balasko, M. , Eross, B. , Dorogi, K. , & Petervari, E. (2024). Obesity paradox in older sarcopenic adults—A delay in aging: A systematic review and meta‐analysis. Ageing Research Reviews, 93, 102164. [DOI] [PubMed] [Google Scholar]
- Ejigu, B. A. , & Tiruneh, F. N. (2023). The link between overweight/obesity and noncommunicable diseases in Ethiopia: Evidence from Nationwide WHO STEPS survey 2015. International Journal of Hypertension, 2023, 2199853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante, A. , Haas, R. W. , & del Rincón, I. (2005). Paradoxical effect of body mass index on survival in rheumatoid arthritis. Archives of Internal Medicine, 165, 1624–1629. [DOI] [PubMed] [Google Scholar]
- Ezzatvar, Y. , Izquierdo, M. , Núñez, J. , Calatayud, J. , Ramirez‐Vélez, R. , & García‐Hermoso, A. (2021). Cardiorespiratory fitness measured with cardiopulmonary exercise testing and mortality in patients with cardiovascular disease: A systematic review and meta‐analysis. Journal of Sport and Health Science, 10(6), 609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall, C. (2009). Maternal nutrition: Effects on health in the next generation. The Indian Journal of Medical Research, 130(5), 593–599. [PubMed] [Google Scholar]
- Faraz, A. , Ashraf, H. , & Ahmad, J. (2021). Clinical features, biochemical profile, and response to standard treatment in lean, normal‐weight, and overweight/obese Indian type 2 diabetes patients. The Review of Diabetic Studies, 17(2), 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felisbino‐Mendes, M. S. , Cousin, E. , Malta, D. C. , Machado, I. E. , Ribeiro, A. L. P. , Duncan, B. B. , Schmidt, M. I. , Silva, D. A. S. , Glenn, S. , Afshin, A. , & Velasquez‐Melendez, G. (2020). The burden of non‐communicable diseases attributable to high BMI in Brazil, 1990–2017: Findings from the global burden of disease study. Population Health Metrics, 18(Suppl 1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal, K. M. , Kit, B. K. , Orpana, H. , & Graubard, B. I. (2013). Association of all‐cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta‐analysis. JAMA, 309(1), 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesaker, M. Q. , Serviente, C. , Troy, L. M. , & Witkowski, S. (2021). The role of cardiorespiratory fitness on quality of life in midlife women. Menopause, 28(4), 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosstveit, S. H. , Lohne‐Seiler, H. , Feron, J. , Lucas, S. J. E. , Ivarsson, A. , & Berntsen, S. (2024). The intensity paradox: A systematic review and meta‐analysis of its impact on the cardiorespiratory fitness of older adults. Scandinavian Journal of Medicine & Science in Sports, 34(2), e14573. [DOI] [PubMed] [Google Scholar]
- Franklin, B. A. , Wedig, I. , Sallis, R. E. , Lavie, C. J. , & Elmer, S. J. (2023). Physical activity and cardiorespiratory fitness as modulators of health outcomes: A compelling research‐based case presented to the medical community. Mayo Clinic Proceedings, 98(2), 316–331. [DOI] [PubMed] [Google Scholar]
- Fuentes, R. , Nilson, E. , Rezende, L. F. M. , Christofaro, D. G. D. , Silva, D. R. , Ferreo‐Hernández, Cristi‐Montero, C. , Marques, A. , Farías‐Valenzuela, C. , & Ferrari, G. (2023). Future burden of non‐communicable diseases attributable to overweight in Chile: A multistate life table modeling study. BMC Public Health, 23(1), 1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganipisetti, V. M. , & Bollimunta, P. (2023). Obesity and set‐point theory. In StatPearls. Bookshelf ID. StatPearls publishing: NBK592402. [PubMed] [Google Scholar]
