Abstract
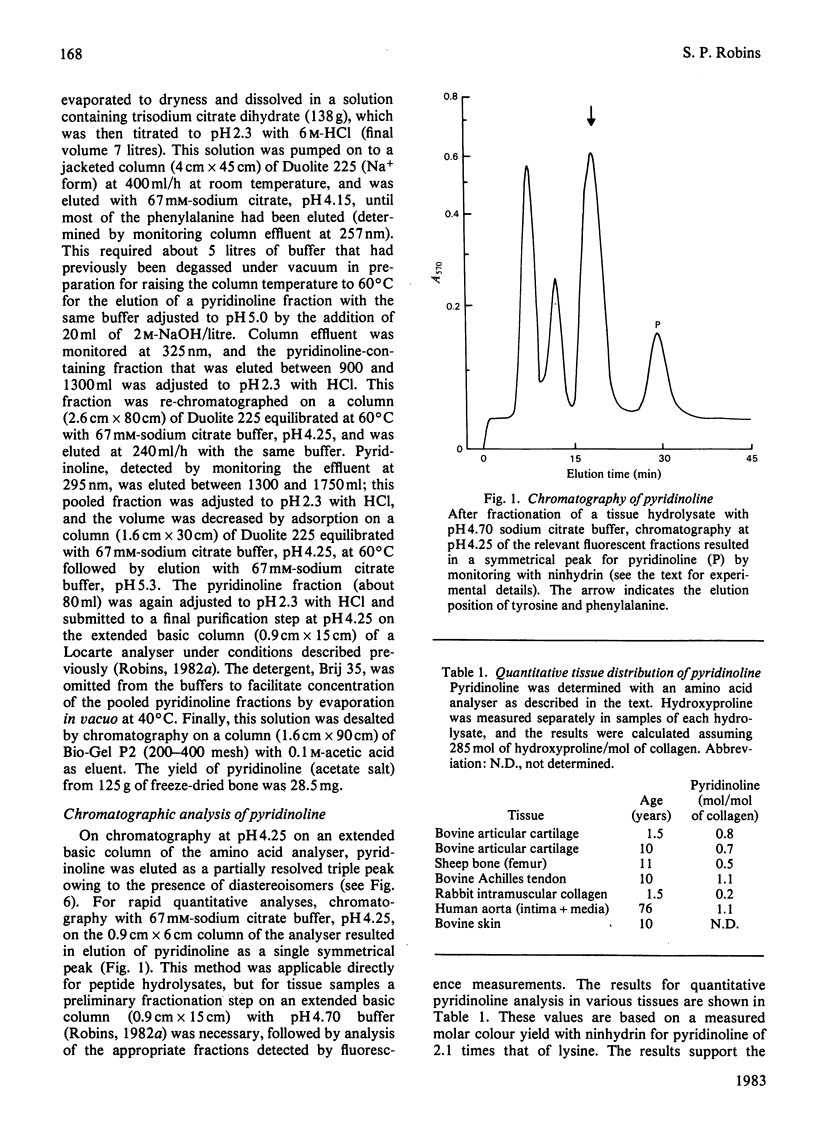
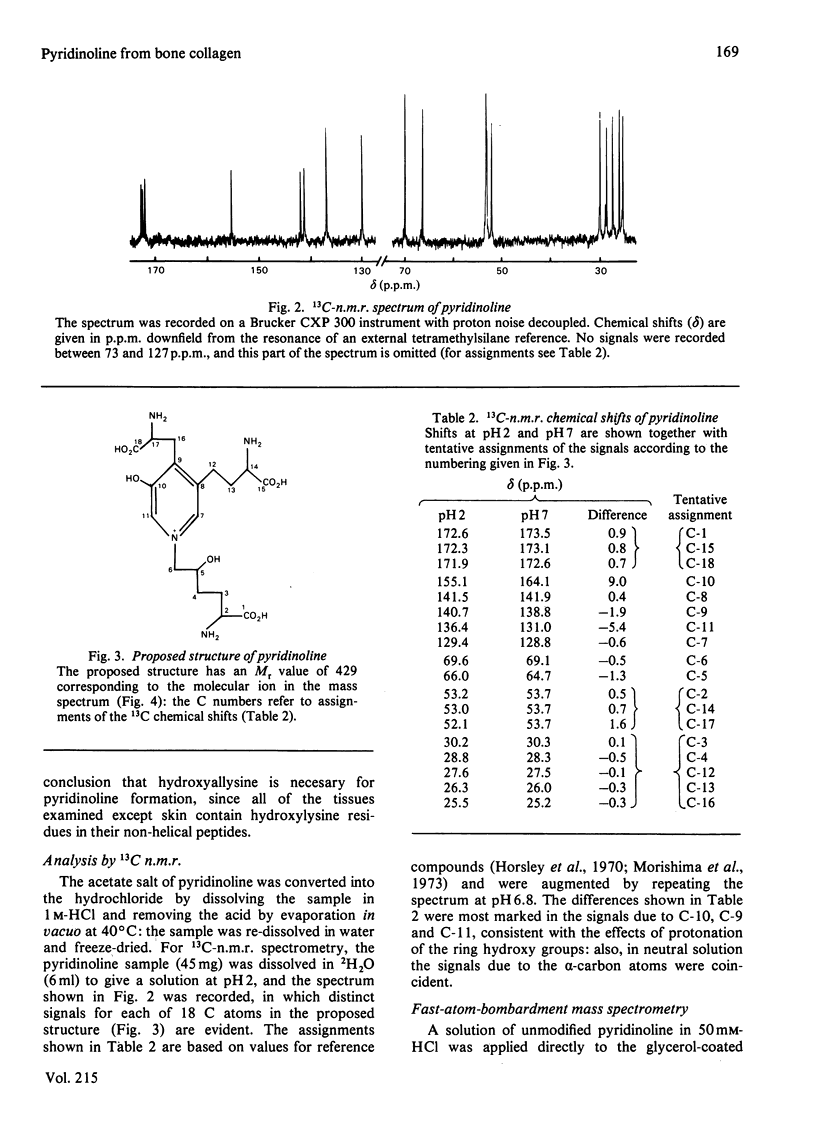
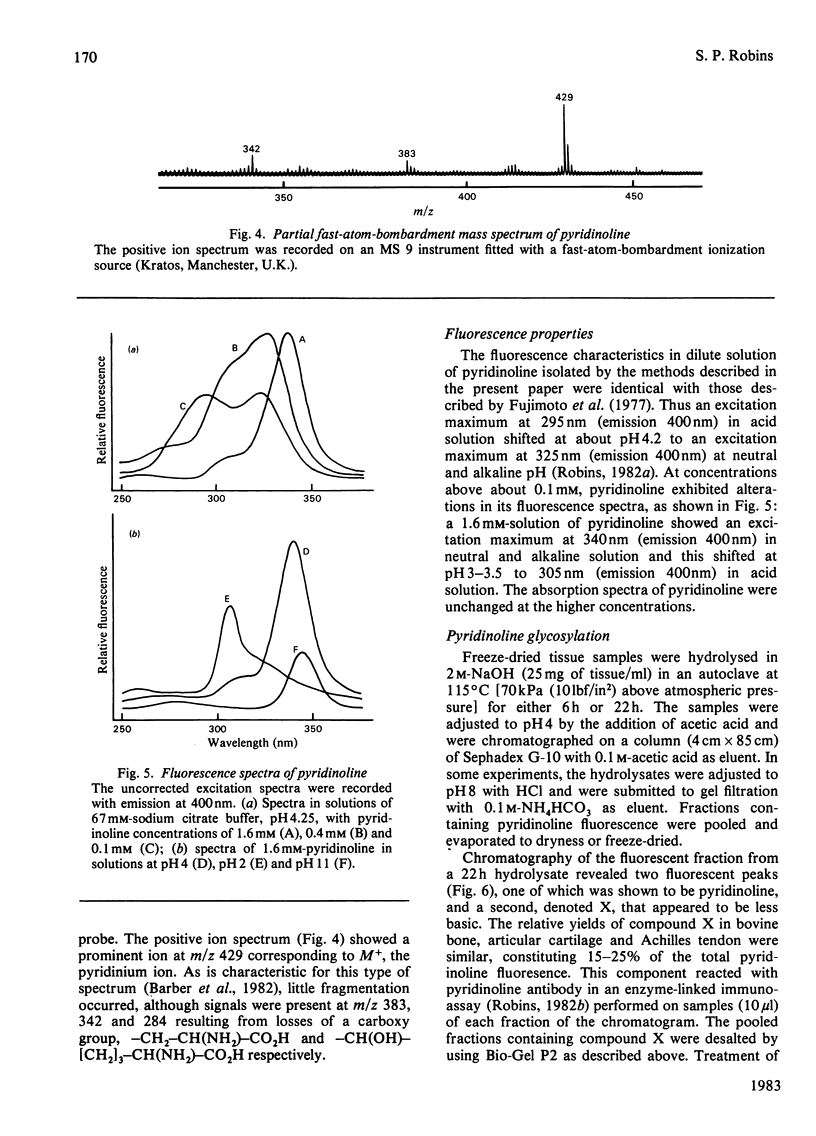
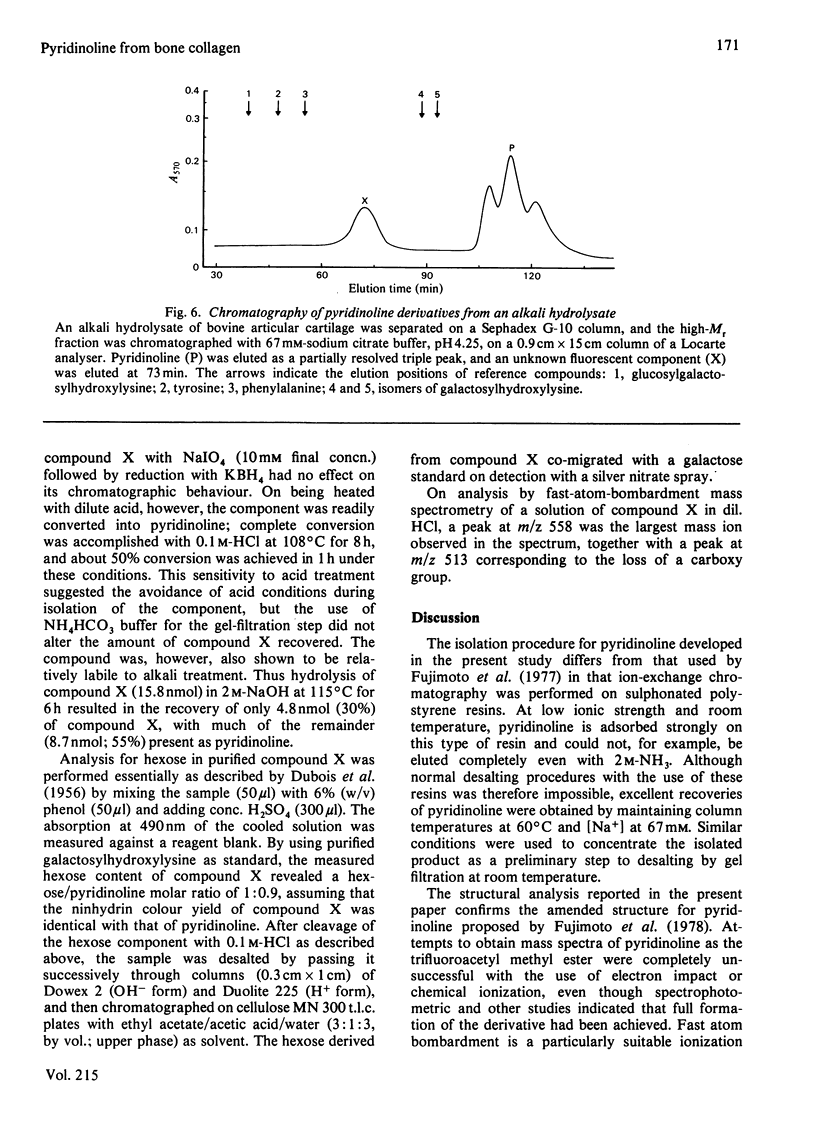
A method for the isolation and purification of pyridinoline from bone collagen was developed, with the use of sulphonated polystyrene resins. The analytical techniques were used to quantify pyridinoline, for which hydroxyallysine is a known precursor, in a wide range of tissues. The structure of pyridinoline proposed by Fujimoto, Moriguchi, Ishida & Hayashi [(1978) Biochem. Biophys. Res. Commun. 84, 52-57] was confirmed by 13C-n.m.r. spectroscopy and fast-atom-bombardment mass spectrometry. At concentrations greater than about 0.1 mM, pyridinoline exhibited altered fluorescence properties that were consistent with excimer formation. From alkali hydrolysates of several different tissues, a fluorescent compound was purified by gel filtration and ion-exchange chromatography and was shown to be galactosylpyridinoline. This derivative was very labile to acid treatment compared with the bifunctional cross-link analogues, and was completely converted into free pyridinoline by heating at 108 degrees C for 8 h in 0.1 M-HCl. Galactosylpyridinoline was