Abstract
Background
Burnout among resident physicians during training has been prevalent, prompting training centers to introduce interventions at the individual or organizational level. However, empirical evidence is crucial before implementing such programs in practice.
Methods
A systematic review and meta-analysis was carried out to evaluate the effectiveness of individual and organizational interventions in reducing burnout among resident physicians. Searching was done across five databases—PubMed, Scopus, ScienceDirect, Embase, and Cochrane Library from 1 December 2023 to 26 August 2024. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used for our reporting of study selection process. Eligibility criteria were randomized or non-randomized designs, with prospective intervention, with a comparator group focused on individual or organizational interventions reducing burnout, in any language and publication date. The Maslach Burnout Inventory scores for emotional exhaustion (EE), depersonalization (DP), and personal accomplishment (PA) were the three outcome measures. Two investigators independently extracted the data. The risk of bias was evaluated using Cochrane risk-of-bias tool for randomized trials (RoB2) and non-randomized studies of interventions (ROBINS-I). Cohen’s d and heterogeneity was estimated using a random-effects DerSimonian-Laird model and visualized by forest plots. Sensitivity analyses were carried out by leave-one-out meta-analysis.
Results
We identified 33 eligible studies (n = 2536), comprising 25 (75.8%) individual intervention studies and 8 (24.2%) organizational intervention studies. Cohen’s d for individual intervention versus control were as follows: EE -0.25 (95% CI -0.40 to -0.11, p < 0.01, I2 = 49.3%), and DP -0.17 (95% CI -0.32 to -0.03, p = 0.02, I2 = 50.0%). The organizational intervention showed no significant association with any domain. Sensitivity analyses were robust in all outcomes, with differences in intervention description and design identified as potential contributors to heterogeneity.
Conclusions
Various interventions, including individual coaching, meditation, and organization interventions, have been implemented to improve resident burnout. The effectiveness of intervention demonstrated none to small practical significance in improving burnout. Data inconsistency and high risk of bias across studies limited the validity of the pooled results. Further studies should focus on a combined approach.
Registration
The study was registered on PROSPERO, under PROSPERO registration number CRD42022349698.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12909-024-06195-3.
Keywords: Burnout, professional; Occupational stress; Controlled clinical trial; Occupational health; Environment health; Internship; Residency
Introduction
Burnout syndrome, defined by the World Health Organization (WHO), is an occupational phenomenon from prolonged exposure to psychosocial risk factors in the work place [1], making it a serious [2–4] and prevalent [5–7] occupational health concern. This phenomenon, characterized by high emotional exhaustion (EE), high depersonalization (DP), and low personal accomplishment (PA), affects physicians and the patients they care for [6]. Its framework, largely influenced by Maslach [8–10], encompasses three domains. The ramifications of burnout are far-reaching: for providers, it can lead to mood disorders, family conflicts, diminished self-esteem, and early career departure; for patients, it associates with increased medical complications, legal challenges, prolonged hospital stay, and reduced satisfaction with healthcare [11–13]. In the United States alone, burnout is estimated to cost the healthcare sector $4.6 billion [3].
The residency period is widely recognized as one of the most stressful stages in a medical career, attributed to factors such as limited autonomy, high workloads, inadequate institutional support, and relatively low income [9, 14–16]. Previous studies, the systematic reviews and meta-analyses, have consistently highlighted the prevalence of burnout among resident physicians, with proportions ranging from 45 to 57% [5, 6, 17] globally. Consequently, over the past two decades, many training centers have initiated various interventions aimed at reducing burnout. These interventions encompass both individual-focused strategies, such as mindfulness training, meditation sessions, self-care courses, and psychological workshops [18–25], as well as organizational initiatives, including providing recreational opportunities, offering healthy food options, implementing rest days following shifts, adjusting shift schedules, and modifying shift duration [26–28].
Previous studies have frequently suggested a reduction in burnout syndrome following interventions targeted at resident physicians. However, there remains a lack of substantial evidence regarding the actual change in intervention effectiveness, which hinders the recommendations for the most suitable approaches. Given the importance of these interventions, a comprehensive and thorough review is essential. Therefore, this study aimed to evaluate the effectiveness of both individual and organizational interventions in reducing burnout among resident physician populations by conducting a systematic review and meta-analysis of existing evidence to assess their effectiveness.