- Garriston, J. D. , & Boudina, S. (2021). The effects of exercise on white and brown adipose tissue cellularity, metabolic activity, and remodeling. Frontiers in Physiology, 12, 772894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gažarová, M. , Bihari, M. , Lorková, M. , Lenártová, P. , Habánová, M. , & Hunag, L.‐T. (2022). The use of different anthropometric induces to assess the body composition of young women in relation to the incidence of obesity, sarcopenia and the premature mortality risk. International Journal of Environmental Research and Public Health, 19(19), 12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genné‐Bacon, E. (2014). Thinking evolutionarily about obesity. The Yale Journal of Biology and Medicine, 87(2), 99–112. [PMC free article] [PubMed] [Google Scholar]
- George, A. M. , Jacob, A. G. , & Fogelfeld, L. (2015). Lean diabetes mellitus: An emerging entity in the era of obesity. World Journal of Diabetes, 6(4), 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour, M. M. , & Vittinghoff, E. (2014). Commentary: Selection bias as an explanation for the obesity paradox: Just because it's possible doesn't mean it's plausible. Epidemiology, 25(1), 4–6. [DOI] [PubMed] [Google Scholar]
- Gujral, U. P. , Pradeepa, R. , Weber, M. B. , Narayan, K. M. V. , & Mohan, V. (2013). Type 2 diabetes in south Asians: Similarities and differences with white Caucasian and other populations. Annals of the New York Academy of Sciences, 1281(1), 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainer, V. , Toplak, H. , & Stich, V. (2009). Fat or fit: What is more important? Diabetes Care, 32(Suppl 2), S392–S397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson, H. , Henriksson, P. , Tynelius, P. , Esktedt, M. , Berglind, D. , Labayen, I. , Ruiz, J. R. , Lavie, C. J. , & Ortega, F. B. (2020). Cardiorespiratory fitness, muscular strength, and obesity in adolescence and later chronic disability due to cardiovascular disease: A cohort study of 1 million men. European Heart Journal, 41(15), 1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkala, S. M. , Motiani, P. , Kivelä, R. , Hemanthkumar, K. A. , Tolavanen, E. , Motiani, K. K. , Eskelinen, J.‐J. , Virtanen, K. A. , Kempainen, J. , Heiskanen, M. A. , Löyttyniemi, E. , Nuutila, P. , Kalliokoski, K. , & Hannukeainen, J. C. (2020). Exercise training improves adipose tissue metabolism and vasculature regardless of baseline glucose tolerance and sex. BMJ Open Diabetes Research & Care, 8, e000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, C.‐N. , & Tain, Y.‐L. (2019). The good, the bad, and the ugly of pregnancy nutrients and developmental programming of adult disease. Nutrients, 11(4), 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, F. B. (2011). Globalization of diabetes: The role of diet, lifestyle, and genes. Diabetes Care, 34(6), 1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovljevic, M. B. , & Milovanovic, O. (2015). Growing burden of non‐communicable diseases in the emerging health markets: The case of BRICS. Frontiers in Public Health, 3, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayabalan, N. , Nair, S. , Nuzhat, S. , Rice, G. E. , Zuñiga, F. A. , Sobrevia, L. , Leiva, A. , Sanhueza, C. , Gutierrez, A. , Lappas, M. , Freeman, D. J. , & Salomon, C. (2017). Cross talk between adipose tissue and placenta in obese and gestational diabetes mellitus pregnancies via exosomes. Frontiers in Endocrinology, 8, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez‐Pavón, D. , Lavie, C. J. , & Blair, S. N. (2019). The role of cardiorespiratory fitness on the risk of sudden cardiac death at the population level: A systematic review and meta‐analysis of the available evidence. Progress in Cardiovascular Diseases, 61(3), 279–287. [DOI] [PubMed] [Google Scholar]
