Abstract
Neutrophils, which originate from the bone marrow and are characterized by a segmented nucleus and a brief lifespan, have a crucial role in the body’s defense against infections and acute inflammation. Recent research has uncovered the complex roles of neutrophils as regulators in tumorigenesis, during which neutrophils exhibit a dualistic nature that promotes or inhibits tumor progression. This adaptability is pivotal within the tumor microenvironment (TME). In this review, we provide a comprehensive characterization of neutrophil plasticity and heterogeneity, aiming to illuminate current research findings and discuss potential therapeutic avenues. By delineating the intricate interplay of neutrophils in the TME, this review further underscores the urgent need to understand the dual functions of neutrophils with particular emphasis on the anti-tumor effects to facilitate the development of effective therapeutic strategies against cancer.
Keywords: Neutrophil, plasticity, tumor microenvironment, immunotherapy
Introduction
Neutrophils, which comprise a significant proportion of circulating leukocytes in humans (50%–70%) and mice (10%–25%)1,2, have pivotal roles in responding to infection and acute inflammation3. However, neutrophil involvement in tumor progression is multifaceted. Neutrophils, which are derived from bone marrow granulocyte monocyte progenitors (GMPs), are characterized by segmented nuclei, a short lifespan, and rapid turnover4,5. These granulocytes are essential in host defense and profoundly impact tumor dynamics by infiltrating tumors, demonstrating remarkable phenotypic traits6.
Recent studies have unveiled the dichotomous nature of neutrophil behavior in tumors with the ability to promote or inhibit tumor growth likely reflecting neutrophil plasticity in response to environmental cues7–10. Of note, some studies have shown that the cytokine and chemokine profiles within the tumor microenvironment (TME) may dictate neutrophil recruitment and functional orientation11–13. While an elevated neutrophil level within solid tumors often correlates with unfavorable clinical outcomes11, emerging evidence suggests the potential of neutrophils to impede tumor progression through mechanisms, such as direct tumor cell cytotoxicity and modulation of innate and adaptive immune responses14,15, which were previously underestimated.
This review aimed to unravel the intricate roles of neutrophils, specifically elucidating how distinct neutrophil subsets exert dual effects on the TME. By meticulously exploring neutrophil heterogeneity and diverse neutrophil functions within the TME, this review offers a comprehensive synthesis of the latest research findings. Moreover, the prospect of leveraging these dynamic neutrophil behaviors to forge innovative therapeutic strategies for cancer treatment is discussed.
Development, maturation, and recruitment of neutrophils
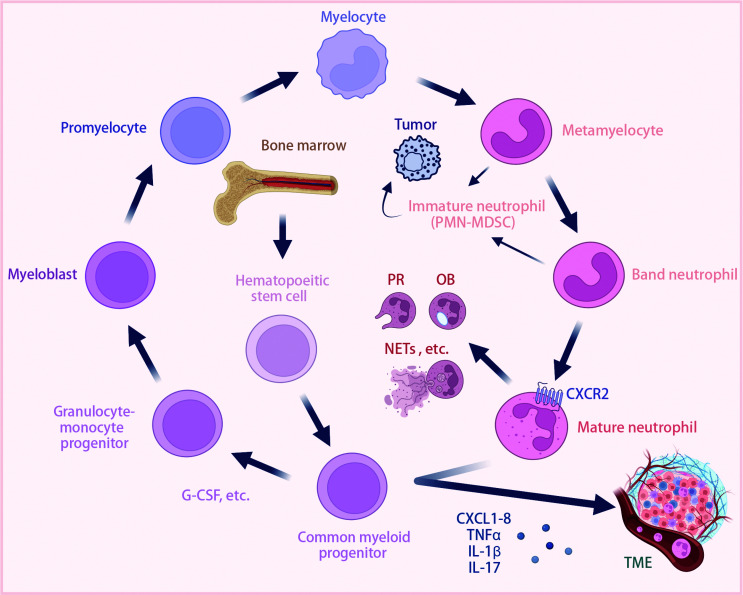
Neutrophil development is initiated within the bone marrow from self-renewing hematopoietic stem cells in a process termed “granulopoiesis” (Figure 1). These stem cells differentiate into multipotent hematopoietic progenitors that subsequently give rise to common myeloid and lymphoid progenitors. In response to increased cytokine levels, such as granulocyte colony-stimulating factor (G-CSF), common myeloid progenitors differentiate into granulocyte or monocyte progenitors16. This orchestrated differentiation leads to the progression of myeloblasts through various stages, including promyelocytes, myelocytes, metamyelocytes, and band neutrophils, culminating in the formation of mature neutrophils17.
Figure 1.
Overview of neutrophil development, maturation, and recruitment. Neutrophils develop from hematopoietic stem cells in the bone marrow through several stages: hematopoietic stem cells; common myeloid progenitors; granulocyte-monocyte progenitors; myeloblasts; promyelocytes; myelocytes; metamyelocytes; band neutrophils; and mature neutrophils. Cytokines, such as G-CSF, have a crucial role in each stage of this differentiation process. Immature neutrophils (PMN-MDSCs) can contribute to TME dynamics with pro-tumor effects. However, mature neutrophils participate in immune responses by PR, OB, and forming NETs. Neutrophils are recruited by chemokines (CXCL1-8) and inflammatory mediators (TNF-α, IL-1β, and IL-17) in the TME. Neutrophils express receptors, such as CXCR2, to facilitate migration and have pro- or anti-tumor effects depending on the context. CXCL1-8, C-X-C motif chemokine ligand 1-8; CXCR2, C-X-C motif chemokine receptor 2; G-CSF, granulocyte colony-stimulating factor; IL-1β, interleukin-1beta; IL-17, interleukin-17; NETs, neutrophil extracellular traps; OB, oxidative burst; PR, pathogen recognition; PMN-MDSCs, polymorphonuclear myeloid-derived suppressor cells; TME, tumor microenvironment; TNF-α, tumor necrosis factor-alpha. Created using BioRender.com.
Mature neutrophils contribute to inflammation resolution through diverse mechanisms, including pathogen recognition and phagocytosis, degranulation, oxidative burst, and the generation of neutrophil extracellular traps (NETs18; Figure 1). Neutrophils are subsequently cleared from the tissue via macrophage phagocytosis19. Beyond the classic role in acute infection resolution, emerging evidence underscores the pivotal involvement of neutrophils in tumor regulation6. Neutrophils populate the TME in various malignancies15. However, the precise neutrophil function remains enigmatic, often contingent upon tumor type, developmental stage, and the interplay with other cellular constituents20.
Moreover, neutrophils undergo a complex journey from the bone marrow to tumor sites that is coordinated by a finely tuned gene expression program (Figure 1). As neutrophils mature, expression of chemokine receptors, such as C-X-C motif chemokine receptor 2 (CXCR2)21,22, is modulated. Changes in these surface receptors direct neutrophil migration toward their chemokine ligands [C-X-C motif chemokine ligand 1 (CXCL1), CXCL2, CXCL3, CXCL5, CXCL6, and CXCL823,24], which are highly expressed in the tumor environment6,25,26. Additionally, inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-17, and IL-1β, have crucial roles in orchestrating neutrophil recruitment into the TME6,27,28.
Finally, tumor-induced stress can trigger “emergency granulopoiesis,” which alters neutrophil maturation and release. This results in the circulation of immature neutrophils, which are sometimes identified as “polymorphonuclear myeloid-derived suppressor cells” (PMN-MDSCs)29. PMN-MDSCs may exert pro-tumor effects within the tumor milieu26 (Figure 1). However, the underlying mechanisms have not been elucidated and discerning the impact of these immature neutrophil populations in vivo is technically challenging.
Despite significant strides in understanding neutrophil biology in tumorigenesis, the multifaceted roles in tumor immunity have not been fully described, primarily due to the inherent neutrophil plasticity and heterogeneity. Neutrophils encompass diverse subsets, each endowed with unique functions within the TME. Deciphering the regulatory mechanisms governing these processes is paramount for bridging the developmental biology of neutrophils with their intricate roles in tumor immunology.
Neutrophil plasticity and heterogeneity in the TME
Historically viewed as short-lived effector cells with limited plasticity, neutrophils have emerged as remarkably heterogeneous and dynamic entities in the TME, challenging traditional perceptions30–32. Extensive evidence underscores the significant plasticity and heterogeneity of neutrophils, reflecting a spectrum of phenotypes and functions akin to the M1/M2 paradigm observed in macrophages. Neutrophils are now classified into anti-tumor (N1) and pro-tumor (N2) subsets, highlighting their diverse roles in tumor progression6,33,34.
