Abstract
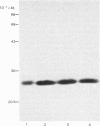
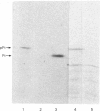
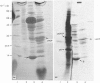
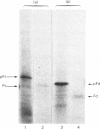
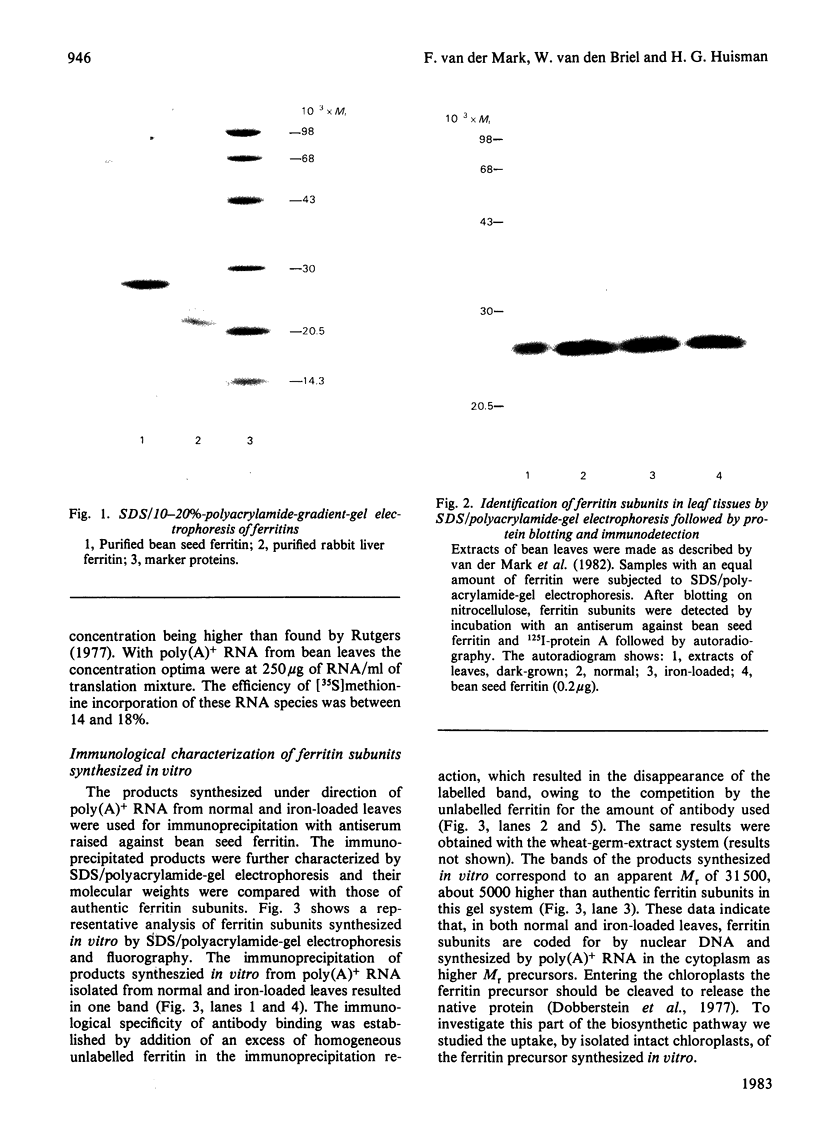
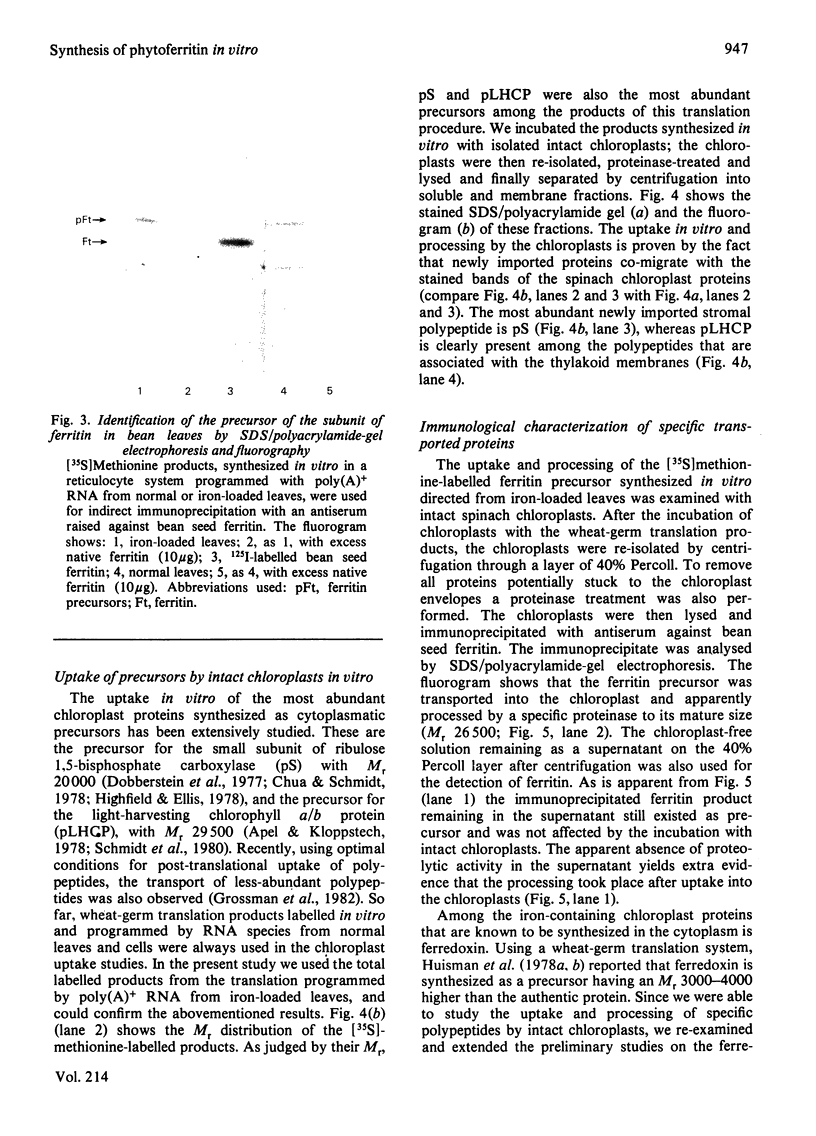
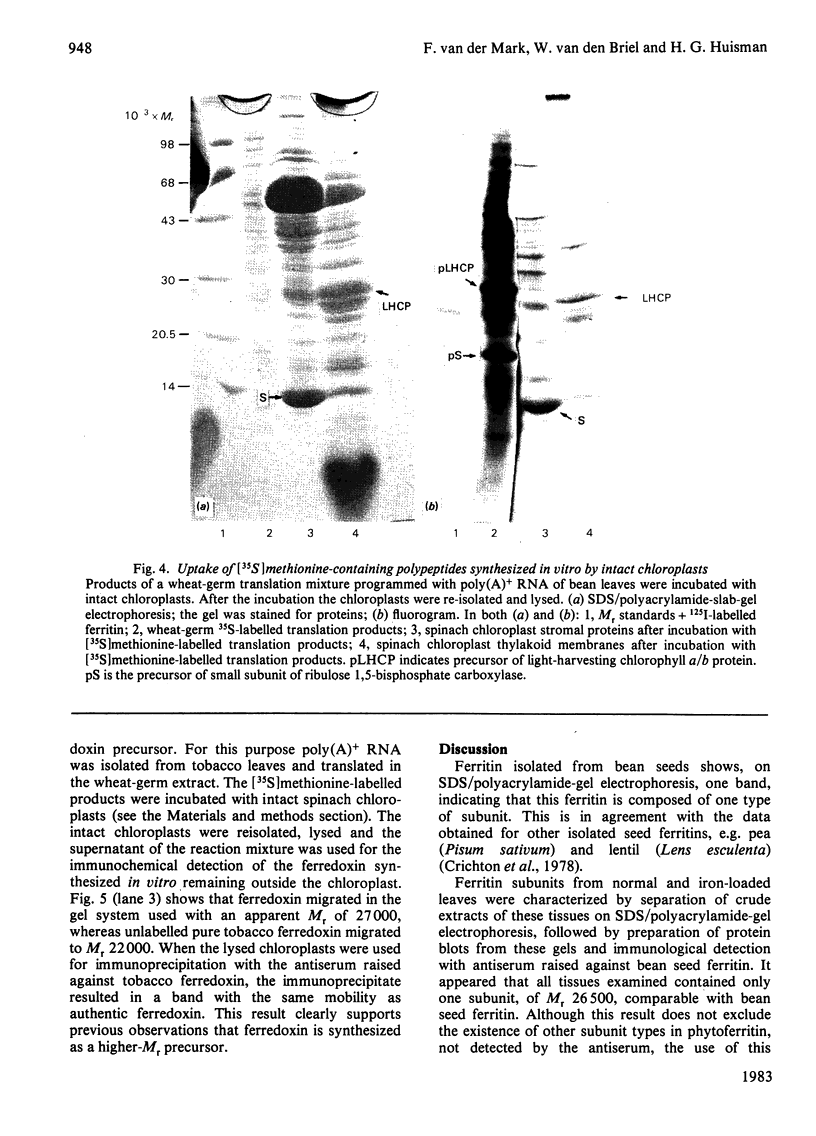
Evidence is presented that French-bean (Phaseolus vulgaris) seed ferritin is composed of one type of subunit with an apparent Mr of 26500. In normal and iron-loaded leaf tissues it is detected immunologically with an antiserum raised against purified bean seed ferritin and migrates in SDS (sodium dodecyl sulphate)/polyacrylamide-gel electrophoresis with the same mobility as the bean seed ferritin subunit. The biosynthetic pathway of ferritin in normal and iron-loaded leaves was investigated. RNA was extracted, fractionated into polyadenylated RNA and translated in a cell-free rabbit reticulocyte lysate and a wheat-germ-extract system. The products were identified by SDS/polyacrylamide-gel electrophoresis after indirect immunoprecipitation. In all cases the ferritin product had an Mr 5000 higher than that of the native subunit. Uptake and processing of the precursor form of ferritin from iron-loaded leaves by intact chloroplasts was demonstrated. This indicates that, in iron-loaded leaves, ferritin acts as a chloroplast protein. We propose that the ferritin precursor in normal leaves follows the same biosynthetic pathway. This suggests that the iron-buffering function of ferritin in plants takes place in the chloroplast and that non-functional cellular iron will accumulate in this cell organelle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Apel K., Kloppstech K. The plastid membranes of barley (Hordeum vulgare). Light-induced appearance of mRNA coding for the apoprotein of the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1978 Apr 17;85(2):581–588. doi: 10.1111/j.1432-1033.1978.tb12273.x. [DOI] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelfranco P. A., Jones O. T. Protoheme turnover and chlorophyll synthesis in greening barley tissue. Plant Physiol. 