Materials and methods
Eligibility criteria
To evaluate any intervention aimed at reducing burnout among resident physicians during their training, regardless of location or specialty. These interventions included randomized controlled trial (RCTs) or non-randomized studies of intervention. Accepted study designs encompassed concurrent non-randomized studies, pre-post studies, or historical control studies. Publications to be included can be in any languages regardless of the publication year, with available online full text. Measurement criteria for evaluation of interventions should utilized the Maslach Burnout Inventory (MBI) [10], with reporting on total scores of each dimension: EE, DP, and PA. Modification to the PROSPERO protocol was made to cover MBI scale other than the 22-item standard version in order to capture all literatures. For analytic purposes, interventions are pre-specified into either individual or organizational categories, the standard definition was derived from the documented types of stress management interventions (SMIs) by the Health and Safety Executive (HSE), United Kingdom [29].
Exclusion criteria
Studies that focused solely on other healthcare personnel (such as nurses, pharmacists, dentists, medical students, and intern physicians), without providing subgroup data specifically for resident physicians and studies that were not available as full-text articles were excluded.
Search strategies and data sources
The search was conducted across five databases: PubMed, Scopus, ScienceDirect, Embase, and the Cochrane Library, spanning December 1 to 21, 2023, with an updated search during the revision between August 19 and 26, 2024. The search process adhered to the PICO framework (Population, Intervention, Comparison, Outcome) and was executed by two investigators (WK and VS), following a stepwise syntax (See Supplementary Appendix 1, Additional File 1). Keywords and medical terms were derived from PubMed [30] and Cochrane Library MeSH (Medical Subject Headings) [31]. Duplicate records were managed using Endnote X9 software.
Study selection
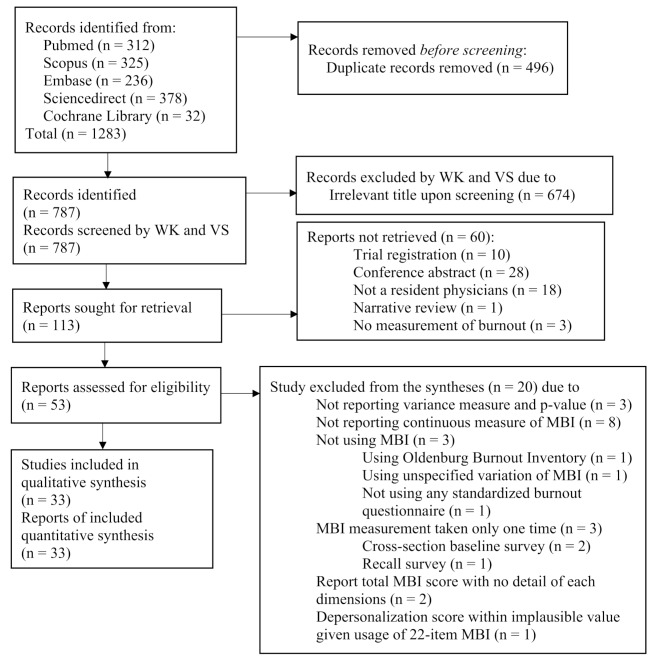
After two investigators (WK and VS) formulate the searching syntax together. These investigators then independently reviewed studies, excluding those without full texts or with irrelevant titles or abstracts. Then, the eligibility of each imported studies of each of the two investigators were deliberated upon, with consensus reached on eligible studies through discussion. In case of disagreement, a third investigator (WS) acted as an adjudicator. The screening process followed the PRISMA 2020 flow diagram (Fig. 1), PRISMA 2020 checklist (See Additional File 2) and PRISMA 2020 abstract checklist (See Additional File 3) [32] to ensure transparency and accuracy.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA 2020) flow diagram of eligible studies
Risk of bias assessment
Two reviewers (WK and VS) utilized the performed the RoB2 (Cochrane risk of bias assessment in randomized trial) [33] for randomized parallel studies and ROBINS-I (Risk of Bias in Non-randomized Studies-of Interventions) [34] for non-randomized studies to assess the risk of bias assessment. Independently, reviewers conducted these assessments between December 21 and 31, 2023. Then, during a discussion session on January 2, 2024, any disparities in findings were thoroughly discussed until a consensus was reached. Although a third investigator (WS) was available to adjudicate in case of disagreements, none arose during the process. Risk of bias assessment for additional studies was carried out during the revision between August 27 and 28, 2024.
