Abstract
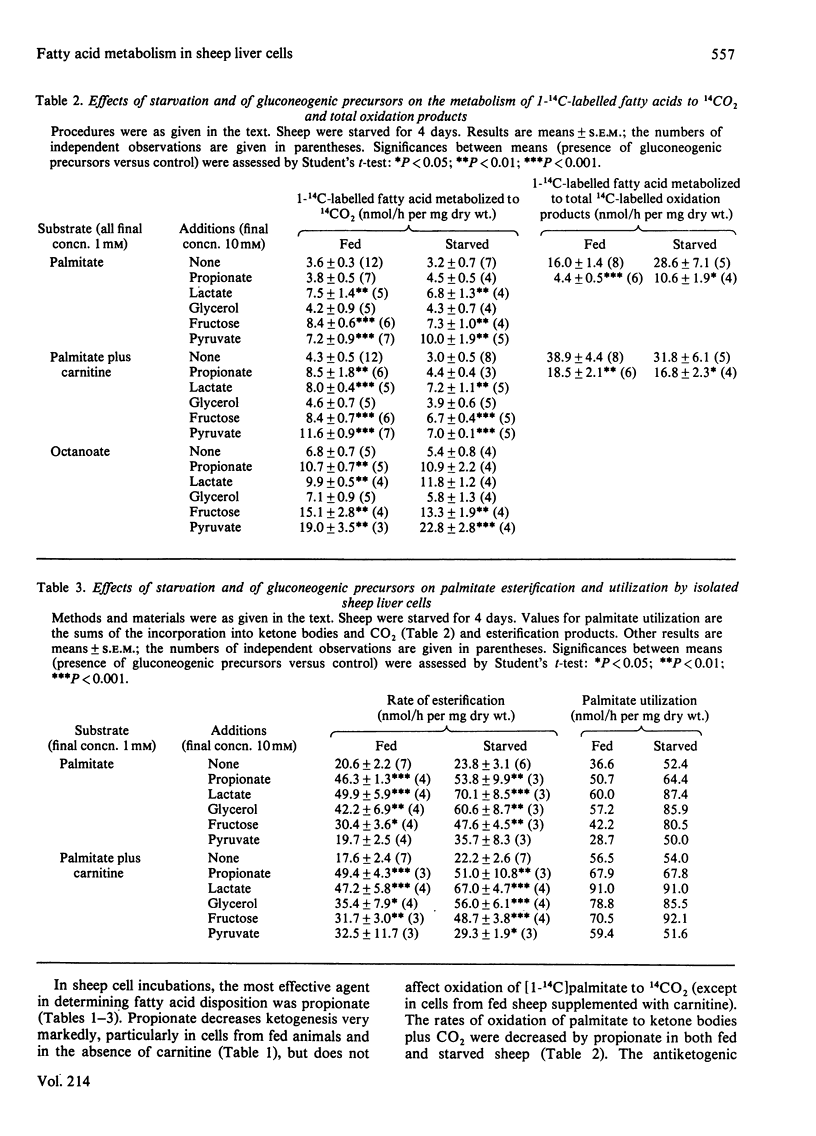
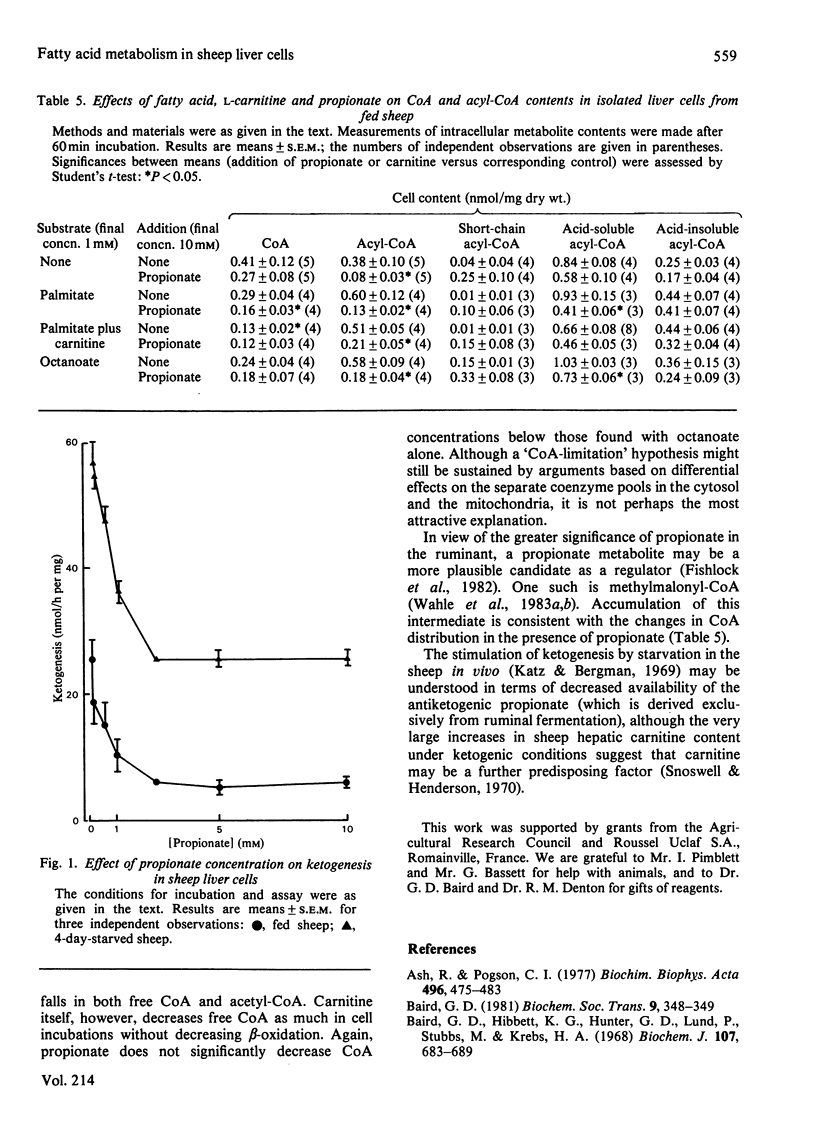
Isolated liver cells prepared from starved sheep converted palmitate into ketone bodies at twice the rate seen with cells from fed animals. Carnitine stimulated palmitate oxidation only in liver cells from fed sheep, and completely abolished the difference between fed and starved animals in palmitate oxidation. The rates of palmitate oxidation to CO2 and of octanoate oxidation to ketone bodies and CO2 were not affected by starvation or carnitine. Neither starvation nor carnitine altered the ratio of 3-hydroxybutyrate to acetoacetate or the rate of esterification of [1-14C]palmitate. Propionate, lactate, pyruvate and fructose inhibited ketogenesis from palmitate in cells from fed sheep. Starvation or the addition of carnitine decreased the antiketogenic effectiveness of gluconeogenic precursors. Propionate was the most potent inhibitor of ketogenesis, 0.8 mM producing 50% inhibition. Propionate, lactate, fructose and glycerol increased palmitate esterification under all conditions examined. Lactate, pyruvate and fructose stimulated oxidation of palmitate and octanoate to CO2. Starvation and the addition of gluconeogenic precursors stimulated apparent palmitate utilization by cells. Propionate, lactate and pyruvate decreased cellular long-chain acylcarnitine concentrations. Propionate decreased cell contents of CoA and acyl-CoA. It is suggested that propionate may control hepatic ketogenesis by acting at some point in the beta-oxidation sequence. The results are discussed in relation to the differences in the regulation of hepatic fatty acid metabolism between sheep and rats.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash R., Pogson C. I. Preparation and biochemical characterisation of isolated parenchymal cells from adult sheep liver. Biochim Biophys Acta. 1977 Feb 28;496(2):475–483. doi: 10.1016/0304-4165(77)90329-4. [DOI] [PubMed] [Google Scholar]
- Baird G. D., Hibbitt K. G., Hunter G. D. Biochemical aspects of bovine ketosis. Biochem J. 1968 May;107(5):683–689. doi: 10.1042/bj1070683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird G. D. Ruminant ketosis. Biochem Soc Trans. 1981 Aug;9(4):348–349. doi: 10.1042/bst0090348. [DOI] [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W., Kronfeld D. S. Gluconeogenesis and lipogenesis in tissue from ruminant and nonruminant animals. Fed Proc. 1969 Jan-Feb;28(1):218–231. [PubMed] [Google Scholar]