1975 Mar;55(3):485–490. doi: 10.1104/pp.55.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R., Ponce-Ortiz Y., Koch M. H., Parfait R., Stuhrmann H. B. Isolation and characterization of phytoferritin from pea (Pisum sativum) and Lentil (Lens esculenta). Biochem J. 1978 May 1;171(2):349–356. doi: 10.1042/bj1710349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C. N., Easterbrook K. Ferritin in the fungus Phycomyces. J Cell Biol. 1971 Jan;48(1):15–28. doi: 10.1083/jcb.48.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A. R., Bartlett S. G., Schmidt G. W., Mullet J. E., Chua N. H. Optimal conditions for post-translational uptake of proteins by isolated chloroplasts. In vitro synthesis and transport of plastocyanin, ferredoxin-NADP+ oxidoreductase, and fructose-1,6-bisphosphatase. J Biol Chem. 1982 Feb 10;257(3):1558–1563. [PubMed] [Google Scholar]
- HYDE B. B., HODGE A. J., KAHN A., BIRNSTIEL M. L. STUDIES ON PHYTOFERRITIN. I. IDENTIFICATION AND LOCALIZATION. J Ultrastruct Res. 1963 Oct;59:248–258. doi: 10.1016/s0022-5320(63)80005-2. [DOI] [PubMed] [Google Scholar]
- Huisman J. G., Moorman A. F., Verkley F. N. In vitro synthesis of chloroplast ferredoxin as a high molecular weight precursor in a cell-free protein synthesizing system from wheat germs. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1121–1131. doi: 10.1016/0006-291x(78)90303-0. [DOI] [PubMed] [Google Scholar]
- Huisman J. G., Stapel S., Muijsers A. O. Two different plant-type ferredoxins in each of two petunia species. FEBS Lett. 1978 Jan 15;85(2):198–202. doi: 10.1016/0014-5793(78)80454-2. [DOI] [PubMed] [Google Scholar]