also partially converted into free pyridinoline by prolonged alkali hydrolysis. This lability, which could also apply to other multifunctional cross-link derivatives, may explain the fact that no disaccharide derivatives of pyridinoline were isolated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLARD E. R., HILL D. W. Analysis of anaesthetic mixtures by gas chromatography. Nature. 1960 Jun 25;186:1045–1045. doi: 10.1038/1861045a0. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Robins S. P., Balian G. Biological significance of the intermolecular crosslinks of collagen. Nature. 1974 Sep 13;251(5471):105–109. doi: 10.1038/251105a0. [DOI] [PubMed] [Google Scholar]
- Barber M., Bordoli R. S., Elliott G. J., Fujimoto D., Scott J. E. The structure(s) of pyridinoline(s). Biochem Biophys Res Commun. 1982 Dec 15;109(3):1041–1046. doi: 10.1016/0006-291x(82)92044-7. [DOI] [PubMed] [Google Scholar]
- Elsden D. F., Light N. D., Bailey A. J. An investigation of pyridinoline, and putative collagen cross-link. Biochem J. 1980 Feb 1;185(2):531–534. doi: 10.1042/bj1850531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Oguchi H. The hydroxypyridinium crosslinks of skeletal collagens: their measurement, properties and a proposed pathway of formation. Biochem Biophys Res Commun. 1980 Jan 29;92(2):403–410. doi: 10.1016/0006-291x(80)90347-2. [DOI] [PubMed] [Google Scholar]
