Abstract
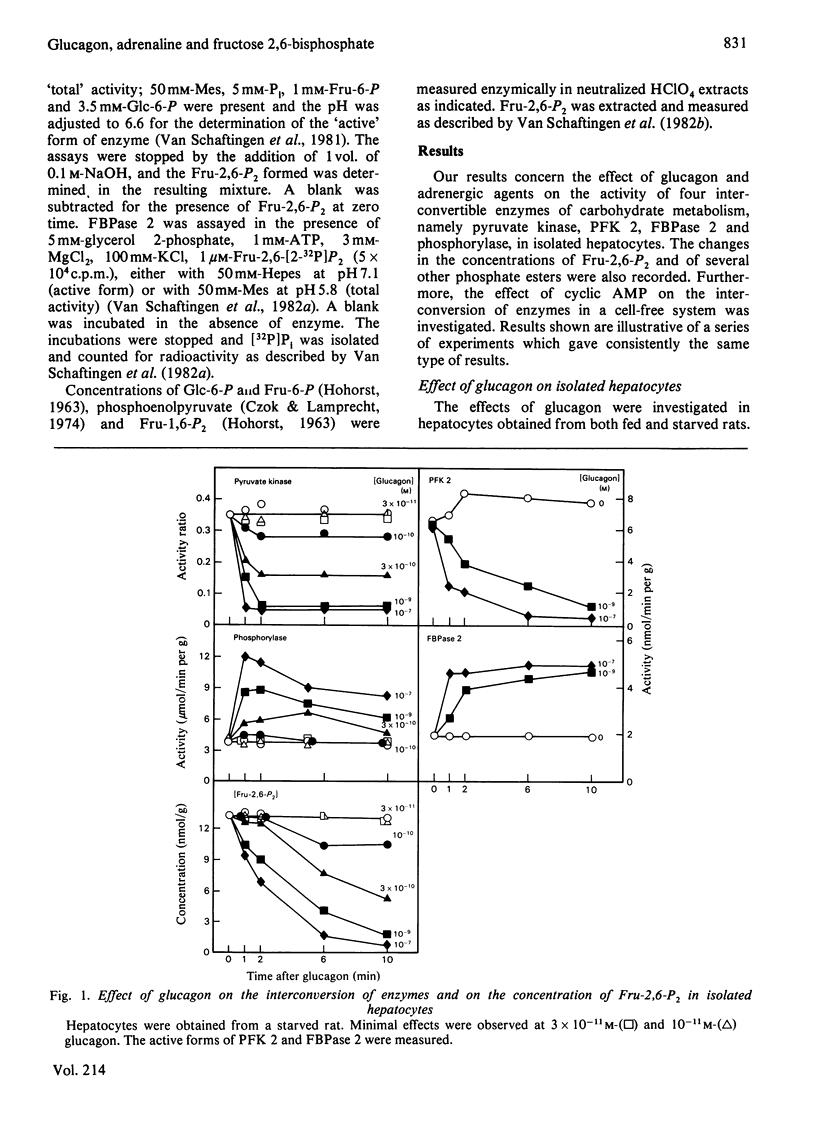
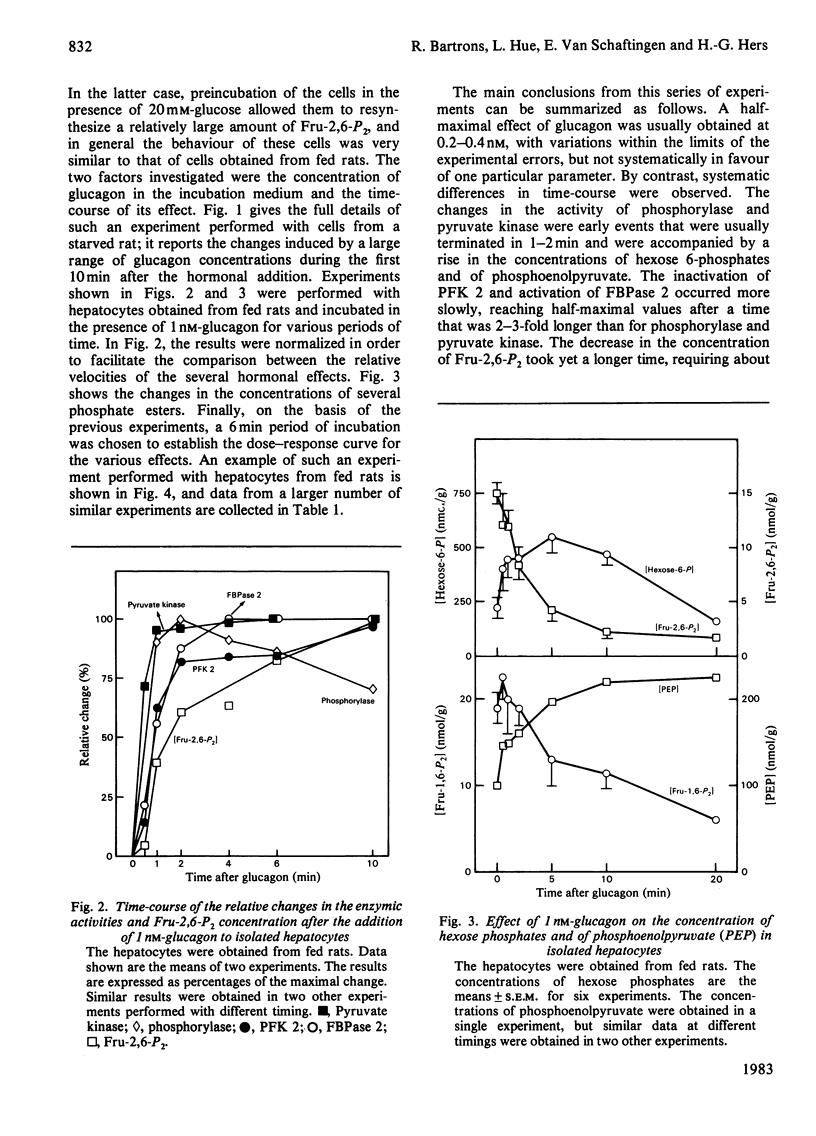
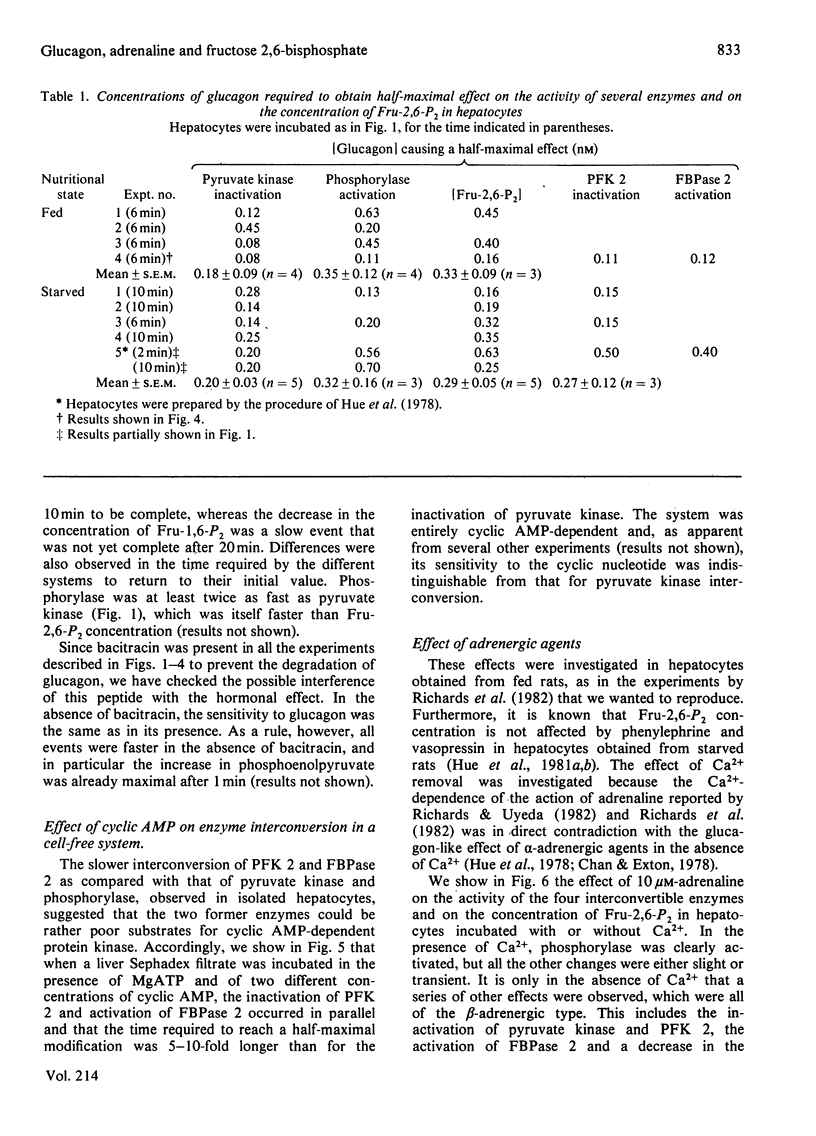
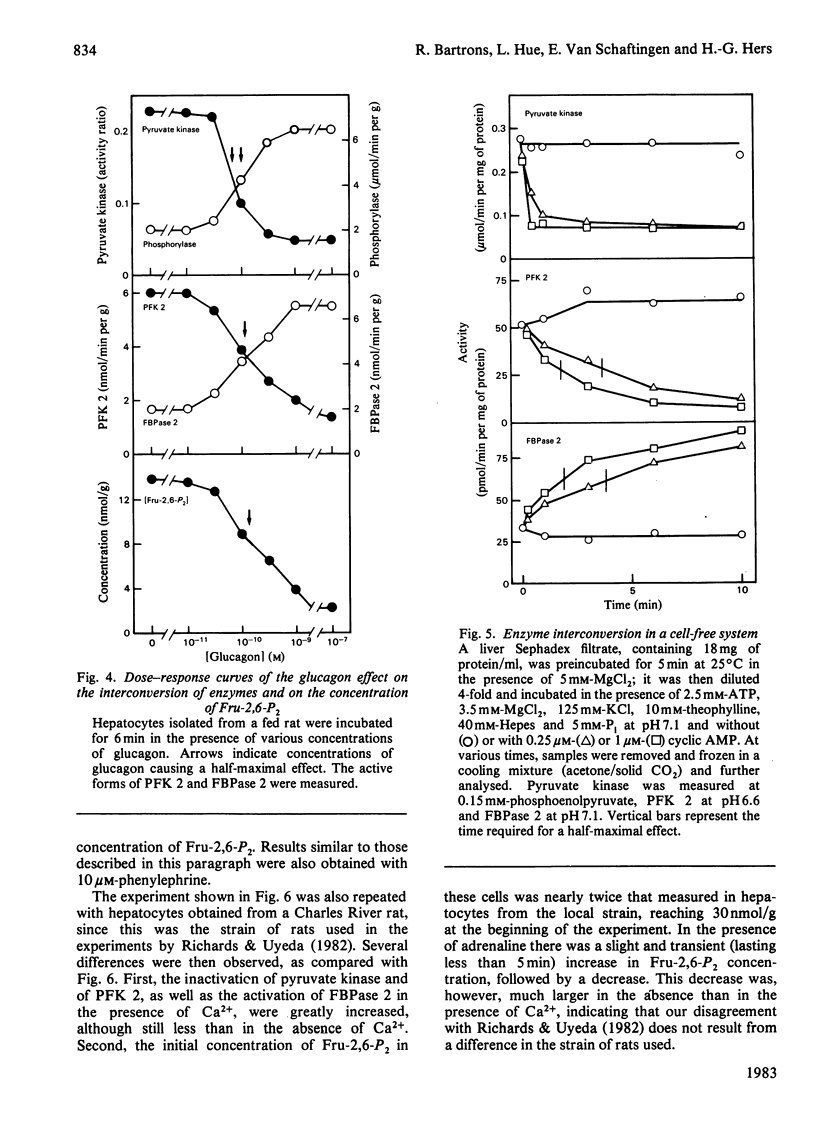
The ability of glucagon and of adrenaline to affect the concentration of fructose 2,6-bisphosphate in isolated hepatocytes was re-investigated because of important discrepancies existing in the literature. We were unable to detect a significant difference in the sensitivity of the hepatocytes with regard to the effect of glucagon to initiate the interconversion of phosphorylase, pyruvate kinase, 6-phosphofructo-2-kinase and fructose 2,6-bisphosphatase, and also to cause the disappearance of fructose 2,6-bisphosphate. In contrast, we have observed differences in the time-course of these various changes, since the interconversions of phosphorylase and of pyruvate kinase were at least twice as fast as those of 6-phosphofructo-2-kinase and of fructose 2,6-bisphosphatase. When measured in a cell-free system in the presence of MgATP, the cyclic AMP-dependent interconversion of pyruvate kinase was 5-10-fold more rapid than those of 6-phosphofructo-2-kinase and of fructose 2,6-bisphosphatase. These data indicate that 6-phosphofructo-2-kinase and fructose 2,6-bisphosphatase are relatively poor substrates for cyclic AMP-dependent protein kinase; they also support the hypothesis that the two catalytic activities belong to a single protein. Adrenaline had only a slight effect on the several parameters under investigation, except for the activation of phosphorylase. In the absence of Ca2+ ions from the incubation medium, however, adrenaline had an effect similar to that of glucagon.