Data extraction
Two investigators (WK and VS) independently retrieved information from January 2 to 10, 2024, and updated upon revision from August 29, and 30, 2024. The extracted data for each study included the author’s name, country, year of publication, study design, intervention name, duration and frequency of sessions, study duration, participant count, specialty, and loss to follow-up. Additionally, outcome data concerning the mean and standard deviation in three domains of the Maslach Burnout Inventory–EE, DP, and PA–was collected at pre-intervention and post-intervention. In cases of incomplete outcome data, standard deviation was calculated from other reported metrics of comparison such as p-value, using the Cochrane Calculator [35, 36]. Furthermore, graphical data with no numerical description of data point were handled by PlotDigitizer.
Data analyses
Analyses were done on STATA version 18.0 (StataCorp LLC, Texas, USA). Heterogeneity was assessed using Cochrane’s Q test and the I-squared statistics (I2) [37]. Due to the expected heterogeneity, the DerSimonian-Laird random-effects model was employed for meta-analysis [38]. Results were presented as post-intervention Cohen’s d standardized mean differences (SMD) and a 95% confidence interval, with visualization carried out by the forest plots. A two-sided p-value of < 0.05 was considered statistically significant. Sensitivity analyses were carried out with subgroup (See Supplementary Appendix 2.1 to 2.6, Additional File 1) and leave-one-out meta-analyses (See Supplementary Appendix 3.1 to 3.6, Additional File 1). Additionally, publication bias was explored by the funnel plots (See Supplementary Appendix 4.1 to 4.6, Additional File 1).
Strength of evidence
Grading Quality of Evidence and Strength of Recommendations (GRADE) [39] approach was used to evaluate the strength of evidence for each outcome, separately for individual and organizational studies. Eight domains were assessed: inconsistency [40], indirectness [41], imprecision [42], risk of bias [33], publication bias, dose-response gradient, magnitude of association, and presence of residual confounding [43].
Results
Study selection and characteristics
We initially identified 1283 studies across five medical databases (See Supplementary Appendix 1, Additional File 1). After removing 496 redundant studies, 787 studies remained for screening. From this screening, 113 studies appeared potentially relevant based on their titles, leading to retrieval of the full paper. Ultimately, 53 studies met the criteria for inclusion as full-paper journal articles. Among them, 33 studies fulfilled the eligibility criteria [44–76]. No additional eligible studies were found through references searches. For a visual representation of the process, refer to the PRISMA 2020 flow diagram [32] (Fig. 1).
Table 1 presents the general characteristics of the thirty-three studies [44–76], including details such as author names, publication year, countries, baseline Maslach Burnout Inventory scores, medical specialties of participants, study designs, intervention descriptions, durations, and frequencies, outcome measurements, and loss to follow-up. Of these studies, 25 (75.8%) focused on individual interventions [50–72, 74, 75], while 8 (24.2%) addressed organizational interventions [44, 45, 47–49, 73, 76]. Among the individual interventions, 16 (64%) centered on coaching and emphasized aspects like self-development, resilience, and coping skills [59–72, 74], while 9 (36%) exclusively utilized meditation [50–58]. Regarding organizational intervention, 6 (75%) primarily targeted work-hour modification through changed in shift lengths and rest days after shift [44, 45, 47, 73, 76], while 2 (25%) focused on creating improved learning environment, such as healthy food delivery programs and workflow modifications [48, 49].
Table 1.