Advanced methodologies, such as single-cell RNA sequencing (scRNA-seq), mass cytometry, and spatial transcriptomics, have unveiled the intricate heterogeneity within neutrophil populations26,35–39. For example, scRNA-seq delineated 11 distinct neutrophil clusters in primary liver tumors, each characterized by unique gene signatures regulated spatiotemporally. Notably, tumor-associated neutrophils (TANs) were predominant, with specific clusters exhibiting functionalities, such as macrophage recruitment via the chemokine (C-C motif) ligand 4 (CCL4)-chemokine (C-C motif) receptor 5 (CCR5) pathway (Neu_11_CCL4) and inhibition of T cell cytotoxicity (Neu_09_IFIT1)40.
Similarly, mass cytometry analysis of melanoma identified 7 neutrophil subsets, including terminally differentiated subsets exhibiting dynamic changes during tumor progression41. Diverse subpopulations within the low-density neutrophil (LDN) and high-density neutrophil (HDN) subsets in lung cancer were identified with implications for disease prognosis41. Remarkably, an intermediate cluster (CD66b+/CD10low/CXCR4+/PD-L1) exclusive to advanced lung cancer was shown to correlate with poorer outcomes42.
Our recent study unveiled diverse transcriptional profiles in neutrophils using scRNA-seq in 17 tumor types from 143 patients15. Noteworthy findings included enrichment of neutrophils linked to antigen presentation (HLA-DR+CD74+) and angiogenesis (VEGFA+SPP1+) in cancerous tissues, while inflammatory clusters (IFIT1+ISG15+ and NFKBIZ+HIF1A+) were prevalent in conditions, such as chronic pancreatitis and COVID-19. Moreover, distinct neutrophil clusters were shown to exhibit associations with specific tumor types and clinical outcomes, underscoring their prognostic significance.
Specifically, VEGFA+SPP1+ neutrophils, which are prevalent in renal cell carcinoma and gastric adenocarcinoma, display enhanced glycan metabolism and are correlated with poor outcomes. Conversely, HLA-DR+CD74+ neutrophils are associated with more favorable prognoses in non-small-cell lung, bladder, and ovarian cancer15.
These findings underscore the pivotal role of neutrophil plasticity and heterogeneity in tumor progression and offer critical insight for the development of prognostic models and therapeutic strategies in cancer management.
The distinct role of neutrophils
Pro-tumor mechanisms of neutrophils
Neutrophils may directly promote tumor progression by enhancing genetic instability, promoting tumor cell proliferation, and facilitating angiogenesis or indirectly by suppressing anti-tumor immune responses and facilitating metastatic spread (Figure 2).
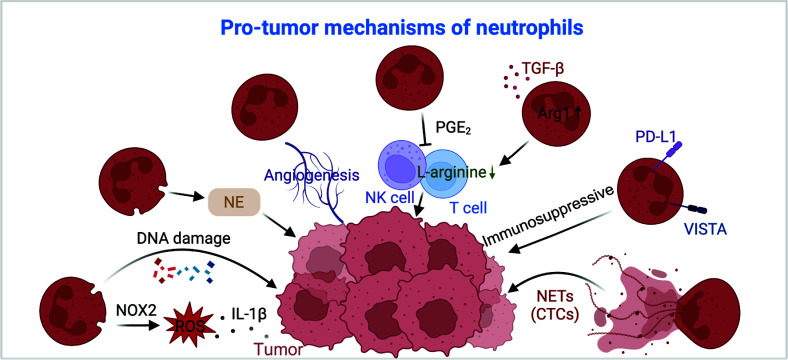
Figure 2.
Overview of neutrophil mechanisms in pro-tumor activities. Neutrophils support tumor progression through multiple mechanisms. Neutrophils produce NOX2-derived ROS, which promotes tumor growth via an IL-1β-dependent pathway. Additionally, neutrophils induce DNA damage and secrete NE, which enhances tumor development. Neutrophils promote tumor development through angiogenesis. Neutrophils also suppress immune responses by releasing PGE2, which negatively affects NK and T cell activity. Moreover, in response to TGF-β, neutrophils enhance Arg-1 expression, depleting L-arginine in T cells and leading to T cell dysfunction. Neutrophils contribute to immunosuppression by expressing PD-L1 and VISTA. Finally, neutrophils release NETs that trap CTCs and aid in metastasis. Arg-1, arginase-1; CTCs, circulating tumor cells; IL-1β, interleukin-1β; NE, neutrophil elastase; NETs, neutrophil extracellular traps; NOX2, NADPH oxidase 2; PGE2, prostaglandin E2; ROS, reactive oxygen species; TGF-β, transforming growth factor-beta; VISTA, v-domain immunoglobulin suppressor of T-cell activation. Created using BioRender.com.
Extensive research has highlighted that neutrophil-derived enzymes, notably reactive oxygen species (ROS) and neutrophil elastase (NE), contribute to tumor initiation43–46. Notably, neutrophil NADPH oxidase 2 (NOX2)-derived ROS has been reported to support tumor colonization through an IL-1β-dependent pathway47. Additionally, ROS can induce DNA damage in lung cells, especially when combined with carcinogens, which accelerates tumor formation48 (Figure 2). In contrast to recent results on the role of human NE49, deletion of NE in prostate and lung cancer murine models resulted in smaller tumors, establishing the crucial role of murine NE in tumor development43,50 (Figure 2). Because murine neutrophils cannot release catalytically active NE49, these effects may be due to changes in neutrophil biology resulting from the absence of NE. Previous research has demonstrated that NE−/− neutrophils have an altered ability to migrate to inflammatory sites and respond to inflammatory challenges51,52.
Moreover, in vivo studies consistently support the tumor-promoting capacity of neutrophils, particularly through mechanisms, such as induction of angiogenesis, which is imperative for sustained tumor growth53. The existence of reprogrammed decoy TNF-related apoptosis-inducing ligand-receptor 1+ (dcTRAIL-R1+) neutrophils within hypoxic and glycolytic tumor niches have been shown to exert pro-angiogenic effects that favor tumor expansion39 (Figure 2).
Furthermore, neutrophil-mediated immunosuppression constitutes a significant mechanism facilitating tumor progression6. This mechanism includes suppression of NK-mediated tumor cell clearance and promotion of disseminated carcinoma cell extravasation54. Our prior work also underscored the critical role of soluble mediators released by neutrophils, notably prostaglandin E2 (PGE2), in subverting the effector functions of T and NK cells55 (Figure 2). Moreover, the substantial production of arginase-1 (Arg-1) by neutrophils in response to transforming growth factor-beta (TGF-β) profoundly impacts T cell metabolism, leading to T cell dysfunction as a result L-arginine depletion33 (Figure 2).
Additional studies have indicated the presence of neutrophils that express PD-L1 or V-domain immunoglobulin suppressor of T-cell activation (VISTA) in human and murine models of hepatocellular carcinoma, melanoma, and gastric cancer56–59. Inhibition of VISTA was reported to lead to a pronounced pro-inflammatory response in myeloid cells and reduce the capacity to suppress immune responses in a murine melanoma model59 (Figure 2). Moreover, brain TANs exhibit distinct immunosuppressive and pro-angiogenic capacities compared to their circulating counterparts60.
Another crucial facet of neutrophil involvement in tumor progression is the release of NETs61. Studies have elucidated the role of NETs in promoting the initiation of metastasis, as evidenced by intravital imaging demonstrating the co-localization of tumor cells with endothelial cell-associated neutrophils62. NETs facilitate metastasis by entrapping circulating tumor cells (CTCs) and promoting adhesion of CTCs at distant sites, with excessive NET formation correlating with shorter progression-free survival63,64 (Figure 2).
The interaction between neutrophils and CTCs in breast cancer enhances the metastatic potential by driving cell cycle progression within the bloodstream65. Finally, the neutrophil-to-lymphocyte ratio (NLR) has emerged as a promising biomarker for tumor patient risk stratification, with alterations in NLR indicating disease recurrence, progression, or response to therapy66,67. Moreover, interferon-stimulated neutrophils serve as a predictor of the immunotherapy response8,68.
Anti-tumor mechanisms of neutrophils
The anti-tumor roles of neutrophils, which are often overlooked, are vital to the immune system arsenal against tumors. In this section we delve into the multifaceted functions and mechanisms of neutrophils in tumor suppression (Figure 3A–C).