- Jin, L. , Diaz‐Canestro, C. , Wang, Y. , Tse, M. A. , & Xu, A. (2024). Exerkines and cardiometabolic benefits of exercise: From bench to clinic. EMBO Molecular Medicine, 16, 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A. G. , & Hattersley, A. T. (2013). The clinical utility of C‐peptide measurement in the care of patients with diabetes. Diabetic Medicine, 30, 803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantar‐Zadeh, K. , Block, G. , Horwich, T. , & Fonarow, G. C. (2004). Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. Journal of the American College of Cardiology, 43(8), 1439–1444. [DOI] [PubMed] [Google Scholar]
- Kalantar‐Zadeh, K. , Block, G. , Humphreys, M. H. , & Kopple, J. D. (2003). Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney International, 63(3), 793–803. [DOI] [PubMed] [Google Scholar]
- Kalantar‐Zadeh, K. , Rhee, C. M. , Chou, J. , Ahmadi, S. F. , Park, J. , Chen, J. L. , & Amin, A. N. (2017). The obesity paradox in kidney disease: How to reconcile it with obesity management. Kidney International Reports, 2(2), 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya, A. M. , Herrington, D. , Vittingoff, E. , Ewing, S. K. , Liu, K. , Blaha, M. J. , Dave, S. S. , Qureshi, F. , & Kandula, N. R. (2014). Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: The MASALA and MESA studies. Diabetes Care, 37(6), 1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, J. H. , Kim, H. , Kim, J. , Seo, J.‐H. , Cha, S. , Oh, H. , Kim, K. , Park, S.‐J. , Kim, E. , Kong, S. , Kong, S. , Lee, J.‐H. , Bae, J. S. , Won, H.‐H. , Joung, J.‐G. , Yang, Y. J. , Kim, J. , & Park, W.‐Y. (2021). Interaction of genetic and environmental factors for body fat mass control: Observational study for lifestyle modification and genotype. Scientific Reports, 11, 13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor, N. (2021). Thin fat Obesity: The tropical phenotype obesity. [updated 2021 mar 14]. In Feingold K. R., Anwalt B., Boyce A., et al. (Eds.), Endotext. MDText.com, Inc. https://www.ncbi.nlm.nih.gov/books/NBK568563 [Google Scholar]
- Karelis, A. D. , St‐Pierre, D. H. , Conus, F. , Rabasa‐Lhoret, R. , & Poehlman, E. (2004). Metabolic and body composition factors in subgroups of obesity: What do we know? The Journal of Clinical Endocrinology and Metabolism, 89(6), 2569–2575. [DOI] [PubMed] [Google Scholar]
- Karla, S. , Das, A. K. , Sahay, R. K. , Bruah, M. P. , Tiwaskar, M. , Das, S. , Chatterjee, S. , Saboo, B. , Bantwal, G. , Bhattacharya, S. , Priya, G. , Chawla, M. , Brar, K. , Raza, S. A. , Amir, A. H. , Shrestha, D. , Somasundaram, N. , Katuland, P. , … Sumanatilleke, M. (2019). Consensus recommendations on GLP‐1 RA use in the management of type 2 diabetes mellitus: South Asian Taks force. Diabetes Therapy, 10, 1645–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibirige, D. , Seitoleko, I. , Lumu, W. , Jones, A. G. , Hattersley, A. T. , Smeeth, L. , & Nyirenda, M. J. (2022). Understanding the pathogenesis of lean non‐autoimmune diabetes in an African population with newly diagnosed diabetes. Diabetologia, 65(4), 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpi, F. , Webbler, L. , Musaigner, A. , Aitsi‐Selmi, A. , Marsh, T. , Rtveladze, K. , McPherson, K. , & Brown, M. (2014). Alarming predictions for obesity and non‐communicable diseases in the Middle East. Public Health Nutrition, 17(5), 1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore, B. K. (2022). Lean diabetes: Epidemiology, pathophysiology, and clinical management. Journal of American Association Physicians of Indian Origin, 2(1), 44–57. [Google Scholar]