Characteristics of thirty-three eligible studies
| Author (year), country Measurement |
Baseline MBI scores |
N control/ intervention, (specialty) |
Design | Intervention | Control | |
|---|---|---|---|---|---|---|
| Intervention Mean (SD) |
Control Mean (SD) |
|||||
| Individual coaching interventions (16 studies) | ||||||
|
Ares (2019), United States (9-item aMBI) |
EE: 7.6 (3.1) DP: 6.5 (4.4) PA: 15.9 (3.3) |
EE: 7.6 (3.1) DP: 6.5 (4.4) PA: 15.9 (3.3) |
25/21 (Neurosurgery) | Historical-control |
Mode: Bimonthly wellness lecture Duration: NA Frequency: 0.5 times per month Length: 12 months Dropout: 0 (0%) |
Mode: Pre-intervention, previous academic year Dropout: 0 (0%) |
|
Bragard (2008), Belgium (22-item MBI) |
EE: 25.2 (9.2) DP: 9.2 (5.3) PA: 37.2 (5.6) |
EE: 26.7 (8.4) DP: 9.1 (5.1) PA: 35.8 (5.5) |
58/57 (Mixed) | Randomized controlled trial |
Mode: 30-hour communication skills and 10-hour stress management skills Duration: 4 h per week Frequency: 10 times per month Length: 5 months Dropout: 9 (16%) |
Mode: Waitlist control Dropout: 10 (18%) |
|
Fainstad (2022), United States (22-item MBI) |
EE: 26.0 (8.1) DP: 10.9 (5.5) PA: 35.8 (5.7) |
EE: 28.2 (8.9) DP: 11.1 (5.6) PA: 33.7 (6.9) |
50/51 (Mixed) | Randomized controlled trial |
Mode: Online group-coaching program Duration: 1 h per week Frequency: 8 times per month Length: 6 months Dropout: 16 (32%) |
Mode: Waitlist control Dropout: 6 (12%) |
|
Hart (2019), United States (22-item MBI) |
EE: 24.3 (9.8) DP: 14.2 (5.4) PA: 33.1 (5.0) |
EE: 24.3 (9.8) DP: 14.2 (5.4) PA: 33.1 (5.0) |
46/46 (Emergency medicine) | Self-control |
Mode: Corporate wellness lectures Duration: 1 h per week Frequency: 1 times per month Length: 6 months Dropout: 22 (48%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 12 (26%) |
| Individual coaching interventions (16 studies), continued | ||||||
|
Huang (2020), China (22-item MBI) |
EE: 16.4 (4.8) DP: 7.0 (3.4) PA: 28.5 (7.1) |
EE: 15.8 (5.5) DP: 6.9 (2.8) PA: 28.1 (7.7) |
18/18 (Mixed) | Randomized controlled trial |
Mode: Balint group Duration: 1 h per week Frequency: 2 times per month Length: 6 months Dropout: 0 (0%) |
Mode: Waitlist control Dropout: 0 (0%) |
|
Martins (2011), Argentina (22-item MBI) |
EE: 22.8 (7.4) DP: 7.3 (3.4) PA: 36.5 (3.5) |
EE: 22.0 (6.4) DP: 6.7 (3.3) PA: 34.8 (3.7) |
37/37 (Pediatrics) | Randomized controlled trial |
Mode: Brief intervention Duration: 3 h per week Frequency: 2 times per month Length: 1 months Dropout: 0 (0%) |
Mode: Waitlist control Dropout: 0 (0%) |
|
Milstein (2012), United States (22-item MBI) |
EE: 26.0 (6.6) DP: 9.1 (6.3) PA: 34.3 (5.9) |
EE: 21.2 (10.1) DP: 12.0 (5.4) PA: 43.6 (3.5) |
7/8 (Pediatrics) | Randomized controlled trial |
Mode: Individual psychotherapeutic toll (brief intervention - BATHE technique) Duration: 0 h per week Frequency: 12 times per month Length: 3 months Dropout: 0 (0%) |
Mode: Waitlist control Dropout: 0 (0%) |
|
Palamara (2021), United States (22-item MBI) |
EE: NA (8.8) DP: NA (8.4) PA: NA |
EE: NA (8.8) DP: NA (8.4) PA: NA |
235/235 (Internal medicine) | Self-control |
Mode: Professional Development Coaching Program Duration: 1 h per week Frequency: 0.3 times per month Length: 8 months Dropout: 117 (50%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 117 (50%) |
| Individual coaching interventions (16 studies), continued | ||||||
|
Riall (2017), United States (16-item MBI) |
EE: 16.8 (8.4) DP: 10.3 (7.9) PA: 27.8 (6.9) |
EE: 16.8 (8.4) DP: 10.3 (7.9) PA: 27.8 (6.9) |