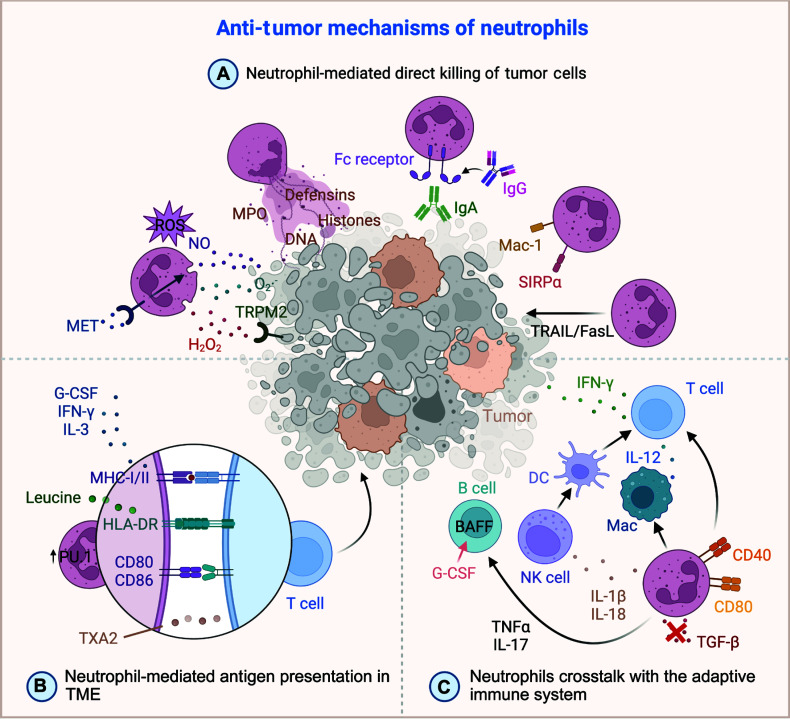
Figure 3.
Overview of neutrophil mechanisms in anti-tumor activities. (A) Direct killing of tumor cells mediated by neutrophils. Neutrophils produce ROS, such as NO, O2·−, and H2O2, which directly damage tumor cells. The production of NO is enhanced by MET receptor signaling. ROS-mediated tumor cell killing hinges on tumor cell TRPM2 expression, an H2O2-dependent calcium channel that triggers a lethal influx of calcium ions into the cell. Additionally, neutrophils release MPO, defensins, histones, and DNA, all of which contribute to the direct killing of tumor cells. Neutrophils also express Fc receptors and Mac-1 adhesion molecules, facilitating ADCC against tumor cells. Notably, inhibiting the CD47-SIRPα interaction is a crucial strategy to boost neutrophil-mediated ADCC. Moreover, through the TRAIL/FasL pathway, neutrophils induce apoptosis and necrosis in tumor cells. (B) Antigen presentation by neutrophils within the TME. Cytokines, such as G-CSF, IFN-γ, and IL-3, along with metabolites (leucine and transcription factor PU.1) influence the antigen-presenting ability of neutrophils. Molecules, such as MHC-I/II, HLA-DR, CD80, and CD86, which are expressed on neutrophils, are involved in antigen presentation to T cells through their corresponding receptors, thereby facilitating the immune response. (C) Crosstalk between neutrophils and the adaptive immune system. Expression of co-stimulatory molecules (CD40 and CD80) on neutrophils enhances the T cell anti-tumor immune response. Blocking TGF-β not only promotes neutrophil recruitment but also facilitates a stronger cytotoxic T cell response. Additionally, neutrophils have a multifaceted role in regulating adaptive immune responses through interactions with various immune cells, such as NK cells, DCs, and B cells. Neutrophils achieve this regulation by secreting cytokines, including IL-1β, IL-17, IL-18, and TNF-α, which significantly influence the broader immune landscape. FasL, fas ligand; Fc, fragment crystallizable; G-CSF, granulocyte colony-stimulating factor; H2O2, hydrogen peroxide; IFN-γ, interferon-gamma; IL-1β, interleukin-1beta; IL-3, interleukin-3; IL-12, interleukin-12; IL-17, interleukin-17; IL-18, interleukin-18; Mac-1, macrophage-1 antigen; MET, mesenchymal-epithelial transition; MHC-I/II, major histocompatibility complex-I/II; MPO, granule enzyme myeloperoxidase; NO, nitric oxide; O2·−, superoxide radical; ROS, reactive oxygen species; SIRP-α, signal regulatory protein-alpha; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor-alpha; TRAIL, tumor necrosis factor-related apoptosis-induced ligand; TRPM2, transient receptor potential cation channel, subfamily M, member 2; TXA2, thromboxane A2. Created using BioRender.com.
Neutrophil-mediated direct killing of tumor cells
Neutrophils exhibit potent anti-tumor effects that are partly achieved through direct cytotoxic and cytostatic mechanisms. Notably, ROS, which include crucial components [hydrogen peroxide (H2O2), superoxide anion (O2·−), and nitric oxide (NO)], have a central role in neutrophil-mediated tumor lysis69 (Figure 3A). Recent studies have unveiled the anti-tumor potential of β-glucan-induced training of granulopoiesis, demonstrating ROS-dependent tumor suppression. Remarkably, the anti-tumor properties of trained neutrophils can be transferred from donor murine bone marrow to recipient naïve mice70. ROS-mediated tumor cell killing hinges on tumor cell expression of transient receptor potential cation channel, subfamily M, member 2 (TRPM2), an H2O2-dependent calcium channel, which triggers a lethal influx of calcium ions into the cell71 (Figure 3A). Furthermore, neutrophil-derived nitric oxide production, which is potentiated by mesenchymal-epithelial transition (MET) receptor signaling, enhances tumor cell killing72 (Figure 3A). NETs also contribute to tumor cell cytotoxicity. NET components, such as myeloperoxidase, can obliterate melanoma cells, while defensins and histones contribute to tumor cell lysis and destruction of supportive blood vessels and epithelial cells, respectively73 (Figure 3A). Moreover, expelled deoxyribonucleic acid (DNA) strands from NETs entrap tumor cells, impairing their metastatic and proliferative capacities74 (Figure 3A).
Surface receptors, such as Fc receptors, enable neutrophils to engage with antibody-opsonized tumor cells, heightening their cytotoxic and phagocytic capabilities. This interaction, which is particularly potent when tumor cells are opsonized with tumor-targeting monoclonal antibodies, facilitates mechanisms, such as antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP75,76; Figure 3A). Neutrophils also demonstrate efficacy in direct tumor cell killing through macrophage-1 antigen (MAC-1)-dependent cellular contacts, which are crucial for cytotoxic activity77,78 (Figure 3A). Additionally, interactions between neutrophils and trastuzumab (IgG)-opsonized cells lead to significant tumor cell destruction in vitro. Compared to IgG, IgA antibodies demonstrate enhanced tumor cell-killing capability (Figure 3A). Notably, inhibiting the CD47-signal regulatory protein-alpha (SIRP-α) interaction, a crucial target, boosts neutrophil-mediated ADCC78–80 (Figure 3A).
Additionally, neutrophils induce tumor cell death via apoptosis and necrosis, as exemplified by the Fas–Fas ligand (FasL) pathway, in which neutrophil-expressed FasL triggers apoptosis upon interaction with the Fas receptor on tumor cells81. Neutrophils stimulated by interferon-gamma (IFN-γ) express TRAIL, inducing apoptosis by interacting with death receptors on tumor cells82 (Figure 3A).
Neutrophil-mediated antigen presentation in the TME
Neutrophils have a pivotal role in orchestrating the immune response against tumors by shuttling tumor antigens from the tumor site to the draining lymph nodes. The ability to present antigens to T cells is indispensable for initiating an effective immune response. This capacity is augmented by cytokines, such as GM-CSF, IFN-γ, and IL-3, which enhance phagocytic activity and the expression of major histocompatibility complex (MHC) molecules necessary for efficient antigen presentation83–85 (Figure 3B). However, optimal antigen presentation by neutrophils requires additional signals from activated T cells because innate cues alone, such as signals from Toll-like receptors (TLRs) or damage-associated molecular patterns (DAMPs), are insufficient to induce high levels of MHC molecule expression86–88.
The role of neutrophils in antigen presentation becomes increasingly crucial in the context of decreased migration of dendritic cells (DCs) and T cells to the tumor site, which is often due to increased thromboxane A2 (TXA2) secretion by neutrophils89,90 (Figure 3B). While neutrophils traditionally lack the robust signaling capabilities of professional antigen-presenting cells, such as DCs, the capacity of neutrophils to effectively activate T cells can be enhanced. For example, endocytosis of antibody-antigen complexes via Fcγ receptors has been shown to elicit CD8+ T cell-dependent anti-tumor immunity in vivo91.