- Jones O. T. Ferrochelatase of spinach chloroplasts. Biochem J. 1968 Mar;107(1):113–119. doi: 10.1042/bj1070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marcu K., Dudock B. Characterization of a highly efficient protein synthesizing system derived from commercial wheat germ. Nucleic Acids Res. 1974 Nov;1(11):1385–1397. doi: 10.1093/nar/1.11.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N., Linder M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol Rev. 1978 Apr;58(2):317–396. doi: 10.1152/physrev.1978.58.2.317. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckbach J. Remarks on ferritin from iron loaded plants. Planta Med. 1972 May;21(3):267–273. doi: 10.1055/s-0028-1099551. [DOI] [PubMed] [Google Scholar]
- Stiefel E. I., Watt G. D. Azotobacter cytochrome b557.5 is a bacterioferritin. Nature. 1979 May 3;279(5708):81–83. doi: 10.1038/279081a0. [DOI] [PubMed] [Google Scholar]
- Terry N. Limiting Factors in Photosynthesis: I. USE OF IRON STRESS TO CONTROL PHOTOCHEMICAL CAPACITY IN VIVO. Plant Physiol. 1980 Jan;65(1):114–120. doi: 10.1104/pp.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Drysdale J. Evidence for distinct mRNAs for ferritin subunits. Biochem Biophys Res Commun. 1981 Jan 30;98(2):507–511. doi: 10.1016/0006-291x(81)90869-x. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Gratzer W. B. Limitations of the detergent-polyacrylamide gel electrophoresis method for molecular weight determination of proteins. J Chromatogr. 1971 Apr 22;57(1):121–125. doi: 10.1016/0021-9673(71)80013-4. [DOI] [PubMed] [Google Scholar]
- Yariv J., Kalb A. J., Sperling R., Bauminger E. R., Cohen S. G., Ofer S. The composition and the structure of bacterioferritin of Escherichia coli. Biochem J. 1981 Jul 1;197(1):171–175. doi: 10.1042/bj1970171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol R. G., van Vloten-Doting L. Translation of alfalfa-mosaic-virus RNA 1 in the mRNA-dependent translation system from rabbit reticulocyte lysates. Eur J Biochem. 1979 Feb 1;93(3):461–468. doi: 10.1111/j.1432-1033.1979.tb12844.x. [DOI] [PubMed] [Google Scholar]