Abstract
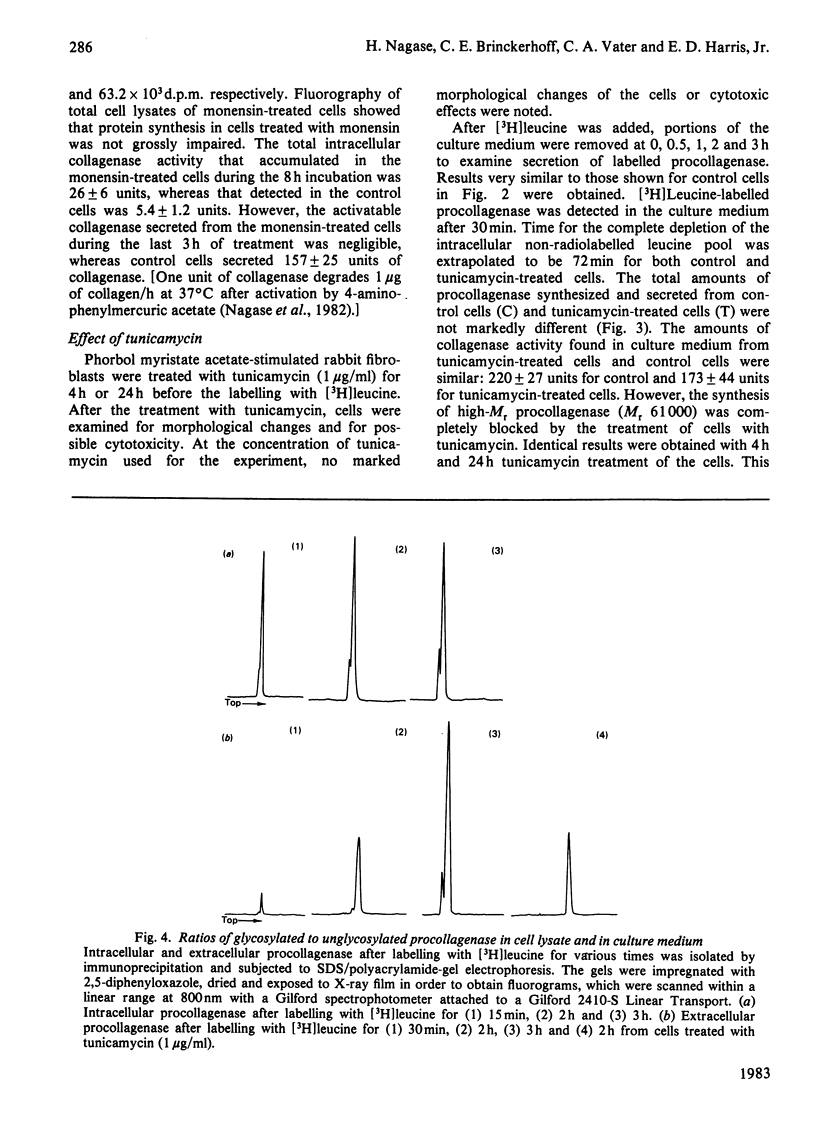
Monolayer cultures of rabbit synovial fibroblasts stimulated with phorbol myristate acetate to produce large amounts of collagenase (EC 3.4.24.7) were used to study the biosynthesis and secretion of this enzyme. [3H]Leucine was added to cell cultures for pulse-chase and continuous-labelling experiments. The labelled procollagenase synthesized was identified by immunoprecipitation followed by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis and fluorography. The amounts of intracellular and extracellular proenzyme were quantified by measuring radioactivity incorporated into the proteins. procollagenase was synthesized as doublet proteins of Mr 57 000 and Mr 61 000. Immunoprecipitable proenzyme proteins were first detected in culture medium 35 min after [3H]leucine was added to the cells. Monensin treatment of the cells inhibited procollagenase secretion and led to intracellular accumulation of the proenzyme. Cells treated with tunicamycin produced only the 57 000-Mr form, indicating that in rabbit synovial cells the 61 000-Mr form was post-translationally modified by addition of oligosaccharides to asparagine residues. The ratios of glycosylated to unglycosylated forms in cell lysates and in culture medium were 0.22:1 and 0.07:1 respectively.