A cluster of N1 neutrophils, which is characterized by CD86 and HLA-DR expression, has been shown to have antigen-presenting capabilities that potentiate the anti-tumor effect of T cells92. In our study using scRNA-seq in multiple tumor types, we observed diverse transcriptional profiles of neutrophils and identified specific subsets with enhanced antigen-presenting capabilities (HLA-DR+CD74+ neutrophils). These subsets, which are significantly involved in amino acid metabolism (especially leucine) were shown to co-localize with CD8+ and CD4+ T cells in the TME and appeared to directly activate T cells through effective ligand-receptor interactions15 (Figure 3B).
Furthermore, the transcription factor, PU.1 (spi-1 proto-oncogene), has a pivotal role in augmenting antigen presentation by neutrophils91 (Figure 3B). In recent years there has been a growing interest in innovative immunotherapeutic strategies, including non-canonical antigen presentation by neutrophils. For example, a combination of radiotherapy and radiodynamic therapy with nanoscale metal–organic frameworks have shown promise in enhancing immune-mediated tumor regression. This approach boosts the expression of co-stimulatory molecules, such as CD80 and CD86, as well as MHC-II molecules on neutrophils, which facilitates effective cross-presentation of antigens and enhances the immune response against tumors93 (Figure 3B).
Neutrophils crosstalk with the adaptive immune system
Neutrophils have a dynamic role in shaping the adaptive immune response, particularly in the initial stages of lung cancer, during which neutrophils bolster T cell functions. This interaction fosters enhanced T cell proliferation and elevated IFN-γ release, thereby amplifying proinflammatory factors and upregulating co-stimulatory molecules on T cells94 (Figure 3C). Notably, in a murine colorectal cancer model, interleukin-1 receptor-associated kinase-M (IRAK-M)-deficient neutrophils have been shown to modulate the TME by diminishing PD-L1 and CD11b expression while enhancing CD40 and CD80 levels, consequently promoting a robust T cell anti-tumor immune response95 (Figure 3C). Subsequent studies showed that blocking TGF-β promotes neutrophil recruitment and also supports cytotoxic T cell responses that exhibited significant anti-tumor activity in lung cancer33,96 (Figure 3C).
In addition to T cells, neutrophils engage in intricate crosstalk with NK cells through various mechanisms97,98. For example, cytokine-stimulated NK cells and neutrophils exchange contact-dependent activation signals mediated by CD18, intercellular adhesion molecule-1 (ICAM-1), and ICAM-397. Additionally, activated neutrophils attract and activate NK cells by releasing IL-1β and IL-18, which triggers a cascade of events culminating in dendritic cell maturation, T cell proliferation, and IFN-γ production98 (Figure 3C). Moreover, neutrophils collaborate with macrophages to enhance IL-12 secretion, which facilitates the polarization of unconventional αβ (UTCαβ) T cells that produce IFN-γ, thereby bolstering anti-tumor immunity99 (Figure 3C).
Furthermore, neutrophils mediate B-cell chemotaxis by secreting TNF-α, especially in the presence of chemokines (CXCL13 or CXCL12)100. Although the direct interaction between neutrophils and follicular B cells remains elusive, evidence suggests that neutrophils accumulate in B-cell zones, where neutrophils secrete B-cell-activating factor (BAFF) through a G-CSF-dependent mechanism and support the accelerated generation of plasma cells101,102 (Figure 3C). Intriguingly, neutrophils also modulate immunoglobulin production by blocking the BAFF receptor on B cells103. Given the multifaceted roles of B cells in anti-tumor immunity and the capacity of B cells to activate other immune cells, such as T and NK cells, elucidating the involvement of neutrophils in this immune crosstalk is of paramount importance104,105.
Recent studies have further underscored the regulatory role of neutrophils in shaping the tumor-associated microbiota via IL-17, thereby fostering B cell activity within the TME and augmenting the overall immune response106 (Figure 3C). These findings highlight the intricate interplay between neutrophils and various components of the adaptive immune system in orchestrating anti-tumor immunity.
Discussion and future perspectives
The increasing interest in the therapeutic potential of neutrophils in tumor treatment has sparked widespread attention107–109. However, recent research has predominantly focused on targeting the immunosuppressive and other pro-tumor functions of neutrophils (Table 1).
Table 1.
Clinical trials based on neutrophil targets
| Targets | Compounds | Clinical trials | Phases | Aims |
|---|---|---|---|---|
| G-CSF mimetics | Pegfilgrastim | NCT00035594 | III | Neutropenia |
| YPEG-rhG-CSF | NCT02005458 | II | Neutropenia | |
| CXCR2 inhibitor | AZD5069 | NCT03177187 | I/II | Metastatic castration-resistant prostate cancer |
| 2020-003346-36 | I/II | Advanced hepatocellular carcinoma | ||
| CXCR1/CXCR2 inhibitors | SX-682 | NCT03161431 | I | Metastatic melanoma |
| NCT05570825 | II | Metastatic non-small cell lung cancer | ||
| NCT06149481 | I/II | Metastatic colorectal cancer | ||
| Reparixin | NCT02001974 | I | HER2-negative metastatic breast cancer | |
| NCT02370238 | II | Metastatic triple-negative breast cancer | ||
| CCR5 antagonist | Maraviroc | NCT01736813 | I | Metastatic colorectal cancer |
| NCT03274804 | I | Metastatic colorectal cancer | ||
| NE inhibitor | Sivelestat | NCT01170845 | NA | Esophageal cancer |
| NSAIDs | Aspirin/ibuprofen | NCT01786200 | NA | Colon cancer |
| Celecoxib | NCT02429427 | III | Breast cancer | |
| NCT04105335 | I | Advanced solid tumors | ||
| TGF-β pathway inhibitors | Fresolimumab | NCT02581787 | I/II | Non-small cell lung cancer |
| Galunisertib | NCT01582269 | II | Recurrent glioblastoma | |
| NCT01682187 | I | Recurrent malignant glioma | ||
| NCT02452008 | II | Metastatic castration-resistant prostate cancer | ||
| NCT02672475 | I | Metastatic triple-negative breast cancer | ||
| NCT02734160 | I | Metastatic pancreatic cancer | ||
| NCT03206177 | I | Ovarian carcinosarcoma | ||
| STAT3 inhibitor | Napabucasin | NCT02358395 | I | Advanced hepatocellular carcinoma |
| NCT02753127 | III | Metastatic colorectal cancer | ||
| β-Glucan | Imucell WGP | NCT00682032 | NA | Non-small cell lung cancer |
| C/EBPα | MTL-CEBPA | NCT02716012 | I | Advanced hepatocellular carcinoma |
| CD47-SIRPα inhibitors | Hu5F9-G4 | NCT02216409 | I | Solid tumors |
| CC-90002 | NCT02367196 | I | Advanced solid and hematologic cancer | |
| IBI188 | NCT03717103 | I | Advanced malignancies | |
| CD40 agonist | CP-870, 893 | NCT00607048 | I | Metastatic solid tumors |
| NCT01103635 | I | Metastatic melanoma | ||
| TRAIL agonists | TRM-1 | NCT00092924 | II | Non-small cell lung cancer |
| AMG 951 | NCT00508625 | II | Non-small cell lung cancer | |
| Mapatumumab | NCT01088347 | I/II | Advanced cervical cancer | |
| CS-1008 | NCT01220999 | I | Metastatic colorectal cancer | |
| Tigatuzumab | NCT01307891 | II | Metastatic triple-negative breast cancer |
CCR5, chemokine (C-C motif) receptor 5; C/EBP-α, CCAAT/enhancer binding protein-alpha; CXCR1, C-X-C motif chemokine receptor 1; CXCR2, C-X-C motif chemokine receptor 2; G-CSF, granulocyte colony-stimulating factor; NE, neutrophil elastase; NSAIDs, non-steroidal anti-inflammatory drugs; SIRP-α, signal regulatory protein-alpha; TGF-β, transforming growth factor-beta; STAT3, signal transducer and activator of transcription 3; TRAIL, tumor necrosis factor-related apoptosis-induced ligand.
For example, prophylactic therapies, such as G-CSF and its mimetics, have been used to mitigate severe chemotherapy-induced neutropenia, thereby enhancing therapeutic outcomes (NCT00035594)110. Moreover, efforts have been made to inhibit neutrophil function due to the pro-tumor phenotype, with a particular focus on targeting the CXCR2 pathway. AZD5069, a CXCR2 inhibitor, is currently being evaluated in a phase I/II study in combination with durvalumab for patients with advanced hepatocellular carcinoma (2020-003346-36)111. Furthermore, clinical trials are investigating SX-682, a small-molecule inhibitor of CXCR1 and CXCR2, in combination with pembrolizumab for various conditions, including metastatic melanoma (NCT03161431) and metastatic non-small cell lung cancer (NCT05570825). Reparixin, another promising agent that antagonizes CXCR1 and CXCR2, is being studied in combination with paclitaxel for treatment of metastatic triple-negative breast cancer (NCT02370238)112.