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackmore P. F., Brumley F. T., Marks J. L., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Relationship between alpha-adrenergic stimulation of calcium efflux and activation of phosphorylase in isolated rat liver parenchymal cells. J Biol Chem. 1978 Jul 25;253(14):4851–4858. [PubMed] [Google Scholar]
- Blair J. B., James M. E., Foster J. L. Adrenergic control of glucose output and adenosine 3':5'-monophosphate levels in hepatocytes from juvenile and adult rats. J Biol Chem. 1979 Aug 25;254(16):7579–7584. [PubMed] [Google Scholar]
- Chan T. M., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. J Biol Chem. 1978 Sep 25;253(18):6393–6400. [PubMed] [Google Scholar]
- Claus T. H., Schlumpf J. R., el-Maghrabi M. R., Pilkis J., Pilkis S. J. Mechanism of action of glucagon on hepatocyte phosphofructokinase activity. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6501–6505. doi: 10.1073/pnas.77.11.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Maghrabi M. R., Claus T. H., Pilkis J., Fox E., Pilkis S. J. Regulation of rat liver fructose 2,6-bisphosphatase. J Biol Chem. 1982 Jul 10;257(13):7603–7607. [PubMed] [Google Scholar]
- El-Maghrabi M. R., Fox E., Pilkis J., Pilkis S. J. Cyclic AMP-dependent phosphorylation of rat liver 6-phosphofructo 2-kinase, fructose 2,6-bisphosphatase. Biochem Biophys Res Commun. 1982 Jun 15;106(3):794–802. doi: 10.1016/0006-291x(82)91780-6. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Mallette L. E., Jefferson L. S., Wong E. H., Friedmann N., Miller T. B., Jr, Park C. R. The hormonal control of hepatic gluconeogenesis. Recent Prog Horm Res. 1970;26:411–461. doi: 10.1016/b978-0-12-571126-5.50014-5. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Molecular mechanisms involved in alpha-adrenergic responses. Mol Cell Endocrinol. 1981 Sep;23(3):233–264. doi: 10.1016/0303-7207(81)90123-4. [DOI] [PubMed] [Google Scholar]
