Abstract
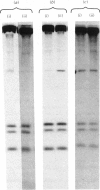
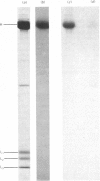
Myosin purified from a murine myeloid leukaemia cell line (M1) that had been incubated with [32P]orthophosphate incorporated 32P into the heavy, but not the light, chain. When the heavy chain was dephosphorylated by bacterial alkaline phosphatase, myosin that had low actin-activated ATPase activity gained higher activity only in the presence of the light-chain kinase. In the absence of the light-chain kinase, however, the Mg2+-stimulated ATPase activity of myosin was not activated by actin, regardless of phosphatase treatment. These results indicate that the activity of M1 myosin ATPase is regulated by phosphorylation of both the light and heavy chains. A scheme for this regulation by phosphorylation is presented and discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Conti M. A. Phosphorylation of platelet myosin increases actin-activated myosin ATPase activity. Nature. 1975 Aug 14;256(5518):597–598. doi: 10.1038/256597a0. [DOI] [PubMed] [Google Scholar]
- Chacko S., Conti M. A., Adelstein R. S. Effect of phosphorylation of smooth muscle myosin on actin activation and Ca2+ regulation. Proc Natl Acad Sci U S A. 1977 Jan;74(1):129–133. doi: 10.1073/pnas.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. H., Korn E. D. Actin activation of Ca2+-sensitive Mg2+-ATPase activity of Acanthamoeba myosin II is enhanced by dephosphorylation of its heavy chains. J Biol Chem. 1980 Sep 10;255(17):8011–8014. [PubMed] [Google Scholar]
- Collins J. H., Korn E. D. Purification and characterization of actin-activatable, Ca2+-sensitive myosin II from Acanthamoeba. J Biol Chem. 1981 Mar 10;256(5):2586–2595. [PubMed] [Google Scholar]
- Fechheimer M., Cebra J. J. Phosphorylation of lymphocyte myosin catalyzed in vitro and in intact cells. J Cell Biol. 1982 May;93(2):261–268. doi: 10.1083/jcb.93.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górecka A., Aksoy M. O., Hartshorne D. J. The effect of phosphorylation of gizzard myosin on actin activation. Biochem Biophys Res Commun. 1976 Jul 12;71(1):325–331. doi: 10.1016/0006-291x(76)90286-2. [DOI] [PubMed] [Google Scholar]
- Hesketh J. E., Virmaux N., Mandel P. Evidence for a cyclic nucleotide-dependant phosphorylation of retinal myosin. FEBS Lett. 1978 Oct 15;94(2):357–360. doi: 10.1016/0014-5793(78)80976-4. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y. Differentiation of a cell line of myeloid leukemia. J Cell Physiol. 1969 Dec;74(3):223–234. doi: 10.1002/jcp.1040740303. [DOI] [PubMed] [Google Scholar]
- Kuczmarski E. R., Spudich J. A. Regulation of myosin self-assembly: phosphorylation of Dictyostelium heavy chain inhibits formation of thick filaments. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7292–7296. doi: 10.1073/pnas.77.12.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Muhlrad A., Oplatka A. Phosphorylation of fibroblast myosin. FEBS Lett. 1977 May 1;77(1):37–40. doi: 10.1016/0014-5793(77)80188-9. [DOI] [PubMed] [Google Scholar]
- Nagata K., Sagara J., Ichikawa Y. Changes in contractile proteins during differentiation of myeloid leukemia cells. I. Polymerization of actin. J Cell Biol. 1980 May;85(2):273–282. doi: 10.1083/jcb.85.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Sagara J., Ichikawa Y. Changes in contractile proteins during differentiation of myeloid leukemia cells. II. Purification and characterization of actin. J Cell Biol. 1982 May;93(2):470–478. doi: 10.1083/jcb.93.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Korn E. D. Acanthamoeba myosin. I. Isolation from Acanthamoeba castellanii of an enzyme similar to muscle myosin. J Biol Chem. 1973 Jul 10;248(13):4682–4690. [PubMed] [Google Scholar]
- Sagara J., Nagata K., Ichikawa Y. A cofactor protein required for actin activation of myosin Mg2+ATPase activity in leukemic myeloblasts. J Biochem. 1982 Dec;92(6):1845–1851. doi: 10.1093/oxfordjournals.jbchem.a134114. [DOI] [PubMed] [Google Scholar]
- Sagara J., Nagata K., Ichikawa Y. Changes in myosin during differentiation of myeloid leukemia cells. J Biochem. 1982 Apr;91(4):1363–1372. doi: 10.1093/oxfordjournals.jbchem.a133824. [DOI] [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Myosin-linked calcium regulation in vertebrate smooth muscle. J Mol Biol. 1976 Mar 25;102(1):75–92. doi: 10.1016/0022-2836(76)90074-7. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stull J. T., Buss J. E. Phosphorylation of cardiac troponin by cyclic adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Feb 10;252(3):851–857. [PubMed] [Google Scholar]
- Trotter J. A., Adelstein R. S. Macrophage myosin. Regulation of actin-activated ATPase, activity by phosphorylation of the 20,000-dalton light chain. J Biol Chem. 1979 Sep 25;254(18):8781–8785. [PubMed] [Google Scholar]
- Trotter J. A. Living macrophages phosphorylate the 20,000 Dalton light chains and heavy chains of myosin. Biochem Biophys Res Commun. 1982 Jun 15;106(3):1071–1077. doi: 10.1016/0006-291x(82)91820-4. [DOI] [PubMed] [Google Scholar]
- Yerna M. J., Dabrowska R., Hartshorne D. J., Goldman R. D. Calcium-sensitive regulation of actin-myosin interactions in baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):184–188. doi: 10.1073/pnas.76.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]