Additionally, one study in which host CCL5 in bone marrow was targeted using nanoparticle-delivered expression silencing coupled with the CCR5 inhibitor, Maraviroc, led to substantial reductions in immunosuppressive myeloid cells and robust anti-tumor responses113. Another promising approach involves combining Sivelestat, an NE inhibitor, with trastuzumab, which may synergistically suppress cancer cell proliferation in HER2-positive breast cancer, offering new therapeutic avenues114. In addition, celecoxib, a COX-2 inhibitor, has shown promise in reducing PD-L1+ neutrophils and restoring T cell cytotoxicity, potentially enhancing the effectiveness of lenvatinib115. Napabucasin, a STAT3 inhibitor, has demonstrated efficacy in protecting the liver and suppressing alcohol-induced pre-metastatic niche formation by inhibiting neutrophil recruitment and cancer cell plasticity116. Pharmacologic inhibition of protein arginine deiminase 4 (PAD4) with JBI-589 in neutrophils has demonstrated efficacy in reducing primary tumor growth and lung metastases, synergistically enhancing the effects of immune checkpoint inhibitors117. Of note, our recent study indicated that mice with KRAS-mutant intrahepatic cholangiocarcinoma treated with anakinra, an interleukin-1 receptor antagonist, had a significantly enhanced anti-tumor immune response due to altered neutrophil recruitment and phenotypes24. Therapies targeting the PD-1/PD-L1 interaction have also shown promise in attenuating pancreatic cancer growth and improving outcomes, especially by modulating neutrophil responses118. Neutrophil nanodecoys are being investigated for their potential to inhibit tumor metastasis by blocking interactions between tumor cells and neutrophils119.
Another emerging research area has harnessed the anti-tumor capabilities of neutrophils in cancer therapy. One promising approach involves MTL-CEBPA, a small activating RNA that is designed to upregulate CCAAT/enhancer binding protein-alpha (C/EBP-α) expression. This upregulation leads to an increase in neutrophil levels. Currently, the anti-tumor effects of MTL-CEBPA are being investigated in a phase 1b trial specifically targeting hepatocellular carcinoma120. Of note, by antagonizing various inhibitory receptors on neutrophils, the anti-tumor function may be bolstered, suggesting a concept akin to neutrophil checkpoint blockade121,122. Neutrophils, which share characteristics with other myeloid immune cells, engage in intricate interactions that mutually reinforce anti-tumor functions. Therapeutic approaches targeting the “don’t eat me” signal mediated by the interaction between SIRP-α expressed on myeloid cells and CD47, which prolongs the lifespan of neutrophils, have shown promise123. Owing to the selective expression of Fcα receptors on neutrophils, artificial IgA antibodies have demonstrated the ability to elicit robust antibody-dependent cytotoxicity, which aid in the eradication of tumor cells124. Additionally, in our study the modulation of leucine metabolism has emerged as a promising avenue to enhance the anti-tumor properties of neutrophils15. Looking ahead, the challenge will be to precisely target neutrophil pro-tumor functions without affecting the anti-tumor and normal functions or directly enhancing anti-tumor capabilities, which are crucial for effective tumor therapy.
Taken together, our research underscores the importance of recognizing the heterogeneity of neutrophils across various tissues and understanding dysfunctional roles within the TME15,24. While our understanding of these neutrophil subsets is growing, the origins and complete lifecycle remain unclear. It is uncertain whether these subsets originate from differentiation in the bone marrow, maturation in the circulation, or reprogramming within the TME. Clarifying the origins and complete lifecycle of neutrophil subsets could provide deeper insight into neutrophil heterogeneity, thereby refining the timing of interventions in neutrophil-targeted immunotherapy and potentially improving patient outcomes.
Furthermore, it is imperative to establish a universally accepted definition and methodology for distinguishing each neutrophil cluster, particularly anti-tumor neutrophils. Similarly, deeper insight into their specific roles in tumor suppression are also needed, including interactions with other immune cells. With precise definitions, separation techniques, and purification methods for each cluster, we could propel new neutrophil immunotherapies closer to the promising possibilities seen with chimeric antigen receptor (CAR)-T cell therapy125. Engineering neutrophils with CARs could enable a targeted attack on tumor-specific antigens, thereby enhancing their precision and efficacy within the TME. Additionally, CRISPR/Cas9-mediated modifications could enhance neutrophil persistence, improve their ability to localize to tumor sites, and augment resistance to immunosuppressive signals. This endeavor will not only redefine the role of neutrophils in cancer therapy but also pave the way for innovative treatments that can modulate neutrophil behavior in a context-dependent manner. Finally, robust biomarkers are necessary for stratifying patient populations likely to benefit from these therapies. Additionally, thoughtful clinical trial design is crucial for assessing the safety, efficacy, and long-term outcomes of these innovative treatments across diverse patient cohorts.
In conclusion, leveraging the dynamic nature of neutrophils in the TME presents multifaceted opportunities for innovative cancer therapies. By targeting neutrophil plasticity, integrating combination therapies, advancing cell engineering, and identifying new biomarkers we can advance toward more effective and personalized treatments that harness the potential of the immune system in combating cancer.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82130077, 81961128025, and 82121002), the Research Projects from the Science and Technology Commission of Shanghai Municipality (Grant Nos. 21JC1401200, 20JC1418900, and 21JC1410100) to QG, the China National Postdoctoral Program for Innovative Talents (Grant No. BX20240090), and the China Postdoctoral Science Foundation (Grant No. 2024M750551) to MZ.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed the analysis: Mao Zhang, Haokai Qin, Yingcheng Wu, and Qiang Gao.
Collected the data: Mao Zhang and Haokai Qin.
Wrote the paper: Mao Zhang, Haokai Qin, and Qiang Gao.
References
- 1.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Zhang Q, Lu L, Xu C, Li J, Zha J, et al. Heterogeneity of neutrophils in cancer: one size does not fit all. Cancer Biol Med. 2022;19:1629–48. doi: 10.20892/j.issn.2095-3941.2022.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaiser R, Gold C, Joppich M, Loew Q, Akhalkatsi A, Mueller TT, et al. Peripheral priming induces plastic transcriptomic and proteomic responses in circulating neutrophils required for pathogen containment. Sci Adv. 2024;10:eadl1710. doi: 10.1126/sciadv.adl1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–70. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Zhu YP, Padgett L, Dinh HQ, Marcovecchio P, Blatchley A, Wu R, et al. Identification of an early unipotent neutrophil progenitor with pro-tumoral activity in mouse and human bone marrow. Cell Rep. 2018;24:2329–41.e8. doi: 10.1016/j.celrep.2018.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20:485–503. doi: 10.1038/s41568-020-0281-y. [DOI] [PubMed] [Google Scholar]
- 7.Kim R, Hashimoto A, Markosyan N, Tyurin VA, Tyurina YY, Kar G, et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022;612:338–46. doi: 10.1038/s41586-022-05443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gungabeesoon J, Gort-Freitas NA, Kiss M, Bolli E, Messemaker M, Siwicki M, et al. A neutrophil response linked to tumor control in immunotherapy. Cell. 2023;186:1448–64.e20. doi: 10.1016/j.cell.2023.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirschhorn D, Budhu S, Kraehenbuehl L, Gigoux M, Schröder D, Chow A, et al. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell. 2023;186:1432–47.e17. doi: 10.1016/j.cell.2023.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Zheng X, Zhang J, Jiang X, Wang J, Li Y, et al. CD300ld on neutrophils is required for tumour-driven immune suppression. Nature. 2023;621:830–9. doi: 10.1038/s41586-023-06511-9. [DOI] [PubMed] [Google Scholar]
- 11.Treffers LW, Hiemstra IH, Kuijpers TW, van den Berg TK, Matlung HL. Neutrophils in cancer. Immunol Rev. 2016;273:312–28. doi: 10.1111/imr.12444. [DOI] [PubMed] [Google Scholar]