- Feliú J. E., Hue L., Hers H. G. Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2762–2766. doi: 10.1073/pnas.73.8.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felíu J. E., Hue L., Hers H. G. Regulation in vitro and in vivo of adenosine 3':5'-monophosphate-dependent inactivation of rat-liver pyruvate kinase type L. Eur J Biochem. 1977 Dec;81(3):609–617. doi: 10.1111/j.1432-1033.1977.tb11988.x. [DOI] [PubMed] [Google Scholar]
- Hers H. G., Van Schaftingen E. Fructose 2,6-bisphosphate 2 years after its discovery. Biochem J. 1982 Jul 15;206(1):1–12. doi: 10.1042/bj2060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Felíu J. E., Hers H. G. Control of gluconeogenesis and of enzymes of glycogen metabolism in isolated rat hepatocytes. A parallel study of the effect of phenylephrine and of glucagon. Biochem J. 1978 Dec 15;176(3):791–797. doi: 10.1042/bj1760791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Van Schaftingen E., Blackmore P. F. Stimulation of glycolysis and accumulation of a stimulator of phosphofructokinase in hepatocytes incubated with vasopressin. Biochem J. 1981 Mar 15;194(3):1023–1026. doi: 10.1042/bj1941023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett M. F., Hue L., Hers H. G. Pyruvate kinase phosphatase. FEBS Lett. 1981 Sep 28;132(2):183–186. doi: 10.1016/0014-5793(81)81156-8. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., Chrisman T. D., El-Maghrabi M. R., Colosia A., Fox E., Pilkis J., Claus T. H. The action of insulin on hepatic fructose 2,6-bisphosphate metabolism. J Biol Chem. 1983 Feb 10;258(3):1495–1503. [PubMed] [Google Scholar]