- CHAIKOFF I. L., GOLDMAN D. S., BROWN G. W., Jr, DAUBEN W. G., GEE M. Acetoacetate formation in liver. I. From palmitic acid-1-C14, 5-C14 and 11-C14. J Biol Chem. 1951 May;190(1):229–240. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Christiansen R. Z., Bremer J. Active transport of butyrobetaine and carnitine into isolated liver cells. Biochim Biophys Acta. 1976 Nov 2;448(4):562–577. doi: 10.1016/0005-2736(76)90110-3. [DOI] [PubMed] [Google Scholar]
- Christiansen R. Z. Regulation of palmitate metabolism by carnitine and glucagon in hepatocytes isolated from fasted and carbohydrate refed rats. Biochim Biophys Acta. 1977 Aug 24;488(2):249–262. doi: 10.1016/0005-2760(77)90182-5. [DOI] [PubMed] [Google Scholar]
- Christiansen R. Z. The effects of antiketogenic agents and pyruvate on the oxidation of palmitate in isolated hepatocytes. FEBS Lett. 1979 Jul 1;103(1):89–92. doi: 10.1016/0014-5793(79)81256-9. [DOI] [PubMed] [Google Scholar]
- Christiansen R., Borrebaek B., Bremer J. The effect of (-)carnitine on the metabolism of palmitate in liver cells isolated from fasted and refed rats. FEBS Lett. 1976 Mar 1;62(3):313–317. doi: 10.1016/0014-5793(76)80083-x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FRITZ I. B. Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev. 1961 Jan;41:52–129. doi: 10.1152/physrev.1961.41.1.52. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems R., Lund P., Krebs H. A. Rapid separation of isolated hepatocytes or similar tissue fragments for analysis of cell constituents. Biochem J. 1975 Jul;150(1):47–50. doi: 10.1042/bj1500047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M. L., Bergman E. N. Hepatic and portal metabolism of glucose, free fatty acids, and ketone bodies in the sheep. Am J Physiol. 1969 Apr;216(4):953–960. doi: 10.1152/ajplegacy.1969.216.4.953. [DOI] [PubMed] [Google Scholar]
- Kean E. A., Pogson C. I. Inhibition of gluconeogenesis in isolated rat liver cells by methylenecyclopropylpyruvate (ketohypoglycin). Biochem J. 1979 Sep 15;182(3):789–796. doi: 10.1042/bj1820789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundakjian P. P., Snoswell A. M. Ketone body and fatty acid metabolism in sheep tissues. 3-Hydroxybutyrate dehydrogenase, a cytoplasmic enzyme in sheep liver and kidney. Biochem J. 1970 Aug;119(1):49–57. doi: 10.1042/bj1190049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V. Metabolism of acetoacetate in animal tissues. 1. Biochem J. 1945;39(5):408–419. [PMC free article] [PubMed] [Google Scholar]