- Fujimoto D. Evidence for natural existence of pyridinoline crosslink in collagen. Biochem Biophys Res Commun. 1980 Apr 14;93(3):948–953. doi: 10.1016/0006-291x(80)91167-5. [DOI] [PubMed] [Google Scholar]
- Fujimoto D. Isolation and characterization of a fluorescent material in bovine achilles tendon collagen. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1124–1129. doi: 10.1016/0006-291x(77)90972-x. [DOI] [PubMed] [Google Scholar]
- Fujimoto D., Moriguchi T., Ishida T., Hayashi H. The structure of pyridinoline, a collagen crosslink. Biochem Biophys Res Commun. 1978 Sep 14;84(1):52–57. doi: 10.1016/0006-291x(78)90261-9. [DOI] [PubMed] [Google Scholar]
- Fujimoto D., Moriguchi T. Pyridinoline, a non-reducible crosslink of collagen. Quantitative determination, distribution, and isolation of a crosslinked peptide. J Biochem. 1978 Mar;83(3):863–867. doi: 10.1093/oxfordjournals.jbchem.a131983. [DOI] [PubMed] [Google Scholar]
- Robins S. P. An enzyme-linked immunoassay for the collagen cross-link pyridinoline. Biochem J. 1982 Dec 1;207(3):617–620. doi: 10.1042/bj2070617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. P. Analysis of the crosslinking components in collagen and elastin. Methods Biochem Anal. 1982;28:329–379. doi: 10.1002/9780470110485.ch8. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Bailey A. J. Isolation and characterization of glycosyl derivatives of the reducible cross-links in collagens. FEBS Lett. 1974 Jan 15;38(3):334–336. doi: 10.1016/0014-5793(74)80085-2. [DOI] [PubMed] [Google Scholar]
- Tsuchikura O., Gotoh Y., Saito S. Pyridinoline fluorescence in cyanogen bromide peptides of collagen. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1203–1208. doi: 10.1016/s0006-291x(81)80139-8. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Ono T., Ogawa T., Kawanishi Y. Pyridinoline is a real moiety of collagen. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1407–1412. doi: 10.1016/0006-291x(82)91406-1. [DOI] [PubMed] [Google Scholar]
- Yamauchi M., Noyes C., Kuboki Y., Mechanic G. L. Collagen structural microheterogeneity and a possible role for glycosylated hydroxylysine in type I collagen. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7684–7688. doi: 10.1073/pnas.79.24.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]