- Kivimaki, M. , Strandberg, T. , Pentti, J. , Nyberg, J. P. , Frank, P. , Jokela, M. , Ervasti, J. , Suominen, S. B. , Vahtera, J. , Sipilä, P. N. , Lindbahm, J. V. , & Ferrie, J. E. (2022). Body‐mass index and risk of obesity‐related complex multimorbidity: An observational multicohort study. The lancet. Diabetes & Endocrinology, 10, 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama, K. , Tojjar, D. , Yamada, S. , Toda, K. , Patel, C. J. , & Butte, A. J. (2013). Ethnic differences in the relationship between insulin sensitivity and insulin response. Diabetes Care, 36(6), 1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama, S. , Saito, K. , Tanaka, S. , Maki, M. , Yachi, Y. , Asumi, M. , Sugawara, A. , Totsuka, K. , Shimano, H. , Ohashi, Y. , Yamada, N. , & Sone, H. (2009). Cardiorespiratory fitness as a quantitative predictor of all‐cause mortality and cardiovascular events in healthy men and women: A meta‐analysis. JAMA, 301(19), 2024–2035. [DOI] [PubMed] [Google Scholar]
- Koliaki, C. , Dalamaga, M. , & Liatis, S. (2023). Update on the obesity epidemic: After the sudden rise, is the upward trajectory beginning to flatten? Current Obesity Reports, 12, 514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunte, P. , Barberio, M. , Tiwari, P. , Sukla, K. , Harmon, B. , Epstein, S. , Bhat, D. , Authelet, K. , Goldberg, M. , Rao, S. , Damle, H. , Freishtat, R. J. , & Yajnik, C. (2024). Neonatal adiposity is associated with microRNAs in adipocyte‐derived extracellular vesicles in maternal and cord blood, a discovery analysis. International Journal of Obesity, 48, 403–413. [DOI] [PubMed] [Google Scholar]
- Lainscak, M. , von Haehling, S. , Doehner, W. , & Anker, S. D. (2012). The obesity paradox in chronic disease: Facts and numbers. Journal of Cachexia, Sarcopenia and Muscle, 3(1), 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, J. J. , Prince, S. A. , Mrucci, K. , Cadenas‐Sanchez, C. , Chaput, J.‐P. , Fraser, B. J. , Manyanga, T. , McGrath, R. , Ortega, F. B. , Singh, B. , & Tomkinson, G. R. (2024). Cardiorespiratory fitness is a strong and consistent predictor of morbidity and mortality among adults: An overview of meta‐analyses representing 20.9 million observations from 199 unique cohort studies. British Journal of Sports Medicine, 58(10), 556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. D. , Blair, S. N. , & Jackson, A. S. (1999). Cardiorespiratory fitness, body composition, and all‐cause and cardiovascular disease mortality in men. The American Journal of Clinical Nutrition, 69(3), 373–380. [DOI] [PubMed] [Google Scholar]
- Lee, S. , Norheim, F. , Langleite, T. M. , Gulseth, H. L. , Birkeland, K. I. , & Drevon, C. A. (2019). Effects of long‐term exercise on plasma adipokines levels and inflammation‐related gene expression in subcutaneous adipose tissue in sedentary dysglycemic, overweight men and sedentary normoglycemic men of healthy weight. Diabetologia, 62, 1048–1064. [DOI] [PubMed] [Google Scholar]
- Lenz, M. , Richter, T. , & Mühlhauser, I. (2009). The morbidity and mortality associated with overweigth and obesity in adulthood: A systematic review. Deutsches Ärzteblatt International, 106(40), 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ler, P. , Ojalehto, E. , Zhan, Y. , Finkel, D. , Aslan, A. K. D. , & Karlsson, I. D. (2024). Conversions between metabolically unhealthy and healthy obesity from midlife to late‐life. Epidemiology and Population Health, 48, 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Fu, Z. , & Zhang, W. (2024). Association of anthropometric measures with all‐cause and cause‐specific mortality in US adults: Revisiting the obesity paradox. MMC Public Health, 24(1), 929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew, C.‐F. , Seah, E.‐S. , Yeo, K.‐P. , Lee, K.‐O. , & Wise, S. D. (2003). Lean, nondiabetic Asian Indians have decreased insulin sensitivity and insulin clearance, and raised leptin compared to Caucasian and Chinese subjects. International Journal of Obesity, 27, 784–789. [DOI] [PubMed] [Google Scholar]
