Abstract
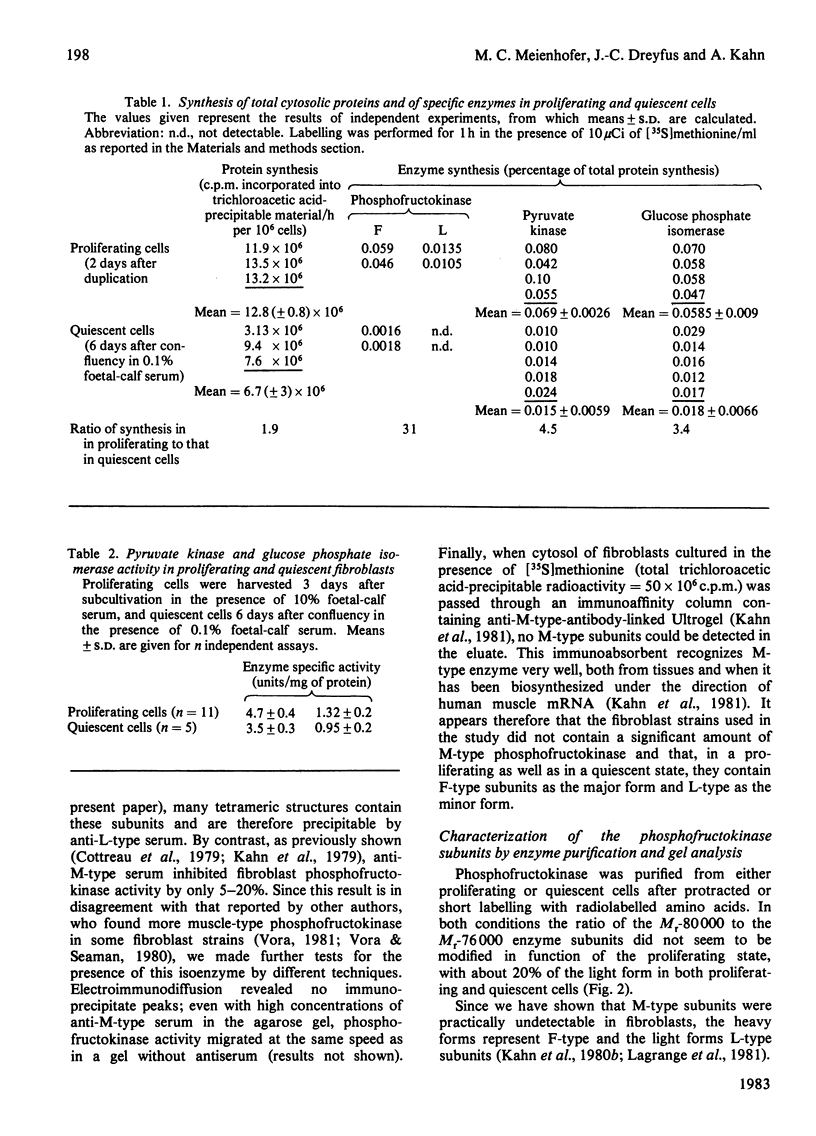
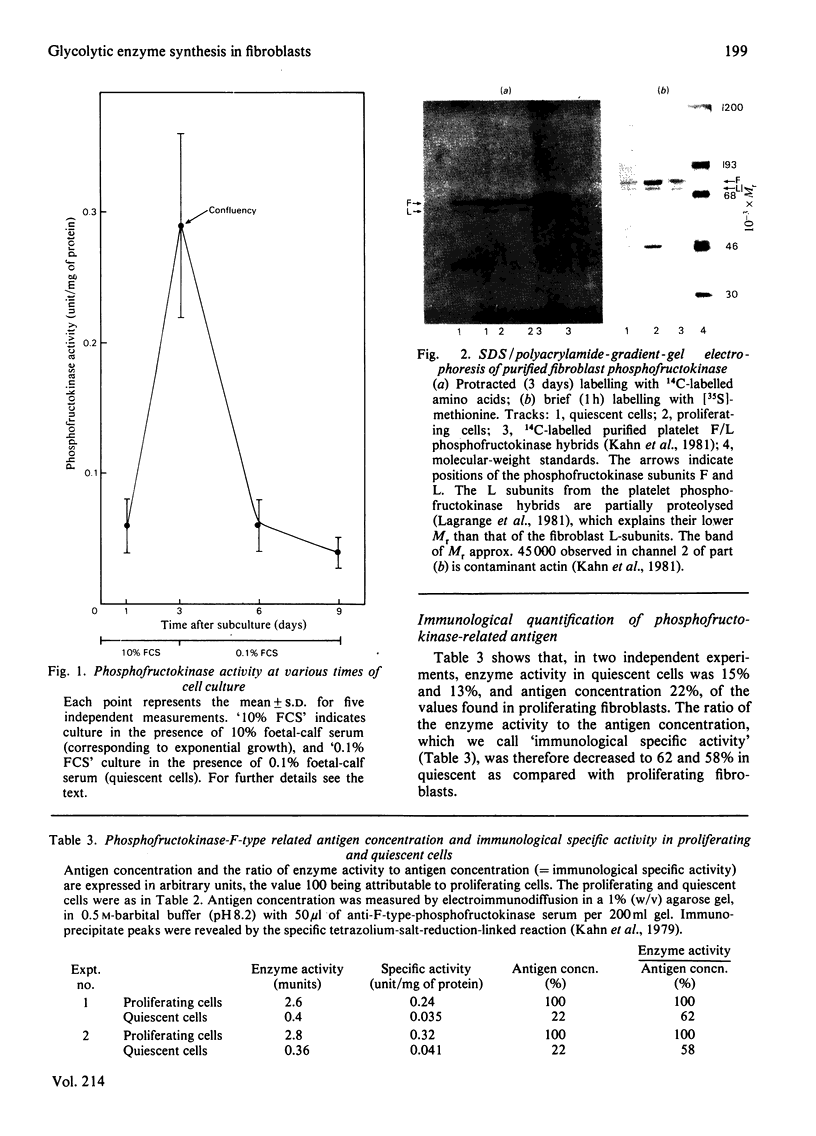
Specific activity of phosphofructokinase is 7-8-fold higher in exponentially growing human fibroblasts than in quiescent cells, but the difference is considerably less pronounced for two other glycolytic enzymes, glucose phosphate isomerase and pyruvate kinase. The ratio of the F-type to L-type phosphofructokinase subunits is essentially the same in growing and resting cells, 4:1. F-type-phosphofructokinase-related antigen concentration is decreased in resting cells as compared with proliferating fibroblasts, but relatively less than the enzyme activity; the ratio of the enzyme activity to the antigen concentration (immunological specific activity) is therefore lower in resting than in growing fibroblasts. Synthesis of phosphofructokinase, as a percentage of the total protein synthesis, is about 30-fold greater during the proliferative phase than in quiescent cells, but this difference is only 3-4-fold for glucose phosphate isomerase and pyruvate kinase. Modulation of the synthesis of phosphofructokinase therefore seems to be responsible for the changes of its specific activity in function of cell proliferation. The appearance of some inactive cross-reacting material in quiescent cells is probably due to post-translational alteration of the pre-synthesized molecules. Compared with other glycolytic enzymes, such as glucose phosphate isomerase and pyruvate kinase, phosphofructokinase seems to be the (or one of the) preferential target of glycolytic induction in proliferating cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besmond C., Benarous R., Kahn A. Cell free synthesis of human prothrombin: immunological characterization of the translation product. Biochem Biophys Res Commun. 1981 Nov 30;103(2):587–594. doi: 10.1016/0006-291x(81)90492-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cottreau D., Levin M. J., Kahn A. Purification and partial characterization of different forms of phosphofructokinase in man. Biochim Biophys Acta. 1979 May 10;568(1):183–194. doi: 10.1016/0005-2744(79)90285-7. [DOI] [PubMed] [Google Scholar]