49/49 (General surgery) | Self-control |
Mode: Energy Leadership executive coaching model Duration: NA Frequency: 1 times per month Length: 12 months Dropout: 10 (20%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 10 (20%) |
|
Sheer (2021), United States (22-item MBI) |
EE: 10.6 (8.3) DP: 10.4 (8.0) PA: 38.5 (6.4) |
EE: 10.6 (8.3) DP: 10.4 (8.0) PA: 38.5 (6.4) |
107/107 (Internal medicine) | Self-control |
Mode: Wellness morning reports by resident and discussion group by senior residents (Grassroot Interventions) Duration: 1 h per week Frequency: 2 times per month Length: 6 months Dropout: 65 (61%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 67 (63%) |
|
Slavin (2016), United States (22-item MBI) |
EE: 29.6 (9.3) DP: 10.2 (4.2) PA: NA |
EE: 29.6 (9.3) DP: 10.2 (4.2) PA: NA |
17/18 (Pediatrics) | Historical-control |
Mode: Small workshop sessions targeted on stress management and life appreciation Duration: 1 h per week Frequency: 0.5 times per month Length: 12 months Dropout: 0 (0%) |
Mode: Pre-intervention, previous academic year Dropout: 0 (0%) |
| Individual coaching interventions (16 studies), continued | ||||||
|
Song (2020), United States (9-item aMBI) |
EE: 7.6 (4.2) DP: 5.2 (4.5) PA: 16.2 (1.8) |
EE: 7.6 (4.2) DP: 5.2 (4.5) PA: 16.2 (1.8) |
25/25 (General surgery) | Self-control |
Mode: Resilience coaching program with workshops Duration: 1 h per week Frequency: 0.7 times per month Length: 8 months Dropout: 0 (0%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 0 (0%) |
| Seeland (2024), United States (22-item MBI) |
EE: 25.5 (9.6) DP: 9.5 (4.3) PA: 39.7 (5.8) |
EE: 25.5 (9.6) DP: 9.5 (4.3) PA: 39.7 (5.8) |
58/58 (Obstetrics and gynecology) | Historical-control |
Wellness Wednesday, wellness week, wellness workshops Duration: NA Frequency: 0.33 time per month Length: 24 months Dropout: 17 (35%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 20 (42%) |
|
Stephanie (2022), Philippines (22-item MBI) |
EE: 30.2 (10.0) DP: 13.7 (5.6) PA: 33.5 (4.9) |
EE: 30.2 (10.0) DP: 13.7 (5.6) PA: 33.5 (4.9) |
59/59 (Mixed) | Self-control |
Mode: I-CARE program (communication skill workshops) Duration: NA Frequency: 2 times per month Length: 6 months Dropout: 42 (71%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 0 (0%) |
|
Wild (2018), United States (22-item MBI, average score) |
EE: 2.6 (1.5) DP: 2.4 (1.6) PA: 5.1 (1.1) |
EE: 2.6 (1.5) DP: 2.4 (1.6) PA: 5.1 (1.1) |
31/31 (Mixed) | Historical-control |
Mode: Patient-centered communication training Duration: 1 h per week Frequency: 4 times per month Length: 36 months Dropout: 0 (0%) |
Mode: Pre-intervention, previous academic year Dropout: 0 (0%) |
| Individual coaching interventions (16 studies), continued | ||||||
|
Winer (2019), United States (22-item MBI) |
EE: 20.0 (9.4) DP: 13.0 (4.8) PA: 38.0 (3.4) |
EE: 20.0 (9.4) DP: 13.0 (4.8) PA: 38.0 (3.4) |
36/36 (General surgery) | Self-control |
Mode: Comprehensive resident curriculum (This Week in SCORE) Duration: 1 h per week Frequency: 4 times per month Length: 12 months Dropout: 19 (53%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 19 (53%) |
| Individual meditation interventions (9 studies) | ||||||
|
Carullo (2021), United States (9-item aMBI) |
EE: 9.9 (3.9) DP: 7.3 (4.3) PA: 13.4 (2.0) |
EE: 9.9 (3.9) DP: 7.3 (4.3) PA: 13.4 (2.0) |
53/53 (Anesthesiology) | Self-control |
Mode: Smartphone meditation application Duration: 1 h per week Frequency: 30 times per month Length: 4 months Dropout: 22 (42%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 22 (42%) |