- 12.Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16:601–620. doi: 10.1038/s41571-019-0222-4. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Guoqiang L, Sun M, Lu X. Targeting and exploitation of tumor-associated neutrophils to enhance immunotherapy and drug delivery for cancer treatment. Cancer Biol Med. 2020;17:32–43. doi: 10.20892/j.issn.2095-3941.2019.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Ma J, Yang X, Nan F, Zhang T, Ji S, et al. Neutrophil profiling illuminates anti-tumor antigen-presenting potency. Cell. 2024;187:1422–39.e24. doi: 10.1016/j.cell.2024.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Görgens A, Radtke S, Möllmann M, Cross M, Dürig J, Horn PA, et al. Revision of the human hematopoietic tree: granulocyte subtypes derive from distinct hematopoietic lineages. Cell Rep. 2013;3:1539–52. doi: 10.1016/j.celrep.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113. doi: 10.3389/fphys.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehman HK, Segal BH. The role of neutrophils in host defense and disease. J Allergy Clin Immunol. 2020;145:1535–44. doi: 10.1016/j.jaci.2020.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology. 2008;125:281–8. doi: 10.1111/j.1365-2567.2008.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Wu W, Du Y, Yin H, Chen Q, Yu W, et al. The evolution and heterogeneity of neutrophils in cancers: origins, subsets, functions, orchestrations and clinical applications. Mol Cancer. 2023;22:148. doi: 10.1186/s12943-023-01843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adrover JM, Del Fresno C, Crainiciuc G, Cuartero MI, Casanova-Acebes M, Weiss LA, et al. A neutrophil timer coordinates immune defense and vascular protection. Immunity. 2019;50:390–402.e10. doi: 10.1016/j.immuni.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423–31. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Ceglie I, Carnevale S, Rigatelli A, Grieco G, Molisso P, Jaillon S. Immune cell networking in solid tumors: focus on macrophages and neutrophils. Front Immunol. 2024;15:1341390. doi: 10.3389/fimmu.2024.1341390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, Huang Y, Pan J, Sang C, Lin Y, Dong L, et al. An inflammatory checkpoint generated by IL1RN splicing offers therapeutic opportunity for KRAS-mutant intrahepatic cholangiocarcinoma. Cancer Discov. 2023;13:2248–69. doi: 10.1158/2159-8290.CD-23-0282. [DOI] [PubMed] [Google Scholar]
- 25.Capucetti A, Albano F, Bonecchi R. Multiple roles for chemokines in neutrophil biology. Front Immunol. 2020;11:1259. doi: 10.3389/fimmu.2020.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carnevale S, Di Ceglie I, Grieco G, Rigatelli A, Bonavita E, Jaillon S. Neutrophil diversity in inflammation and cancer. Front Immunol. 2023;14:1180810. doi: 10.3389/fimmu.2023.1180810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–23. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SenGupta S, Hein LE, Parent CA. The recruitment of neutrophils to the tumor microenvironment is regulated by multiple mediators. Front Immunol. 2021;12:734188. doi: 10.3389/fimmu.2021.734188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–52. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2012;2:120134. doi: 10.1098/rsob.120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng LG, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat Rev Immunol. 2019;19:255–65. doi: 10.1038/s41577-019-0141-8. [DOI] [PubMed] [Google Scholar]
- 32.Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22:173–87. doi: 10.1038/s41577-021-00571-6. [DOI] [PubMed] [Google Scholar]
- 33.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” tan. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sionov RV, Fridlender ZG, Granot Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2015;8:125–58. doi: 10.1007/s12307-014-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montaldo E, Lusito E, Bianchessi V, Caronni N, Scala S, Basso-Ricci L, et al. Cellular and transcriptional dynamics of human neutrophils at steady state and upon stress. Nat Immunol. 2022;23:1470–83. doi: 10.1038/s41590-022-01311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salcher S, Sturm G, Horvath L, Untergasser G, Kuempers C, Fotakis G, et al. High-resolution single-cell atlas reveals diversity and plasticity of tissue-resident neutrophils in non-small cell lung cancer. Cancer Cell. 2022;40:1503–20.e8. doi: 10.1016/j.ccell.2022.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng H, Wu X, Liu S, He M, Tang C, Wen Y, et al. Cellular dynamics in tumour microenvironment along with lung cancer progression underscore spatial and evolutionary heterogeneity of neutrophil. Clin Transl Med. 2023;13:e1340. doi: 10.1002/ctm2.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorin M, Rezanejad M, Karimi E, Fiset B, Desharnais L, Perus LJM, et al. Single-cell spatial landscapes of the lung tumour immune microenvironment. Nature. 2023;614:548–54. doi: 10.1038/s41586-022-05672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng MSF, Kwok I, Tan L, Shi C, Cerezo-Wallis D, Tan Y, et al. Deterministic reprogramming of neutrophils within tumors. Science. 2024;383:eadf6493. doi: 10.1126/science.adf6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue R, Zhang Q, Cao Q, Kong R, Xiang X, Liu H, et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature. 2022;612:141–7. doi: 10.1038/s41586-022-05400-x. [DOI] [PubMed] [Google Scholar]
- 41.Awasthi D, Sarode A. Neutrophils at the crossroads: unraveling the multifaceted role in the tumor microenvironment. Int J Mol Sci. 2024;25:2929. doi: 10.3390/ijms25052929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaul ME, Eyal O, Guglietta S, Aloni P, Zlotnik A, Forkosh E, et al. Circulating neutrophil subsets in advanced lung cancer patients exhibit unique immune signature and relate to prognosis. FASEB J. 2020;34:4204–18. doi: 10.1096/fj.201902467R. [DOI] [PubMed] [Google Scholar]
- 43.Lerman I, Garcia-Hernandez ML, Rangel-Moreno J, Chiriboga L, Pan C, Nastiuk KL, et al. Infiltrating myeloid cells exert protumorigenic actions via neutrophil elastase. Mol Cancer Res. 2017;15:1138–52. doi: 10.1158/1541-7786.MCR-17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taya M, Garcia-Hernandez ML, Rangel-Moreno J, Minor B, Gibbons E, Hammes SR. Neutrophil elastase from myeloid cells promotes TSC2-null tumor growth. Endocr Relat Cancer. 2020;27:261–74. doi: 10.1530/ERC-19-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Song M, Zhang B, Zhang Y. Reactive oxygen species regulate T cell immune response in the tumor microenvironment. Oxid Med Cell Longev. 2016;2016:1580967. doi: 10.1155/2016/1580967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao Z, Chua D, Tan NS. Reactive oxygen species: a volatile driver of field cancerization and metastasis. Mol Cancer. 2019;18:65. doi: 10.1186/s12943-019-0961-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong J, Li Q, Luo H, Holmdahl R. Neutrophil-derived reactive oxygen species promote tumor colonization. Commun Biol. 2021;4:865. doi: 10.1038/s42003-021-02376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wculek SK, Bridgeman VL, Peakman F, Malanchi I. Early neutrophil responses to chemical carcinogenesis shape long-term lung cancer susceptibility. iScience. 2020;23:101277. doi: 10.1016/j.isci.2020.101277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cui C, Chakraborty K, Tang XA, Zhou G, Schoenfelt KQ, Becker KM, et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. 2021;184:3163–77.e21. doi: 10.1016/j.cell.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16:219–23. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kessenbrock K, Fröhlich L, Sixt M, Lämmermann T, Pfister H, Bateman A, et al. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J Clin Invest. 2008;118:2438–47. doi: 10.1172/JCI34694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colom B, Bodkin JV, Beyrau M, Woodfin A, Ody C, Rourke C, et al. Leukotriene b4-neutrophil elastase axis drives neutrophil reverse transendothelial cell migration in vivo. Immunity. 2015;42:1075–86. doi: 10.1016/j.immuni.