Abstract
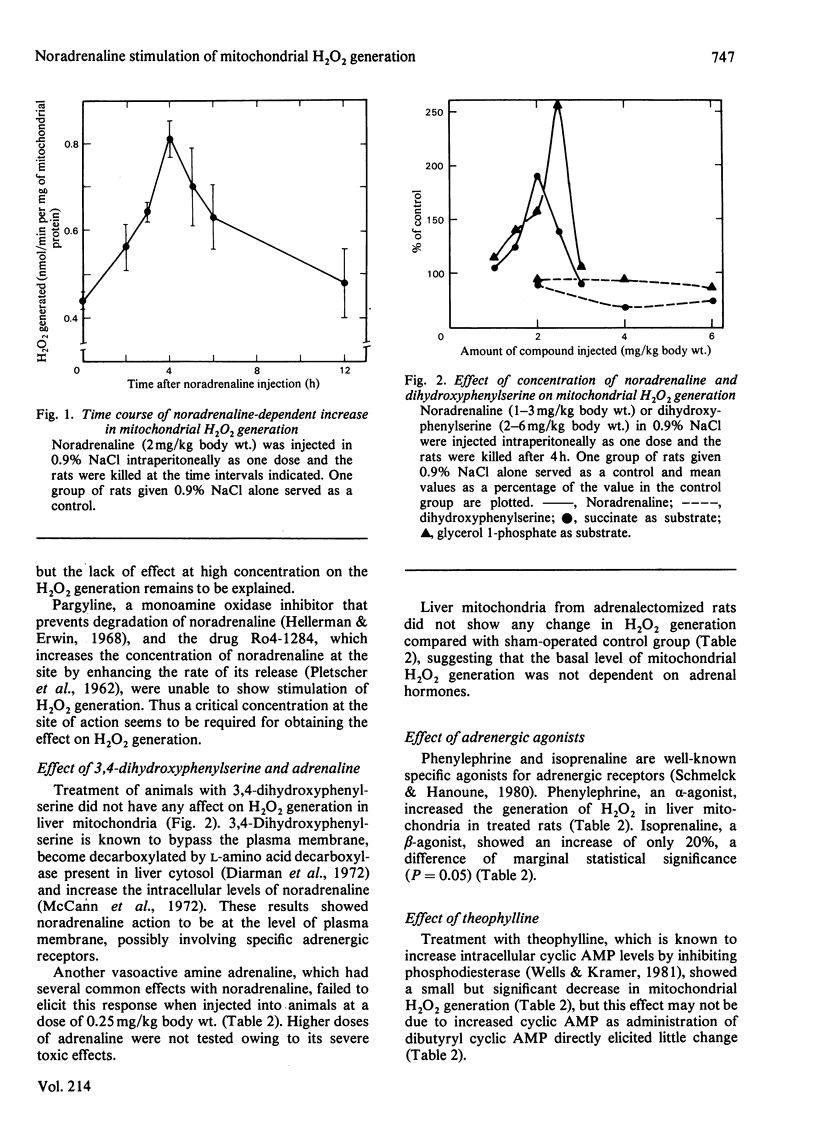
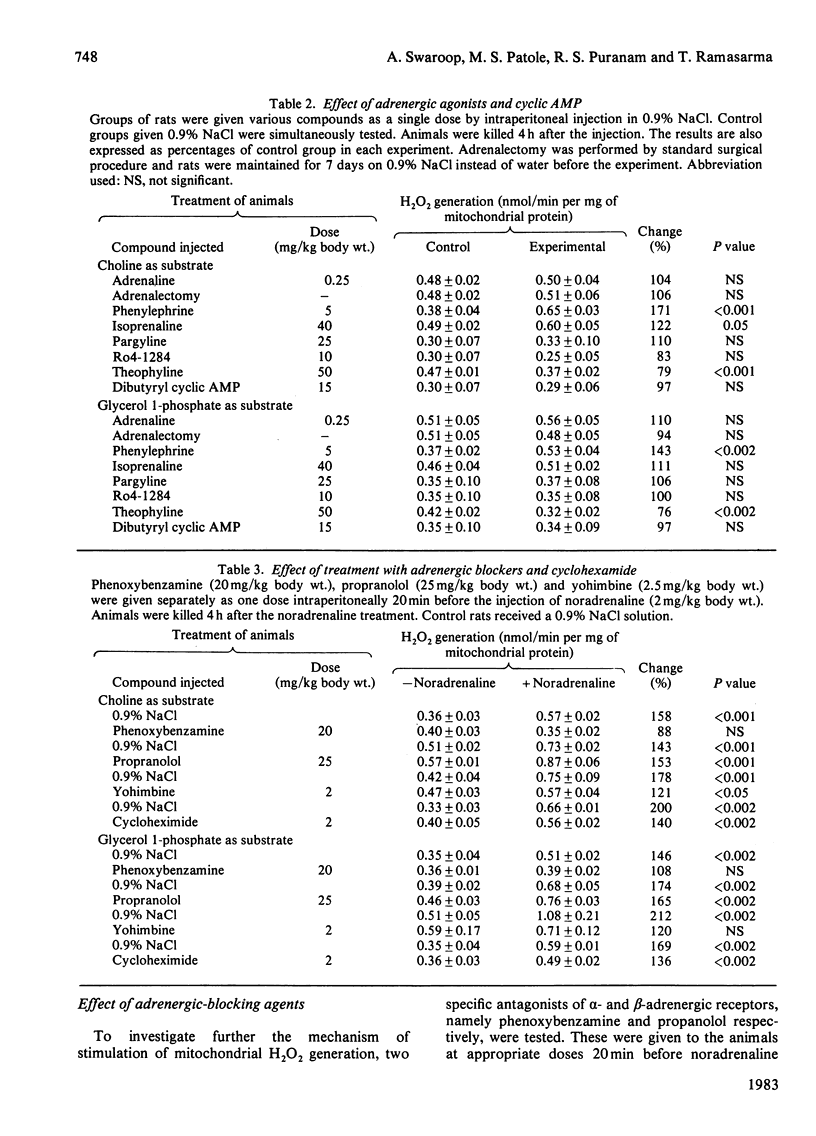
Treatment of rats with noradrenaline stimulated H2O2 generation in liver mitochondria using succinate, choline or glycerol 1-phosphate as substrate. The dehydrogenase activity with either succinate or choline as substrate showed no change, whereas that with glycerol 1-phosphate increased. The effect was obtained with noradrenaline, but not with dihydroxyphenylserine. Phenoxybenzamine and yohimbine, but not propranolol, prevented the response to noradrenaline treatment. Phenylephrine could stimulate H2O2 generation, whereas isoprenaline had only a marginal effect. Theophylline treatment slightly decreased the generation of H2O2 in liver mitochondria, but treatment with pargyline, Ro4-1284 and dibutyryl cyclic AMP had little effect. These studies showed that noradrenaline might possibly be acting through the alpha 2-adrenergic system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boveris A., Cadenas E., Stoppani A. O. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem J. 1976 May 15;156(2):435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972 Jul;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Dairman W., Christenson J. G., Udenfriend S. Changes in tyrosine hydroxylase and dopa decarboxylase induced by pharmacological agents. Pharmacol Rev. 1972 Jun;24(2):269–289. [PubMed] [Google Scholar]
- Dri P., Bellavite P., Berton G., Rossi F. Interrelationship between oxygen consumption, superoxide anion and hydrogen peroxide formation in phagocytosing guinea pig polymorphonuclear leucocytes. Mol Cell Biochem. 1979 Jan 26;23(2):109–122. [PubMed] [Google Scholar]
- George R., Ramasarma T. Nature of the stimulation of biogenesis of cholesterol in the liver by noradrenaline. Biochem J. 1977 Mar 15;162(3):493–499. doi: 10.1042/bj1620493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govier W. C., Lovenberg W., Sjoerdsma A. Studies on the role of catecholamines as regulators of tyrosine aminotransferase. Biochem Pharmacol. 1969 Oct;18(10):2661–2666. doi: 10.1016/0006-2952(69)90196-8. [DOI] [PubMed] [Google Scholar]