Full text
PDF

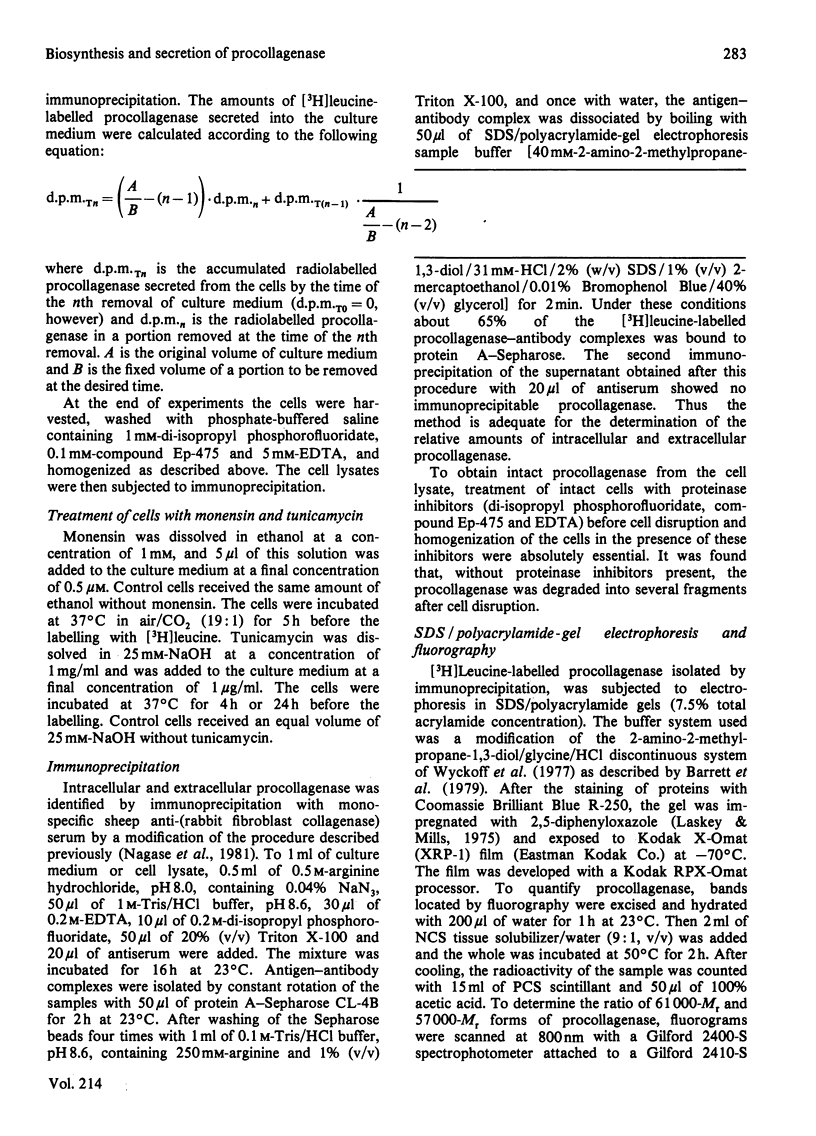
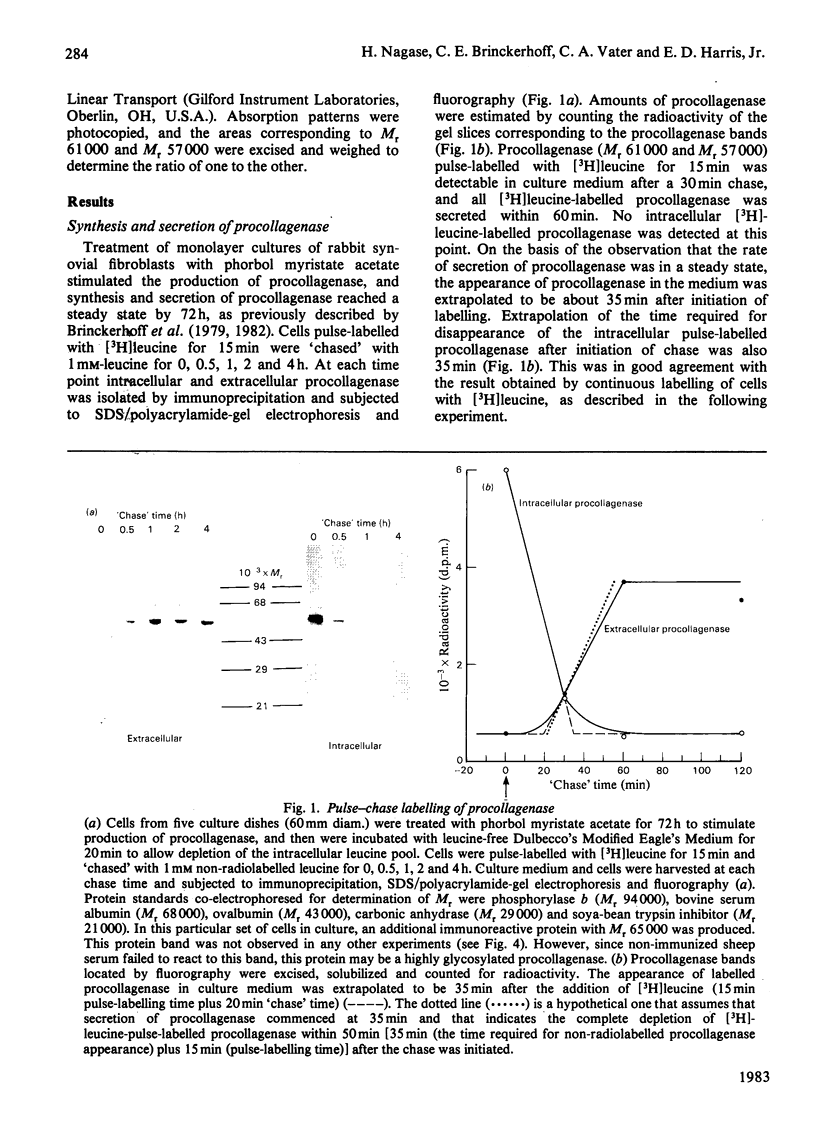
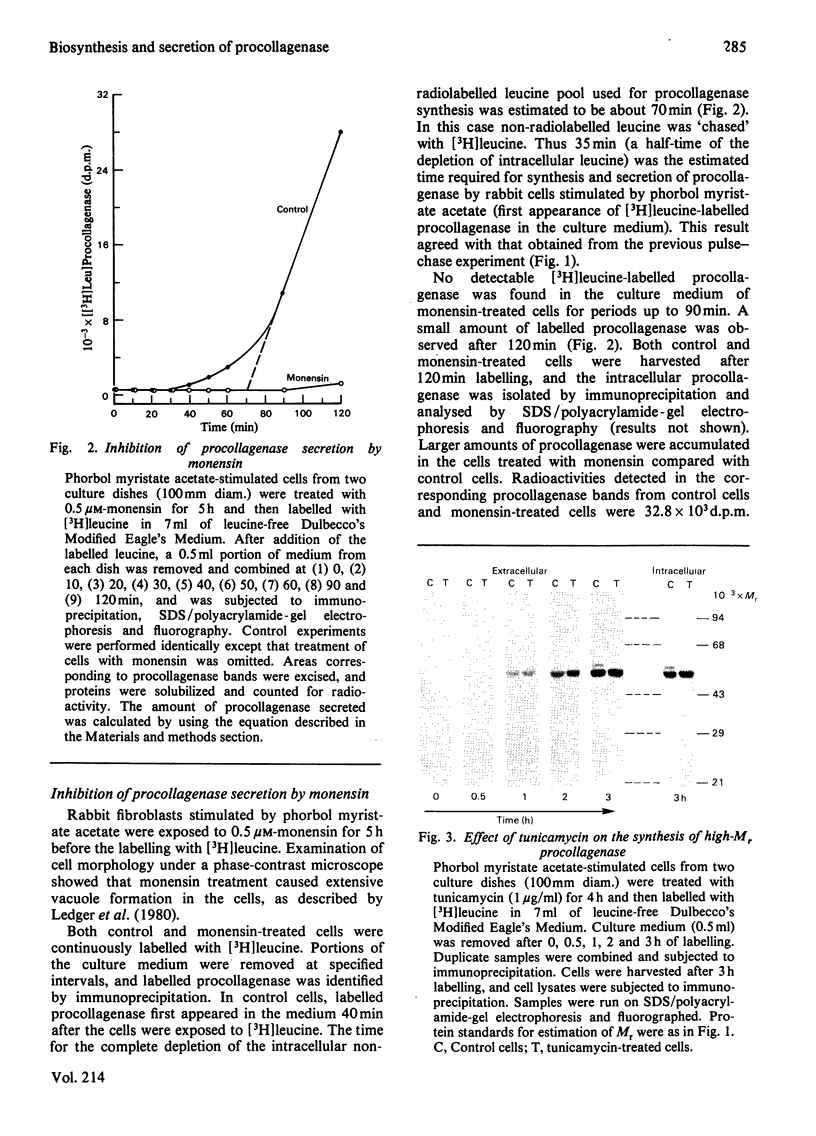


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Brown M. A., Sayers C. A. The electrophoretically 'slow' and 'fast' forms of the alpha 2-macroglobulin molecule. Biochem J. 1979 Aug 1;181(2):401–418. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Cobb C. M., Taylor R. E., Fullmer H. M. Synthesis and release of procollagenase by cultured fibroblasts. J Biol Chem. 1976 May 25;251(10):3162–3168. [PubMed] [Google Scholar]
- Brinckerhoff C. E., Gross R. H., Nagase H., Sheldon L., Jackson R. C., Harris E. D., Jr Increased level of translatable collagenase messenger ribonucleic acid in rabbit synovial fibroblasts treated with phorbol myristate acetate or crystals of monosodium urate monohydrate. Biochemistry. 1982 May 25;21(11):2674–2679. doi: 10.1021/bi00540a015. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., McMillan R. M., Fahey J. V., Harris E. D., Jr Collagenase production by synovial fibroblasts treated with phorbol myristate acetate. Arthritis Rheum. 1979 Oct;22(10):1109–1116. doi: 10.1002/art.1780221010. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Galloway W. A., Mercer E., Murphy G., Reynolds J. J. Purification of rabbit bone inhibitor of collagenase. Biochem J. 1981 Apr 1;195(1):159–165. doi: 10.1042/bj1950159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977 Jul 15;166(1):21–31. doi: 10.1042/bj1660021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper E., Bloch K. J., Gross J. The zymogen of tadpole collagenase. Biochemistry. 1971 Aug 3;10(16):3035–3041. doi: 10.1021/bi00792a008. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (first of three parts). N Engl J Med. 1974 Sep 12;291(11):557–563. doi: 10.1056/NEJM197409122911105. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (second of three parts). N Engl J Med. 1974 Sep 19;291(12):605–609. doi: 10.1056/NEJM197409192911205. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (third of three parts). N Engl J Med. 1974 Sep 26;291(13):652–661. doi: 10.1056/NEJM197409262911305. [DOI] [PubMed] [Google Scholar]