- Fodge D. W., Rubin H. Activation of phosphofructokinase by stimulants of cell multiplication. Nat New Biol. 1973 Dec 12;246(154):181–183. doi: 10.1038/newbio246181a0. [DOI] [PubMed] [Google Scholar]
- Grégori C., Besmond C., Kahn A., Dreyfus J. C. Characterization of messenger RNA for aldolase B in adult and fetal human liver. Biochem Biophys Res Commun. 1982 Jan 29;104(2):369–375. doi: 10.1016/0006-291x(82)90646-5. [DOI] [PubMed] [Google Scholar]
- Kahn A., Boivin P., Vibert M., Cottreau D., Dreyfus J. C. Post-translational modifications of human glucose-6-phosphate dehydrogenase. Biochimie. 1974;56(10):1395–1407. doi: 10.1016/s0300-9084(75)80026-5. [DOI] [PubMed] [Google Scholar]
- Kahn A., Boyer C., Cottreau D., Marie J., Boivin P. Immunologic study of the age-related loss of activity of six enzymes in the red cells from newborn infants and adults--evidence for a fetal type of erythrocyte phosphofructokinase. Pediatr Res. 1977 Apr;11(4):271–276. doi: 10.1203/00006450-197704000-00001. [DOI] [PubMed] [Google Scholar]
- Kahn A., Cottreau D., Boivin P. Molecular mechanism of glucose-6-phosphate dehydrogenase deficiency. Humangenetik. 1974;25(2):101–109. doi: 10.1007/BF00283310. [DOI] [PubMed] [Google Scholar]
- Kahn A., Cottreau D., Dreyfus J. C. Phosphofructokinase in human fetus. Pediatr Res. 1980 Nov;14(11):1162–1167. doi: 10.1203/00006450-198011000-00003. [DOI] [PubMed] [Google Scholar]
- Kahn A., Cottreau D., Meienhofer M. C. Purification of F4 phosphofructokinase from human platelets and comparison with the other phosphofructokinase forms. Biochim Biophys Acta. 1980 Jan 11;611(1):114–126. doi: 10.1016/0005-2744(80)90047-9. [DOI] [PubMed] [Google Scholar]
- Kahn A., Meienhofer M. C., Cottreau D., Lagrange J. L., Dreyfus J. C. Phosphofructokinase (PFK) isozymes in man. I. Studies of adult human tissues. Hum Genet. 1979 Apr 17;48(1):93–108. doi: 10.1007/BF00273280. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin M. J., Daegelen D., Meienhofer M. C., Dreyfus J. C., Kahn A. Two different species of messenger RNAs specify synthesis of M1 and M2 pyruvate kinase subunits. Biochim Biophys Acta. 1982 Nov 30;699(2):77–83. doi: 10.1016/0167-4781(82)90140-3. [DOI] [PubMed] [Google Scholar]