- Lindsay D. B. Gluconeogenesis in ruminants. Biochem Soc Trans. 1978;6(6):1152–1156. doi: 10.1042/bst0061152. [DOI] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Regulation of fat metabolism of the liver. Nature. 1967 Aug 12;215(5102):716–718. doi: 10.1038/215716a0. [DOI] [PubMed] [Google Scholar]
- Mayes P. A., Laker M. E. Regulation of ketogenesis in the liver. Biochem Soc Trans. 1981 Aug;9(4):339–341. doi: 10.1042/bst0090339. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Effects of exogenous fatty acid concentration on glucagon-induced changes in hepatic fatty acid metabolism. Diabetes. 1980 Mar;29(3):236–240. doi: 10.2337/diab.29.3.236. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. In support of the roles of malonyl-CoA and carnitine acyltransferase I in the regulation of hepatic fatty acid oxidation and ketogenesis. J Biol Chem. 1979 Sep 10;254(17):8163–8168. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Robles-Valdes C., Foster D. W. Role of carnitine in hepatic ketogenesis. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4385–4388. doi: 10.1073/pnas.72.11.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J., Wright P. H., Foster D. W. Hormonal control of ketogenesis. Rapid activation of hepatic ketogenic capacity in fed rats by anti-insulin serum and glucagon. J Clin Invest. 1975 Jun;55(6):1202–1209. doi: 10.1172/JCI108038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N. C., Fleischer S. Beta-hydroxybutyrate dehydrogenase: lack in ruminant liver mitochondria. Science. 1969 Nov 21;166(3908):1017–1019. doi: 10.1126/science.166.3908.1017. [DOI] [PubMed] [Google Scholar]
- Nosadini R., Datta H., Hodson A., Alberti K. G. A possible mechanism for the anti-ketogenic action of alanine in the rat. Biochem J. 1980 Aug 15;190(2):323–332. doi: 10.1042/bj1900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontko J. A. Metabolism of free fatty acids in isolated liver cells. Factors affecting the partition between esterification and oxidation. J Biol Chem. 1972 Mar 25;247(6):1788–1800. [PubMed] [Google Scholar]