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Itatani Y, Yamamoto T, Zhong C, Molinolo AA, Ruppel J, Hegde P, et al. Suppressing neutrophil-dependent angiogenesis abrogates resistance to anti-VEGF antibody in a genetic model of colorectal cancer. Proc Natl Acad Sci USA. 2020;117:21598–608. doi: 10.1073/pnas.2008112117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 2016;6:630–49. doi: 10.1158/2159-8290.CD-15-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin J, Dai Y, Sang C, Song G, Xiang B, Zhang M, et al. Multimodule characterization of immune subgroups in intrahepatic cholangiocarcinoma reveals distinct therapeutic vulnerabilities. J Immunother Cancer. 2022;10:e004892. doi: 10.1136/jitc-2022-004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-l1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-l1 pathway. Gut. 2017;66:1900–11. doi: 10.1136/gutjnl-2016-313075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng Y, Li H, Deng Y, Tai Y, Zeng K, Zhang Y, et al. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9:422. doi: 10.1038/s41419-018-0458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu W, Dong J, Zheng Y, Zhou J, Yuan Y, Ta HM, et al. Immune-checkpoint protein vista regulates antitumor immunity by controlling myeloid cell-mediated inflammation and immunosuppression. Cancer Immunol Res. 2019;7:1497–510. doi: 10.1158/2326-6066.CIR-18-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maas RR, Soukup K, Fournier N, Massara M, Galland S, Kornete M, et al. The local microenvironment drives activation of neutrophils in human brain tumors. Cell. 2023;186:4546–66.e27. doi: 10.1016/j.cell.2023.08.043. [DOI] [PubMed] [Google Scholar]
- 61.Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front Immunol. 2020;11:1749. doi: 10.3389/fimmu.2020.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 2012;72:3919–27. doi: 10.1158/0008-5472.CAN-11-2393. [DOI] [PubMed] [Google Scholar]
- 63.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–58. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petrova M, Parvanov D, Ganeva R, Metodiev D, Bachurska S, Eneva M, et al. Tumor neutrophil extracellular traps and pretreatment neutrophils in association with progression-free survival in patients with metastatic non-small cell lung cancer receiving pembrolizumab alone or with chemotherapy. J Clin Oncol. 2022;40:e21099 [Google Scholar]
- 65.Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–7. doi: 10.1038/s41586-019-0915-y. [DOI] [PubMed] [Google Scholar]
- 66.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 67.de Jong MC, Mihai R, Khan S. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as possible prognostic markers for patients undergoing resection of adrenocortical carcinoma. World J Surg. 2021;45:754–64. doi: 10.1007/s00268-020-05868-6. [DOI] [PubMed] [Google Scholar]
- 68.Benguigui M, Cooper TJ, Kalkar P, Schif-Zuck S, Halaban R, Bacchiocchi A, et al. Interferon-stimulated neutrophils as a predictor of immunotherapy response. Cancer Cell. 2024;42:253–65.e12. doi: 10.1016/j.ccell.2023.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Que H, Fu Q, Lan T, Tian X, Wei X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim Biophys Acta Rev Cancer. 2022;1877:188762. doi: 10.1016/j.bbcan.2022.188762. [DOI] [PubMed] [Google Scholar]
- 70.Kalafati L, Kourtzelis I, Schulte-Schrepping J, Li X, Hatzioannou A, Grinenko T, et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell. 2020;183:771–85.e12. doi: 10.1016/j.cell.2020.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gershkovitz M, Caspi Y, Fainsod-Levi T, Katz B, Michaeli J, Khawaled S, et al. TRPM2 mediates neutrophil killing of disseminated tumor cells. Cancer Res. 2018;78:2680–90. doi: 10.1158/0008-5472.CAN-17-3614. [DOI] [PubMed] [Google Scholar]
- 72.Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522:349–53. doi: 10.1038/nature14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garley M, Jabłońska E, Dąbrowska D. NETs in cancer. Tumour Biol. 2016;37:14355–61. doi: 10.1007/s13277-016-5328-z. [DOI] [PubMed] [Google Scholar]
- 74.Schedel F, Mayer-Hain S, Pappelbaum KI, Metze D, Stock M, Goerge T, et al. Evidence and impact of neutrophil extracellular traps in malignant melanoma. Pigment Cell Melanoma Res. 2020;33:63–73. doi: 10.1111/pcmr.12818. [DOI] [PubMed] [Google Scholar]
- 75.Hernandez-Ilizaliturri FJ, Jupudy V, Ostberg J, Oflazoglu E, Huberman A, Repasky E, et al. Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin’s lymphoma severe combined immunodeficiency mouse model. Clin Cancer Res. 2003;9:5866–73. [PubMed] [Google Scholar]
- 76.Behrens LM, van Egmond M, van den Berg TK. Neutrophils as immune effector cells in antibody therapy in cancer. Immunol Rev. 2023;314:280–301. doi: 10.1111/imr.13159. [DOI] [PubMed] [Google Scholar]
- 77.Metelitsa LS, Gillies SD, Super M, Shimada H, Reynolds CP, Seeger RC. Antidisialoganglioside/granulocyte macrophage-colony-stimulating factor fusion protein facilitates neutrophil antibody-dependent cellular cytotoxicity and depends on FcgammaRII (CD32) and Mac-1 (CD11b/CD18) for enhanced effector cell adhesion and azurophil granule exocytosis. Blood. 2002;99:4166–73. doi: 10.1182/blood.v99.11.4166. [DOI] [PubMed] [Google Scholar]
- 78.Matlung HL, Babes L, Zhao XW, van Houdt M, Treffers LW, van Rees DJ, et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 2018;23:3946–59.e6. doi: 10.1016/j.celrep.2018.05.082. [DOI] [PubMed] [Google Scholar]
- 79.Brandsma AM, Bondza S, Evers M, Koutstaal R, Nederend M, Jansen JHM, et al. Potent Fc receptor signaling by IgA leads to superior killing of cancer cells by neutrophils compared to igg. Front Immunol. 2019;10:704. doi: 10.3389/fimmu.2019.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Treffers LW, Ten Broeke T, Rösner T, Jansen JHM, van Houdt M, Kahle S, et al. IgA-mediated killing of tumor cells by neutrophils is enhanced by CD47-SIRPα checkpoint inhibition. Cancer Immunol Res. 2020;8:120–30. doi: 10.1158/2326-6066.CIR-19-0144. [DOI] [PubMed] [Google Scholar]
- 81.Sun B, Qin W, Song M, Liu L, Yu Y, Qi X, et al. Neutrophil suppresses tumor cell proliferation via Fas/Fas ligand pathway mediated cell cycle arrested. Int J Biol Sci. 2018;14:2103–13. doi: 10.7150/ijbs.29297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koga Y, Matsuzaki A, Suminoe A, Hattori H, Hara T. Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils. Cancer Res. 2004;64:1037–43. doi: 10.1158/0008-5472.can-03-1808. [DOI] [PubMed] [Google Scholar]
- 83.Gosselin EJ, Wardwell K, Rigby WF, Guyre PM. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J Immunol. 1993;151:1482–90. [PubMed] [Google Scholar]
- 84.Smith WB, Guida L, Sun Q, Korpelainen EI, van den Heuvel C, Gillis D, et al. Neutrophils activated by granulocyte-macrophage colony-stimulating factor express receptors for interleukin-3 which mediate class II expression. Blood. 1995;86:3938–44. [PubMed] [Google Scholar]
- 85.Reinisch W, Lichtenberger C, Steger G, Tillinger W, Scheiner O, Gangl A, et al. Donor dependent, interferon-gamma induced HLA-DR expression on human neutrophils in vivo. Clin Exp Immunol. 2003;133:476–84. doi: 10.1046/j.1365-2249.2003.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol Rev. 2016;273:329–43. doi: 10.1111/imr.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vono M, Lin A, Norrby-Teglund A, Koup RA, Liang F, Loré K. Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo. Blood. 2017;129:1991–2001. doi: 10.1182/blood-2016-10-744441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patidar A, Selvaraj S, Sarode A, Chauhan P, Chattopadhyay D, Saha B. DAMP-TLR-cytokine axis dictates the fate of tumor. Cytokine. 2018;104:114–23. doi: 10.1016/j.cyto.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 89.Yang CW, Strong BS, Miller MJ, Unanue ER. Neutrophils influence the level of antigen presentation during the immune response to protein antigens in adjuvants. J Immunol. 2010;185:2927–34. doi: 10.4049/jimmunol.1001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang CW, Unanue ER. Neutrophils control the magnitude and spread of the immune response in a thromboxane A2-mediated process. J Exp Med. 2013;210:375–87. doi: 10.1084/jem.20122183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mysore V, Cullere X, Mears J, Rosetti F, Okubo K, Liew PX, et al. FcγR engagement reprograms neutrophils into antigen cross-presenting cells that elicit acquired anti-tumor immunity. Nat Commun. 2021;12:4791. doi: 10.1038/s41467-021-24591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singhal S, Bhojnagarwala PS, O’Brien S, Moon EK, Garfall AL, Rao AS, et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016;30:120–35. doi: 10.1016/j.ccell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo N, Ni K, Luo T, Lan G, Arina A, Xu Z, et al. Reprogramming of neutrophils as non-canonical antigen presenting cells by radiotherapy-radiodynamic therapy to facilitate immune-mediated tumor regression. ACS Nano. 2021;15:17515–27. doi: 10.1021/acsnano.1c04363. [DOI] [PubMed] [Google Scholar]