|
Dunne (2019), United States (22-item MBI) |
EE: 26.0 (4.0) DP: 9.4 (1.7) PA: 36.7 (7.0) |
EE: 26.5 (5.2) DP: 8.8 (1.0) PA: 35.8 (8.8) |
29/29 (Emergency medicine) | Randomized controlled trial |
Mode: Attention-based training program (mantra meditation) Duration: 4 h per week Frequency: 2 times per month Length: 2 months Dropout: 12 (41%) |
Mode: Waitlist control Dropout: 4 (14%) |
|
Loewenthal (2021), United States (22-item MBI) |
EE: 3.4 (1.2) DP: 2.8 (1.5) PA: NA |
EE: 3.2 (1.9) DP: 3.1 (2.1) PA: NA |
38/18 (Mixed) | Randomized controlled trial |
Mode: RISE program (Mindfulness-Based Stress Reduction by Yoga) Duration: 1 h per week Frequency: 4 times per month Length: 2 months Dropout: 12 (32%) |
Mode: Waitlist control Dropout: 2 (11%) |
| Individual meditation interventions (9 studies), continued | ||||||
|
Pandit (2022), United Kingdom (9-item aMBI) |
EE: 7.5 (4.8) DP: 5.0 (1.2) PA: 15.0 (6.0) |
EE: 7.5 (4.8) DP: 5.0 (1.2) PA: 15.0 (6.0) |
21/21 (Neurosurgery) | Self-control |
Mode: Mindfulness course Duration: 2 h per week Frequency: 4 times per month Length: 2 months Dropout: 0 (0%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 0 (0%) |
|
Peterson (2021), United States (22-item MBI) |
EE: 21.1 (12.2) DP: 8.3 (6.2) PA: 42.2 (3.4) |
EE: 21.1 (12.2) DP: 8.3 (6.2) PA: 42.2 (3.4) |
14/14 (Obstetrics and gynecology) | Self-control |
Mode: Mindfulness course Duration: 2 h per week Frequency: 2 times per month Length: 3 months Dropout: 2 (14%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 0 (0%) |
|
Purdie (2023), United States (9-item aMBI) |
EE: 10.7 (4.8) DP: 6.0 (4.8) PA: 13.8 (3.9) |
EE: 10.6 (4.0) DP: 5.9 (4.0) PA: 13.9 (3.1) |
27/39 (Pediatrics) | Randomized controlled trial |
Mode: Mindfulness Awareness Practices (MAPs) Duration: 2 h per week Frequency: 3 times per month Length: 1 months Dropout: 0 (0%) |
Mode: Waitlist control Dropout: 0 (0%) |
|
Schmeusser (2023), United States (22-item MBI) |
EE: 14.5 (5.2) DP: 14.2 (7.2) PA: 37.7 (5.7) |
EE: 14.5 (5.2) DP: 14.2 (7.2) PA: 37.7 (5.7) |
24/24 (Obstetrics and gynecology) | Historical-control |
Mode: Wellness program (meditation, guided reflection, and yoga) Duration and frequency: NA Length: 12 months Dropout: 6 (25%) |
Mode: Pre-intervention, previous academic year Dropout: 5 (21%) |
| Individual meditation interventions (9 studies), continued | ||||||
|
Verweij (2017), Netherlands (20-item MBI) |
EE: 16.5 (7.8) DP: 4.8 (3.0) PA: 32.8 (5.1) |
EE: 14.5 (7.1) DP: 5.5 (3.9) PA: 32.9 (5.0) |
80/68 (Mixed) | Randomized controlled trial |
Mode: Mindfulness-Based Stress Reduction (MBSR) Duration: 3 h per week Frequency: 4 times per month Length: 2 months Dropout: 9 (11%) |
Mode: Waitlist control Dropout: 1 (1%) |
|
Weitzman (2021), United States (22-item MBI) |
EE: NA (0.3) DP: NA (0.3) PA: NA (0.4) |
EE: NA (0.3) DP: NA (0.3) PA: NA (0.4) |
18/18 (Otolaryngology) | Self-control |
Mode: Virtual reality meditation program Duration: 0 h per week Frequency: 1 times per month Length: 4 months Dropout: 0 (0%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 0 (0%) |
| Organizational work-hour interventions (6 studies) | ||||||
|
Burgos (2014), Argentina (22-item MBI) |
EE: 29.0 (11.6) DP: 19.0 (12.3) PA: 31.0 (5.8) |
EE: 29.0 (11.6) DP: 19.0 (12.3) PA: 31.0 (5.8) |
25/25 (Cardiology) | Historical-control |
Mode: Day of rest after shift Duration: NA Frequency: NA Length: 12 months Dropout: 2 (8%) |