- Pilkis S. J., Claus T. H., Johnson R. A., Park C. R. Hormonal control of cyclic 3':5'-AMP levels and gluconeogenesis in isolated hepatocytes from fed rats. J Biol Chem. 1975 Aug 25;250(16):6328–6336. [PubMed] [Google Scholar]
- Richards C. S., Furuya E., Uyeda K. Regulation of fructose 2,6-P2 concentration in isolated hepatocytes. Biochem Biophys Res Commun. 1981 Jun;100(4):1673–1679. doi: 10.1016/0006-291x(81)90711-7. [DOI] [PubMed] [Google Scholar]
- Richards C. S., Uyeda K. Hormonal regulation of fructose-6-P-2-kinase and fructose-2,6-P2 by two mechanisms. J Biol Chem. 1982 Aug 10;257(15):8854–8861. [PubMed] [Google Scholar]
- Richards C. S., Yokoyama M., Furuya E., Uyeda K. Reciprocal changes in fructose-6-phosphate,2-kinase and fructose-2,6-bisphosphatase activity in response to glucagon and epinephrine. Biochem Biophys Res Commun. 1982 Feb 11;104(3):1073–1079. doi: 10.1016/0006-291x(82)91359-6. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973 Dec;82(2):391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- Studer R. K., Borle A. B. Differences between male and female rats in the regulation of hepatic glycogenolysis. The relative role of calcium and cAMP in phosphorylase activation by catecholamines. J Biol Chem. 1982 Jul 25;257(14):7987–7993. [PubMed] [Google Scholar]