- 94.Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466–80. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, Diao N, Lee CK, Chu HW, Bai L, Li L. Neutrophils deficient in innate suppressor IRAK-M enhances anti-tumor immune responses. Mol Ther. 2020;28:89–99. doi: 10.1016/j.ymthe.2019.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lang M, Borgmann M, Oberhuber G, Evstatiev R, Jimenez K, Dammann KW, et al. Thymoquinone attenuates tumor growth in ApcMin mice by interference with Wnt-signaling. Mol Cancer. 2013;12:41. doi: 10.1186/1476-4598-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Costantini C, Calzetti F, Perbellini O, Micheletti A, Scarponi C, Lonardi S, et al. Human neutrophils interact with both 6-sulfo LacNAc+ DC and NK cells to amplify NK-derived IFN{gamma}: role of CD18, ICAM-1, and ICAM-3. Blood. 2011;117:1677–86. doi: 10.1182/blood-2010-06-287243. [DOI] [PubMed] [Google Scholar]
- 98.Riise RE, Bernson E, Aurelius J, Martner A, Pesce S, Della Chiesa M, et al. TLR-stimulated neutrophils instruct NK cells to trigger dendritic cell maturation and promote adaptive T cell responses. J Immunol. 2015;195:1121–8. doi: 10.4049/jimmunol.1500709. [DOI] [PubMed] [Google Scholar]
- 99.Ponzetta A, Carriero R, Carnevale S, Barbagallo M, Molgora M, Perucchini C, et al. Neutrophils driving unconventional T cells mediate resistance against murine sarcomas and selected human tumors. Cell. 2019;178:346–60.e24. doi: 10.1016/j.cell.2019.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shaul ME, Zlotnik A, Tidhar E, Schwartz A, Arpinati L, Kaisar-Iluz N, et al. Tumor-associated neutrophils drive B-cell recruitment and their differentiation to plasma cells. Cancer Immunol Res. 2021;9:811–24. doi: 10.1158/2326-6066.CIR-20-0839. [DOI] [PubMed] [Google Scholar]
- 101.Hampton HR, Chtanova T. The lymph node neutrophil. Semin Immunol. 2016;28:129–36. doi: 10.1016/j.smim.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 102.Tomay F, Wells K, Duong L, Tsu JW, Dye DE, Radley-Crabb HG, et al. Aged neutrophils accumulate in lymphoid tissues from healthy elderly mice and infiltrate T- and B-cell zones. Immunol Cell Biol. 2018;96:831–40. doi: 10.1111/imcb.12046. [DOI] [PubMed] [Google Scholar]
- 103.Parsa R, Lund H, Georgoudaki AM, Zhang XM, Ortlieb Guerreiro-Cacais A, Grommisch D, et al. BAFF-secreting neutrophils drive plasma cell responses during emergency granulopoiesis. J Exp Med. 2016;213:1537–53. doi: 10.1084/jem.20150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hansen MH, Nielsen HV, Ditzel HJ. Translocation of an intracellular antigen to the surface of medullary breast cancer cells early in apoptosis allows for an antigen-driven antibody response elicited by tumor-infiltrating B cells. J Immunol. 2002;169:2701–11. doi: 10.4049/jimmunol.169.5.2701. [DOI] [PubMed] [Google Scholar]
- 105.Downs-Canner SM, Meier J, Vincent BG, Serody JS. B cell function in the tumor microenvironment. Annu Rev Immunol. 2022;40:169–93. doi: 10.1146/annurev-immunol-101220-015603. [DOI] [PubMed] [Google Scholar]
- 106.Triner D, Devenport SN, Ramakrishnan SK, Ma X, Frieler RA, Greenson JK, et al. Neutrophils restrict tumor-associated microbiota to reduce growth and invasion of colon tumors in mice. Gastroenterology. 2019;156:1467–82. doi: 10.1053/j.gastro.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang J, Tannous BA, Poznansky MC, Chen H. CXCR4 antagonist AMD3100 (plerixafor): from an impurity to a therapeutic agent. Pharmacol Res. 2020;159:105010. doi: 10.1016/j.phrs.2020.105010. [DOI] [PubMed] [Google Scholar]
- 108.He J, Zhou M, Yin J, Wan J, Chu J, Jia J, et al. METTL3 restrains papillary thyroid cancer progression via m6A/c-Rel/IL-8-mediated neutrophil infiltration. Mol Ther. 2021;29:1821–37. doi: 10.1016/j.ymthe.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feng M, Wang F, Liu X, Hao T, Zhang N, Deng M, et al. Neutrophils as key regulators of tumor immunity that restrict immune checkpoint blockade in liver cancer. Cancer Biol Med. 2023;20:421–37. doi: 10.20892/j.issn.2095-3941.2023.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lyman GH, Reiner M, Morrow PK, Crawford J. The effect of filgrastim or pegfilgrastim on survival outcomes of patients with cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2015;26:1452–58. doi: 10.1093/annonc/mdv174. [DOI] [PubMed] [Google Scholar]
- 111.Evans TRJ, Basu B, Hubner R, Ma YT, Meyer T, Palmer DH, et al. A phase I/II study of the CXCR2 inhibitor, AZD5069, in combination with durvalumab, in patients (pts) with advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2023;41:TPS631 [Google Scholar]
- 112.Schott AF, Goldstein LJ, Cristofanilli M, Ruffini PA, McCanna S, Reuben JM, et al. Phase Ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2-negative metastatic breast cancer. Clin Cancer Res. 2017;23:5358–65. doi: 10.1158/1078-0432.CCR-16-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ban Y, Mai J, Li X, Mitchell-Flack M, Zhang T, Zhang L, et al. Targeting autocrine CCL5-CCR5 axis reprograms immunosuppressive myeloid cells and reinvigorates antitumor immunity. Cancer Res. 2017;77:2857–68. doi: 10.1158/0008-5472.CAN-16-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nawa M, Osada S, Morimitsu K, Nonaka K, Futamura M, Kawaguchi Y, et al. Growth effect of neutrophil elastase on breast cancer: favorable action of sivelestat and application to anti-HER2 therapy. Anticancer Res. 2012;32:13–9. [PubMed] [Google Scholar]
- 115.Deng H, Kan A, Lyu N, He M, Huang X, Qiao S, et al. Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma. J Immunother Cancer. 2021;9:e002305. doi: 10.1136/jitc-2020-002305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qiu X, Zhou J, Xu H, Li Y, Ma S, Qiao H, et al. Alcohol reshapes a liver premetastatic niche for cancer by extra- and intrahepatic crosstalk-mediated immune evasion. Mol Ther. 2023;31:2662–80. doi: 10.1016/j.ymthe.2023.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deng H, Lin C, Garcia-Gerique L, Fu S, Cruz Z, Bonner EE, et al. A novel selective inhibitor JBI-589 targets PAD4-mediated neutrophil migration to suppress tumor progression. Cancer Res. 2022;82:3561–72. doi: 10.1158/0008-5472.CAN-21-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nielsen SR, Strøbech JE, Horton ER, Jackstadt R, Laitala A, Bravo MC, et al. Suppression of tumor-associated neutrophils by lorlatinib attenuates pancreatic cancer growth and improves treatment with immune checkpoint blockade. Nat Commun. 2021;12:3414. doi: 10.1038/s41467-021-23731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zeng W, Wang Y, Zhang Q, Hu C, Li J, Feng J, et al. Neutrophil nanodecoys inhibit tumor metastasis by blocking the interaction between tumor cells and neutrophils. ACS Nano. 2024;18:7363–78. doi: 10.1021/acsnano.3c08946. [DOI] [PubMed] [Google Scholar]
- 120.Zhou J, Li H, Xia X, Herrera A, Pollock N, Reebye V, et al. Anti-inflammatory activity of MTL-CEBPA, a small activating RNA drug, in LPS-stimulated monocytes and humanized mice. Mol Ther. 2019;27:999–1016. doi: 10.1016/j.ymthe.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med. 2020;217:e20190354. doi: 10.1084/jem.20190354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hailemichael Y, Johnson DH, Abdel-Wahab N, Foo WC, Bentebibel SE, Daher M, et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell. 2022;40:509–23.e6. doi: 10.1016/j.ccell.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Russ A, Hua AB, Montfort WR, Rahman B, Riaz IB, Khalid MU, et al. Blocking “don’t eat me” signal of CD47-SIRPα in hematological malignancies, an in-depth review. Blood Rev. 2018;32:480–9. doi: 10.1016/j.blre.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peter HH, Ochs HD, Cunningham-Rundles C, Vinh DC, Kiessling P, Greve B, et al. Targeting FcRn for immunomodulation: benefits, risks, and practical considerations. J Allergy Clin Immunol. 2020;146:479–91.e5. doi: 10.1016/j.jaci.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liang Y, Xu Q, Gao Q. Advancing car-based immunotherapies in solid tumors: car-macrophages and neutrophils. Front Immunol. 2023;14:1291619. doi: 10.3389/fimmu.2023.1291619. [DOI] [PMC free article] [PubMed] [Google Scholar]