- Hellerman L., Erwin V. G. Mitochondrial monoamine oxidase. II. Action of various inhibitors for the bovine kidney enzyme. Catalytic mechanism. J Biol Chem. 1968 Oct 25;243(20):5234–5243. [PubMed] [Google Scholar]
- Himms-Hagen J. Cellular thermogenesis. Annu Rev Physiol. 1976;38:315–351. doi: 10.1146/annurev.ph.38.030176.001531. [DOI] [PubMed] [Google Scholar]
- Hutson N. J., Brumley F. T., Assimacopoulos F. D., Harper S. C., Exton J. H. Studies on the alpha-adrenergic activation of hepatic glucose output. I. Studies on the alpha-adrenergic activation of phosphorylase and gluconeogenesis and inactivation of glycogen synthase in isolated rat liver parenchymal cells. J Biol Chem. 1976 Sep 10;251(17):5200–5208. [PubMed] [Google Scholar]
- Janský L. Non-shivering thermogenesis and its thermoregulatory significance. Biol Rev Camb Philos Soc. 1973 Feb;48(1):85–132. doi: 10.1111/j.1469-185x.1973.tb01115.x. [DOI] [PubMed] [Google Scholar]
- Kurup C. K., Aithal H. N., Ramasarma T. Increase of hepatic mitochondria on administration of ethyl alpha-p-chlorophenoxyisobutyrate to the rat. Biochem J. 1970 Mar;116(5):773–779. doi: 10.1042/bj1160773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Duncan H., Hooker N., Morse A. Hydrogen peroxide induces spawning in mollusks, with activation of prostaglandin endoperoxide synthetase. Science. 1977 Apr 15;196(4287):298–300. doi: 10.1126/science.403609. [DOI] [PubMed] [Google Scholar]
- Mukherjee S. P. Mediation of the antilipolytic and lipogenic effects of insulin in adipocytes by intracellular accumulation of hydrogen peroxide. Biochem Pharmacol. 1980 May 1;29(9):1239–1246. doi: 10.1016/0006-2952(80)90280-4. [DOI] [PubMed] [Google Scholar]
- Mukherjee S. P., Mukherjee C. Stimulation of pyruvate dehydrogenase activity in adipocytes by oxytocin: evidence for mediation of the insulin-like effect by endogenous hydrogen peroxide independent of glucose transport. Arch Biochem Biophys. 1982 Mar;214(1):211–222. doi: 10.1016/0003-9861(82)90024-8. [DOI] [PubMed] [Google Scholar]