- Marie J., Simon M. P., Dreyfus J. C., Kahn A. One gene, but two messenger RNAs encode liver L and red cell L' pyruvate kinase subunits. Nature. 1981 Jul 2;292(5818):70–72. doi: 10.1038/292070a0. [DOI] [PubMed] [Google Scholar]
- Meienhofer M. C., Cottreau D., Dreyfus J. C., Kahn A. Kinetic properties of human F4 phosphofructokinase: a poor regulatory enzyme. FEBS Lett. 1980 Feb 11;110(2):219–222. doi: 10.1016/0014-5793(80)80077-9. [DOI] [PubMed] [Google Scholar]
- Meienhofer M. C., Lagrange J. L., Cottreau D., Lenoir G., Dreyfus J. C., Kahn A. Phosphofructokinase in human blood cells. Blood. 1979 Aug;54(2):389–400. [PubMed] [Google Scholar]
- Pouysségur J., Franchi A., Silvestre P. Relationship between increased aerobic glycolysis and DNA synthesis initiation studied using glycolytic mutant fibroblasts. Nature. 1980 Oct 2;287(5781):445–447. doi: 10.1038/287445a0. [DOI] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Rubinson H., Kahn A., Boivin P., Schapira F., Gregori C., Dreyfus J. C. Aging and accuracy of protein synthesis in man: search for inactive enzymatic cross-reacting material in granulocytes of aged people. Gerontology. 1976;22(6):438–448. doi: 10.1159/000212156. [DOI] [PubMed] [Google Scholar]
- Vora S. Isozymes of human phosphofructokinase in blood cells and cultured cell lines: molecular and genetic evidence for a trigenic system. Blood. 1981 Apr;57(4):724–732. [PubMed] [Google Scholar]
- Vora S., Seaman C., Durham S., Piomelli S. Isozymes of human phosphofructokinase: identification and subunit structural characterization of a new system. Proc Natl Acad Sci U S A. 1980 Jan;77(1):62–66. doi: 10.1073/pnas.77.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora S., Wims L. A., Durham S., Morrison S. L. Production and characterization of monoclonal antibodies to the subunits of human phosphofructokinase: new tools for the immunochemical and genetic analyses of isozymes. Blood. 1981 Oct;58(4):823–829. [PubMed] [Google Scholar]
- Weil D., Cottreau D., Nguyen Van Cong, Rebourcet R., Foubert C., Gross M. S., Dreyfus J. C., Kahn A. Assignment of the gene for F-type phosphofructokinase to human chromosome 10 by somatic cell hybridization and specific immunoprecipitation. Ann Hum Genet. 1980 Jul;44(Pt 1):11–16. doi: 10.1111/j.1469-1809.1980.tb00941.x. [DOI] [PubMed] [Google Scholar]