- Liu, C. , Wong, P. Y. , Chung, Y. L. , Chow, S. K.‐H. , Cheung, W. H. , Law, S. W. , Chan, J. C. N. C. , & Wong, R. M. Y. (2022). Deciphering the “obesity paradox” in the elderly: A systematic review and meta‐analysis of sarcopenic obesity. Obesity Reviews, 24(2), e13534. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Fox, C. S. , Hickson, D. , Bidulescu, A. , Carr, J. J. , & Taylor, H. A. (2011). Fatty liver, abdominal visceral fat and cardiometabolic risk factors: The Jackson heart study. Arteriosclerosis, Thrombosis, and Vascular Biology, 31(11), 2715–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobstein, T. , & Brinsden, H. (2014). Symposium report: The prevention of obesity and NCDs: Challenges and opportunities for governments. Obesity Reviews, 15(8), 630–639. [DOI] [PubMed] [Google Scholar]
- Lung, T. , Jan, S. , Tan, E. J. , Killedar, A. , & Hayes, A. (2019). Impact of overweight, obesity and severe obesity on life expectancy of Australian adults. International Journal of Obesity, 43(4), 782–789. [DOI] [PubMed] [Google Scholar]
- Ma, R. C. W. , & Chan, J. C. N. (2013). Type 2 diabetes in east Asians: Similarities and differences with populations in Europe and the United States. Annals of the New York Academy of Sciences, 1281(1), 64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro, B. S. , & Dias, D. P. M. (2024). Brown and beige adipose tissue: New therapeutic targets for metabolic disorders. Health Science Reviews, 10, 100148. [Google Scholar]
- Muniyappa, R. , Madari, R. , & Varghese, R. T. (2021). Assessing insulin sensitivity and resistance in humans. In Feingold K. R., Anawalt B., Boyce A., et al. (Eds.), Endotext MDText.com, Inc.; 2000. https://www.ncbi.nlm.nih.gov/books/NBK278954/ [PubMed] [Google Scholar]
- Narayan, K. M. V. , & Kanaya, A. M. (2020). Why are south Asians prone to type 2 diabetes? A hypothesis based on underexplored pathways. Diabetologia, 63, 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan, K. M. V. , Kondal, D. , Daya, N. , Gujral, U. P. , Mohan, D. , Patel, S. A. , Shivashankar, R. , Anjana, R. M. , Staimez, L. R. , Ali, M. K. , Chang, H. H. , Kadir, M. , Prabhakaran, D. , Selvin, E. , Mohan, V. , & Tandon, N. (2021). Incidence and pathophysiology of diabetes in south Asian adults living in India and Pakistan compared with US blacks and whites. BMJ Open Diabetes Research & Care, 9, e001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndubuisi, N. B. (2021). Noncommunicable diseases prevention in low‐ and middle‐income countries: An overview of health in all policies (HiPA). Inquiry, 58, 004695020927885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeland, I. J. , Ayers, C. R. , Rohatgi, A. K. , Turer, A. T. , Berry, J. D. , Das, S. R. , Vega, G. L. , Khera, A. , McGuire, D. K. , Grundy, S. M. , & de Lemos, J. A. (2013). Association of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese subjects. Obesity (Silver Spring), 21(9), E439–E447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg, S. T. , Batty, G. D. , Pentti, J. , Virtanen, M. , Afredsson, L. , Fransson, E. I. , Goldberg, M. , et al. (2018). Obesity and loss of disease‐free years owing to major non‐communicable diseases: A multicohort study. The Lancet Public Health, 