- RENDINA G., SINGER T. P. Studies on choline dehydrogenase. I. Extraction in soluble form, assay, and some properties of the enzyme. J Biol Chem. 1959 Jun;234(6):1605–1610. [PubMed] [Google Scholar]
- RUEGAMER W. R., WESTERFELD W. W., RICHERT D. A. ALPHA-GLYCEROPHOSPHATE DEHYDROGENASE RESPONSE TO THYROXINE IN THYROIDECTOMIZED, THIOURACIL-FED AND TEMPERATURE-ADAPTED RATS. Endocrinology. 1964 Dec;75:908–916. doi: 10.1210/endo-75-6-908. [DOI] [PubMed] [Google Scholar]
- Ramasarma T. Generation of H2O in biomembranes. Biochim Biophys Acta. 1982 Aug 11;694(1):69–93. doi: 10.1016/0304-4157(82)90014-4. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Boveris A., Bonner W. D., Jr, Moore A. L. Hydrogen peroxide generation by the alternate oxidase of higher plants. Biochem Biophys Res Commun. 1976 Aug 9;71(3):695–703. doi: 10.1016/0006-291x(76)90887-1. [DOI] [PubMed] [Google Scholar]
- Schmelck P. H., Hanoune J. The hepatic adrenergic receptors. Mol Cell Biochem. 1980 Dec 10;33(1-2):35–48. doi: 10.1007/BF00224570. [DOI] [PubMed] [Google Scholar]
- Sitaramam V., Panini S. R., Rau M., Ramasarma T. Nature of the inhibition by noradrenaline of induction by cortisol of hepatic tryptophan pyrrolase. Biochem Pharmacol. 1979;28(1):77–81. doi: 10.1016/0006-2952(79)90273-9. [DOI] [PubMed] [Google Scholar]
- Sitaramam V., Ramasarma T. Inhibition of cortisol-mediated induction of hepatic tryptophan-2,3-dioxygenase by norepinephrine. Biochem Biophys Res Commun. 1974 Jul 24;59(2):578–583. doi: 10.1016/s0006-291x(74)80019-7. [DOI] [PubMed] [Google Scholar]
- Taurog A. Thyroid peroxidase and thyroxine biosynthesis. Recent Prog Horm Res. 1970;26:189–247. doi: 10.1016/b978-0-12-571126-5.50009-1. [DOI] [PubMed] [Google Scholar]
- Wells J. N., Kramer G. L. Phosphodiesterase inhibitors as tools in cyclic nucleotide research: a precautionary comment. Mol Cell Endocrinol. 1981 Jul;23(1):1–9. doi: 10.1016/0303-7207(81)90112-x. [DOI] [PubMed] [Google Scholar]
- Zeisberger E., Brück K. The significance of central adrenergic alpha-receptive structures in the control of thermogenesis and in cold adaptation. Isr J Med Sci. 1976 Sep;12(9):1103–1106. [PubMed] [Google Scholar]