- Hiti-Harper J., Wohl H., Harper E. Platelet factor 4: an inhibitor of collagenase. Science. 1978 Mar 3;199(4332):991–992. doi: 10.1126/science.203038. [DOI] [PubMed] [Google Scholar]
- Horwitz A. L., Kelman J. A., Crystal R. G. Activation of alveolar macrophage collagenase by a neutral protease secreted by the same cell. Nature. 1976 Dec 23;264(5588):772–774. doi: 10.1038/264772a0. [DOI] [PubMed] [Google Scholar]
- Kuettner K. E., Hiti J., Eisenstein R., Harper E. Collagenase inhibition by cationic proteins derived from cartilage and aorta. Biochem Biophys Res Commun. 1976 Sep 7;72(1):40–46. doi: 10.1016/0006-291x(76)90957-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Ledger P. W., Uchida N., Tanzer M. L. Immunocytochemical localization of procollagen and fibronectin in human fibroblasts: effects of the monovalent ionophore, monensin. J Cell Biol. 1980 Dec;87(3 Pt 1):663–671. doi: 10.1083/jcb.87.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehle L., Tanner W. The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 1976 Nov 15;72(1):167–170. doi: 10.1016/0014-5793(76)80922-2. [DOI] [PubMed] [Google Scholar]
- McCroskery P. A., Richards J. F., Harris E. D., Jr Purification and characterization of a collagenase extracted from rabbit tumours. Biochem J. 1975 Oct;152(1):131–142. doi: 10.1042/bj1520131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan R. M., Vater C. A., Hasselbacher P., Hahn J., Harris E. D., Jr Induction of collagenase and prostaglandin synthesis in synovial fibroblasts treated with monosodium urate crystals. J Pharm Pharmacol. 1981 Jun;33(6):382–383. doi: 10.1111/j.2042-7158.1981.tb13809.x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cawston T. E., Reynolds J. J. An inhibitor of collagenase from human amniotic fluid. Purification, characterization and action on metalloproteinases. Biochem J. 1981 Apr 1;195(1):167–170. doi: 10.1042/bj1950167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H., Cawston T. E., De Silva M., Barrett A. J. Identification of plasma kallikrein as an activator of latent collagenase in rheumatoid synovial fluid. Biochim Biophys Acta. 1982 Mar 18;702(1):133–142. doi: 10.1016/0167-4838(82)90036-x. [DOI] [PubMed] [Google Scholar]