- Van Schaftingen E., Davies D. R., Hers H. G. Inactivation of phosphofructokinase 2 by cyclic AMP - dependent protein kinase. Biochem Biophys Res Commun. 1981 Nov 16;103(1):362–368. doi: 10.1016/0006-291x(81)91701-0. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hers H. G. Formation of fructose 2,6-bisphosphate from fructose 1,6-bisphosphate by intramolecular cyclisation followed by alkaline hydrolysis. Eur J Biochem. 1981 Jul;117(2):319–323. doi: 10.1111/j.1432-1033.1981.tb06339.x. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hers H. G. Inhibition of fructose-1,6-bisphosphatase by fructose 2,6-biphosphate. Proc Natl Acad Sci U S A. 1981 May;78(5):2861–2863. doi: 10.1073/pnas.78.5.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Control of the fructose-6-phosphate/fructose 1,6-bisphosphate cycle in isolated hepatocytes by glucose and glucagon. Role of a low-molecular-weight stimulator of phosphofructokinase. Biochem J. 1980 Dec 15;192(3):887–895. doi: 10.1042/bj1920887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Fructose 2,6-bisphosphate, the probably structure of the glucose- and glucagon-sensitive stimulator of phosphofructokinase. Biochem J. 1980 Dec 15;192(3):897–901. doi: 10.1042/bj1920897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Hoek J. B. Role of calcium in the hormonal regulation of liver metabolism. Biochim Biophys Acta. 1981 Dec 30;639(3-4):243–295. doi: 10.1016/0304-4173(81)90012-4. [DOI] [PubMed] [Google Scholar]
- van Schaftingen E., Davies D. R., Hers H. G. Fructose-2,6-bisphosphatase from rat liver. Eur J Biochem. 1982 May;124(1):143–149. doi: 10.1111/j.1432-1033.1982.tb05917.x. [DOI] [PubMed] [Google Scholar]
- van de Werve G., Hue L., Hers H. G. Hormonal and ionic control of the glycogenolytic cascade in rat liver. Biochem J. 1977 Jan 15;162(1):135–142. doi: 10.1042/bj1620135. [DOI] [PMC free article] [PubMed] [Google Scholar]