- Patterson D. S. Depôt fat mobilization and liver lipogenesis and ketogenesis in ovine pregnancy toxaemia and the effects of corticotrophin administration. Res Vet Sci. 1966 Oct;7(4):484–492. [PubMed] [Google Scholar]
- Siess E. A., Kientsch-Engel R. I., Wieland O. H. Role of free oxaloacetate in ketogenesis. Derivation from the direct measurement of mitochondrial [3-hydroxybutyrate]/[acetoacetate] ratio in hepatocytes. Eur J Biochem. 1982 Jan;121(3):493–499. doi: 10.1111/j.1432-1033.1982.tb05814.x. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Elliott K. R., Pogson C. I. Differential effects of tryptophan on glucose synthesis in rats and guinea pigs. Biochem J. 1978 Dec 15;176(3):817–825. doi: 10.1042/bj1760817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoswell A. M., Henderson G. D. Aspects of carnitine ester metabolism in sheep liver. Biochem J. 1970 Aug;119(1):59–65. doi: 10.1042/bj1190059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoswell A. M., Koundakjian P. P. Relationships between carnitine and coenzyme A esters in tissues of normal and alloxan-diabetic sheep. Biochem J. 1972 Mar;127(1):133–141. doi: 10.1042/bj1270133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. E., Gardner J. W., Bell A. W. The oxygen consumption, fatty acid and glycerol uptake of the liver in fed and fasted sheep during cold exposure. Q J Exp Physiol Cogn Med Sci. 1975 Apr;60(2):107–121. doi: 10.1113/expphysiol.1975.sp002297. [DOI] [PubMed] [Google Scholar]
- Varnam G. C., Jeacock M. K., Shepherd D. A. Hepatic ketone-body metabolism in developing sheep and pregnant ewes. Br J Nutr. 1978 Sep;40(2):359–367. doi: 10.1079/bjn19780132. [DOI] [PubMed] [Google Scholar]
- WEINHOUSE S., MILLINGTON R. H., VOLK M. E. Oxidation of isotopic palmitic acid in animal tissues. J Biol Chem. 1950 Jul;185(1):191–200. [PubMed] [Google Scholar]
- Whitelaw E., Williamson D. H. Effects of lactation of ketogenesis from oleate or butyrate in rat hepatocytes. Biochem J. 1977 Jun 15;164(3):521–528. doi: 10.1042/bj1640521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witters L. A., Kowaloff E. M., Avruch J. Glucagon regulation of protein phosphorylation. Identification of acetyl coenzyme A carboxylase as a substrate. J Biol Chem. 1979 Jan 25;254(2):245–248. [PubMed] [Google Scholar]