- Nagase H., Jackson R. C., Brinckerhoff C. E., Vater C. A., Harris E. D., Jr A precursor form of latent collagenase produced in a cell-free system with mRNA from rabbit synovial cells. J Biol Chem. 1981 Dec 10;256(23):11951–11954. [PubMed] [Google Scholar]
- Smilowitz H. Monovalent ionophores inhibit acetylcholinesterase release from cultured chick embryo skeletal muscle cells. Mol Pharmacol. 1979 Jul;16(1):202–214. [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Tamai M., Hanada K., Adachi T., Oguma K., Kashiwagi K., Omura S., Ohzeki M. Papain inhibitions by optically active E-64 analogs. J Biochem. 1981 Jul;90(1):255–257. doi: 10.1093/oxfordjournals.jbchem.a133458. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P., Détraz M. Comparative studies of intracellular transport of secretory proteins. J Cell Biol. 1978 Dec;79(3):694–707. doi: 10.1083/jcb.79.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Uchida N., Smilowitz H., Ledger P. W., Tanzer M. L. Kinetic studies of the intracellular transport of procollagen and fibronectin in human fibroblasts. Effects of the monovalent ionophore, monensin. J Biol Chem. 1980 Sep 25;255(18):8638–8644. [PubMed] [Google Scholar]
- Uchida N., Smilowitz H., Tanzer M. L. Monovalent ionophores inhibit secretion of procollagen and fibronectin from cultured human fibroblasts. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1868–1872. doi: 10.1073/pnas.76.4.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaes G., Eeckhout Y., Lenaers-Claeys G., François-Gillet C., Druetz J. E. The simultaneous release by bone explants in culture and the parallel activation of procollagenase and of a latent neutral proteinase that degrades cartilage proteoglycans and denatured collagen. Biochem J. 1978 May 15;172(2):261–274. doi: 10.1042/bj1720261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle K. J., Bauer E. A. Biosynthesis of collagenase by human skin fibroblasts in monolayer culture. J Biol Chem. 1979 Oct 25;254(20):10115–10122. [PubMed] [Google Scholar]