3(10), e490–e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaogun, I. , Farag, M. , & Hamid, P. (2020). The pathophysiology of type 2 diabetes mellitus in non‐obese individuals: An overview of the current understanding. Cureus, 12(4), e7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega, F. B. , Cadenas‐Sanchez, C. , Migueles, J. H. , Labayen, I. , Ruiz, J. R. , Sui, X. , Blair, S. N. , Martinez‐Vizcaino, V. , & Lavie, C. J. (2018). Role of physical activity and fitness in the characterization and prognosis of the metabolically healthy obesity phenotype: A systematic review and meta‐analysis. Progress in Cardiovascular Diseases, 61(2), 190–205. [DOI] [PubMed] [Google Scholar]
- Peeters, A. , Barendregt, J. , Willekens, F. , Mackenbach, J. P. , Al Mamun, A. , & Bonneux, L. (2003). Obesity in adulthood and its consequences for life expectancy: A life‐table analysis. Annals of Internal Medicine, 138(1), 24–32. [DOI] [PubMed] [Google Scholar]
- Pomeroy, E. , Mushrif‐Tripathy, V. , Cole, T. J. , Wells, J. C. K. , & Stock, J. T. (2019). Ancient origins of low lean mass among south Asians and implications for modern type 2 diabetes susceptibility. Scientific Reports, 9, 10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuveer, G. , Hartz, J. , Lubans, D. R. , Takken, T. , Wiltz, J. L. , Mietus‐Snyder, M. , Perak, A. M. , Baker‐Smith, C. , Pietris, N. , & Edwards, N. M. (2020). Cardiorespiratory fitness in youth: An important marker of health: A scientific statement from the American Heart Association. Circulation, 142(7), e101–e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji, A. , Seely, E. W. , Arky, R. A. , & Simonson, D. C. (2001). Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. The Journal of Clinical Endocrinology & Metabolism, 86, 5366–5371. [DOI] [PubMed] [Google Scholar]
- Remais, J. V. , Zeng, G. , Li, G. , Tian, L. , & Engelgau, M. M. (2013). Convergence of non‐communicable and infectious diseases in low‐ and middle‐income countries. International Journal of Epidemiology, 42(1), 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero‐Corral, A. , & Somers, V. (2010). Normal weight obesity: A risk factor for cardiometabolic dysregulation and cardiovascular mortality. European Heart Journal, 31(6), 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, R. , Blair, S. N. , Arena, R. , Church, T. S. , Després, J.‐P. , Franklin, B. A. , Haskell, W. L. , et al. (2016). Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign: A scientific statement from the American Heart Association. Circulation, 134, e653–e699. [DOI] [PubMed] [Google Scholar]
- Rush, E. C. , Freitas, I. , & Plank, L. D. (2009). Body size, body composition and fat distribution: Comparative analysis of European, Maori, Pacific Island and Asian Indian adults. The British Journal of Nutrition, 102(4), 632–641. [DOI] [PubMed] [Google Scholar]
- Sakaguchi, M. (2024). Adipose tissue dynamics, thermogenesis, and interorgan connections for prevailing obesity and metabolic disorders. JMA Journal, 7(2), 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore, T. , Galiero, R. , Caturano, A. , Rinaldi, L. , Criscuolo, L. , Di Martino, A. , Albanese, G. , Vetrano, E. , Catalini, C. , Sardu, C. , Docimo, G. , Marfella, R. , & Sasso, F. C. (2023). Current knowledge on the pathophysiology of lean/normal‐weight type 2 diabetes. International Journal of Molecular Sciences, 24, 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam, S. (2018). Differential effect of subcutaneous abdominal and visceral adipose tissue on cardiometabolic risk. (2018). Horm Mol biol . The Clinical Investigator, 33(1), 20180014. [DOI] [PubMed] [Google Scholar]
- Simati, S. , Kokkinos, A. , Dalamaga, M. , & Argyrakopoulou, G. (2023). Obesity paradox: Fact or fiction? Current Obesity Reports, 12(2), 75–85. [DOI] [PubMed] [Google Scholar]
- Solomon, C. G. , & Manson, J. E. (1997). Obesity and mortality: A review of the epidemiologic data. The American Journal of Clinical Nutrition, 66(Suppl 4), 1044S–1050S. [DOI] [PubMed] [Google Scholar]
- Sommer, I. , Teufer, B. , Szelag, M. , Nussbaumer‐Streit, B. , Titscher, V. , Klerings, I. , & Garlener, G. (2020). The performance of anthropometric tools to determine obesity: A systematic review and meta‐analysis. Scientific Reports, 10, 12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen, T. A. , Martinez, A. R. , & Jørgensen, T. S. H. (2022). Epidemiology of obesity. Handbook of Experimental Pharmacology, 274, 3–27. [DOI] [PubMed] [Google Scholar]
- Speakman, J. R. , & Elmquist, J. (2022). Obesity: An evolutionary context. Life Metabolism, 1(1), 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperrin, M. , Candlish, J. , Badrick, E. , Renehan, A. , & Buchan, I. (2016). Collider bias is only a partial explanation for the obesity paradox. Epidemiology, 27, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, M. , Foshag, P. , Jehn, U. , Brzek, A. , Littwitz, H. , & Leischik, R. (2021). Higher cardiorespiratory fitness is strongly associated with lower cardiovascular risk factors in firefighters: A cross‐sectional study in German fire brigade. Scientific Reports, 11, 2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroh, A. M. , & Stanford, K. I. (2023). Exercise‐induced regulation of adipose tissue. Current Opinion in Genetics & Development, 81, 102058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, L. , Pan, Y. , & Chen, H. (2022). The harm of metabolically healthy obese and the effect of exercise on their health promotion. Frontiers in Physiology, 13, 924649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui, X. , Hooker, S. P. , Lee, I.‐M. , Church, T. S. , Colabianchi, N. , Lee, C.‐D. , & Blair, S. N. (2008). A prospective study of cardiorespiratory fitness and risk of type 2 diabetes in women. Diabetes Care, 31(3), 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, B. J. , Morais, J. A. , & Santosa, S. (2020). Obesity and aging: Two sides of the same coin. Obesity Reviews, 21(4), e12991. [DOI] [PubMed] [Google Scholar]
- Tan, V. M. H. , Lee, Y. S. , Venkataraman, K. , Khoo, E. Y. H. , Tai, E. S. , Chong, Y. S. , Gluckman, P. , Leow, M. K. S. , & Khoo, C. M. (2015). Ethnic differences in insulin sensitivity and beta‐cell function among Asian men. Nutrition & Diabetes, 5, e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar, A. S. , Tallapragada, D. S. , Nongmaithem, S. S. , Shrestha, S. , Yajnik, C. S. , & Chandak, G. R. (2015). Intrauterine programming of diabetes and adiposity. Metabolism, 4, 418–428. [DOI] [PubMed] [Google Scholar]
- Tönnies, T. , Kahl, S. , & Kuss, O. (2022). Collider bias in observational studies. Deutsches Ärzteblatt International, 119(7), 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsoulis, A. , & Paschou, S. A. (2020). Metabolically healthy obesity: Criteria, epidemiology, controversies, and consequences. Current Obesity Reports, 9(2), 109–120. [DOI] [PubMed] [Google Scholar]