- Vater C. A., Hahn J. L., Harris E. D., Jr Preparation of a monospecific antibody to purified rabbit synovial fibroblast collagenase. Coll Relat Res. 1981 Nov;1(6):527–542. doi: 10.1016/s0174-173x(81)80034-9. [DOI] [PubMed] [Google Scholar]
- Vater C. A., Mainardi C. L., Harris E. D., Jr Inhibitor of human collagenase from cultures of human tendon. J Biol Chem. 1979 Apr 25;254(8):3045–3053. [PubMed] [Google Scholar]
- Vogt M., Dulbecco R. VIRUS-CELL INTERACTION WITH A TUMOR-PRODUCING VIRUS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):365–370. doi: 10.1073/pnas.46.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Olsen C. E., Sandberg A. L., Mergenhagen S. E. Prostaglandin regulation of macrophage collagenase production. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4955–4958. doi: 10.1073/pnas.74.11.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Stricklin G. P., Eisen A. Z., Bauer E. A., Cooney R. V., Jeffrey J. J. A specific inhibitor of vertebrate collagenase produced by human skin fibroblasts. J Biol Chem. 1979 Mar 25;254(6):1938–1943. [PubMed] [Google Scholar]
- Werb Z., Burleigh M. C., Barrett A. J., Starkey P. M. The interaction of alpha2-macroglobulin with proteinases. Binding and inhibition of mammalian collagenases and other metal proteinases. Biochem J. 1974 May;139(2):359–368. doi: 10.1042/bj1390359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr A latent form of collagenase in the involuting rat uterus and its activation by a serine proteinase. Biochem J. 1977 Mar 1;161(3):535–542. doi: 10.1042/bj1610535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley D. E., Lindberg K. A., Glanville R. W., Evanson J. M. Action of rheumatoid synovial collagenase on cartilage collagen. Different susceptibilities of cartilage and tendon collagen to collagenase attack. Eur J Biochem. 1975 Jan 2;50(2):437–444. doi: 10.1111/j.1432-1033.1975.tb09821.x. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Roberts D. R., Evanson J. M. Small molecular weight beta 1 serum protein which specifically inhibits human collagenases. Nature. 1976 May 27;261(5558):325–327. doi: 10.1038/261325a0. [DOI] [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]