- Van Vliet‐Ostaptchouk, J. V. , Snieder, H. , & Lagou, V. (2012). Gene‐lifestyle interactions in obesity. Current Nutrition Reports, 1, 184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers, D. , Hens, W. , Taeymans, J. , Beeyens, J.‐P. , Poortmans, J. , & Gaal, L. V. (2013). The effect of exercise on visceral adipose tissue in overweight adults: A systematic review and meta‐analysis. PLoS One, 8(2), e56415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.‐S. , Xa, P.‐F. , Ma, M.‐N. , Geng, T.‐T. , Zhng, Y.‐B. , Tu, Z.‐Z. , Jiang, L. , Zhou, L.‐R. , Zhang, B.‐F. , Tong, W.‐W. , Shan, Z. , Liu, G. , Yang, K. , & Pan, A. (2023). Trends in the prevalence of metabolically healthy obesity among US adults, 1999‐2018. JAMA Network Open, 6(3), e232145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber, L. , Kilpi, F. , Marsh, T. , Rtveladze, K. , Brown, M. , & McPherson, K. (2012). High rates of obesity and non‐communicable diseases predicted across Latin America. PLoS One, 7(8), e39589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, M. , Kampert, J. B. , Barlow, C. E. , Nichaman, M. Z. , Gibbons, L. W. , Paffenbarger, R. S. , & Blair, S. N. (1999). Relationship between low cardiorespiratory fitness and mortality in Normal‐weight, overweight, and obese men. JAMA, 282(16), 1547–1553. [DOI] [PubMed] [Google Scholar]
- Wells, J. C. K. (2012). The evolution of human adiposity and obesity: Where did it all go wrong? Disease Models & Mechanisms, 5(5), 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, J. C. K. , Pomeroy, E. , Walimbe, S. R. , Popkin, B. M. , & Yajnik, C. S. (2016). The elevated susceptibility to diabetes in India: An evolutionary perspective. Frontiers in Public Health, 4, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman, R. P. , Munter, P. , Reynolds, K. , McGinn, A. P. , Rajpathak, S. , Wylie‐Rosett, J. , & Sowers, M. R. (2008). The obese without cardiometabolic risk factor clustering and the Normal weight with cardiometabolic risk factor clustering. Archives of Internal Medicine, 168(15), 1617–1624. [DOI] [PubMed] [Google Scholar]
- World Health Organization Non‐Communicable Diseases. https://www.who.int/news‐room/fact‐sheets/detail/noncommunicable‐diseases
- Wu, L. , Zhu, W. , Qiao, Q. , Huang, L. , Li, Y. , & Chen, L. (2021). Novel and traditional anthropometric indices for identifying metabolic syndrome in non‐overweight/obese adults. Nutrition & Metabolism (London), 18, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajnik, C. S. , Deshpande, S. S. , Jackson, A. A. , Refsum, H. , Rao, S. , Fisher, D. J. , Bhat, D. S. , Naik, S. S. , Coyaji, K. J. , Joglekar, C. V. , Joshi, N. , Lubree, H. G. , Deshpande, V. U. , Rege, S. S. , & Fall, C. H. D. (2008). Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The Pune maternal nutrition study. Diabetologia, 51, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajnik, C. S. , Fall, C. H. D. , Coyaji, K. J. , Hirve, S. S. , Rao, S. , Barker, D. J. P. , Joglekar, C. , & Kellinggray, S. (2003). Neonatal anthropometry: The thin‐fat Indian baby. The Pune maternal nutrition study. International Journal of Obesity and Related Metabolic Disorders, 27(2), 173–180. [DOI] [PubMed] [Google Scholar]
- Yajnik, C. S. , & Yudkin, J. S. (2004). The Y‐Y paradox. Lancet, 363, 163. [DOI] [PubMed] [Google Scholar]
- Zatońska, K. , Psikus, P. , Basiak‐Rasala, A. , Stepnicka, Z. , Gawel‐Dabrowska, D. , Wolyniec, M. , Gibka, J. , Suba, A. , & Poltyn‐Zaradna, K. (2021). Obesity and chosen non‐communicable diseases in PURE Poland cohort study. International Journal of Environmental Research and Public Health, 18(5), 2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Liu, J. , Ni, Y. , Yi, C. , Fang, Y. , Ning, Q. , Shen, B. , Zhang, K. , Liu, Y. , Yang, L. , Li, K. , Liu, Y. , Huang, R. , & Li, Z. (2024). Global prevalence of overweight and obesity in children and adolescents: A systematic review and meta‐analysis. JAMA Pediatrics, 178(8), 800–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable—no new data generated.