Mode: Pre-intervention, previous academic year Dropout: 6 (24%) |
|
Parshuram (a) (2015), Canada (22-item MBI) |
EE: 26.2 (11.0) DP: 13.0 (4.8) PA: 37.3 (4.9) |
EE: 23.7 (10.2) DP: 9.8 (4.9) PA: 36.9 (7.4) |
17/15 (Mixed) | Randomized controlled trial |
Mode: Shift length modification from 24 to 12 h Duration: NA Frequency: NA Length: 2 months Dropout: 3 (18%) |
Mode: 24-hour shift Length: 2 months Dropout: 2 (13%) |
| Organizational work-hour interventions (6 studies), continued | ||||||
|
Parshuram (b) (2015), Canada (22-item MBI) |
EE: 26.4 (9.6) DP: 11.4 (7.4) PA: 35.3 (5.4) |
EE: 23.7 (10.2) DP: 9.8 (4.9) PA: 36.9 (7.4) |
15/15 (Mixed) | Randomized controlled trial |
Mode: Shift length modification from 24 to 16 h Duration: NA Frequency: NA Length: 2 months Dropout: 1 (7%) |
Mode: 24-hour shift Length: 2 months Dropout: 2 (13%) |
| Heppe (2024), United States (22-item MBI) |
EE: 25 (IQR, 19–30) DP: 11 (IQR, 8–15) PA 38 (IQR, 33–41) |
EE: 25 (IQR, 19–30) DP: 11 (IQR, 8–15) PA 38 (IQR, 33–41) |
313/313 (Internal Medicine) | Historical-control |
Mode: Alternate 4 + 4 block schedule (4 inpatient on-call weeks plus 4 outpatient off-call weeks) Duration: 24 months Frequency: NA Dropout: 97 (31%) |
Mode: No alternate on-call and off-call schedule Duration: 24 months Frequency: NA Dropout: 97 (31%) |
|
Schuh (2011), United States (22-item MBI) |
EE: 23.3 (12.4) DP: 8.7 (6.6) PA: 35.6 (8.1) |
EE: 23.3 (12.4) DP: 8.7 (6.6) PA: 35.6 (8.1) |
34/34 (Neurology) | Self-control |
Mode: Work hour limitation Duration: NA Frequency: NA Length: 1 months Dropout: 11 (32%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 10 (29%) |
|
Stevens (2020), United States (22-item MBI) |
EE: 2.7 (1.2) DP: 1.7 (0.9) PA: 4.6 (0.9) |
EE: 2.7 (1.2) DP: 1.7 (0.9) PA: 4.6 (0.9) |
19/19 (Otolaryngology) | Self-control |
Mode: 2-hour protected nonclinical time Duration: 2 h per week Frequency: 4 times per month Length: 4 months Dropout: 0 (0%) |
Mode: Pre-intervention baseline characteristics of the participants Dropout: 0 (0%) |
| Organizational improved learning environment interventions (2 studies) | ||||||
|
Bisgaard (2021), United States (22-item MBI) |
EE: 23.5 (11.2) DP: 9.6 (4.4) PA: 32.8 (6.4) |
EE: 23.5 (11.2) DP: 9.6 (4.4) PA: 32.8 (6.4) |
59/59 (General surgery) | Historical-control |
Mode: Healthy snacks delivery Duration: NA Frequency: 4 times per month Length: 24 months Dropout: 32 (54%) |
Mode: Pre-intervention, previous academic year Dropout: 28 (47%) |
|
Ogunyemi (2021), United States (22-item MBI) |
EE: 28.1 (10.6) DP: 12.5 (6.6) PA: 38.5 (6.3) |
EE: 28.1 (10.6) DP: 12.5 (6.6) PA: 38.5 (6.3) |
130/130 (Mixed) | Historical-control |
Mode: Learning environment and workflow streamlining Duration: NA Frequency: NA Length: 24 months Dropout: 9 (7%) |
Mode: Pre-intervention, previous academic year Dropout: 0 (0%) |
The majority of studies employed non-randomized, non-concurrent designs, with 9 (27.2%) using historical controls [44, 48, 49, 56, 59, 68, 71, 75, 76] and 13 (39.4%) utilizing self-control studies [47, 50, 53, 54, 58, 61, 65–67, 69, 70, 72, 73]. Eleven (33.3%) studies were randomized, controlled, concurrent trials [45, 51, 52, 55, 57, 60–64, 74]. Outcome measurements were conducted using various versions of the validated MBI. Specifically, 26 studies (78.7%) used the 22-item MBI [44, 45, 47–49, 51, 52, 54, 56, 58, 60–65, 67, 68, 70–76], 5 studies (15.2%) employed the 9-item MBI [50, 53, 55, 59, 69], 1 study (3.0%) used the 20-item Dutch version of the MBI [57], and 1 study (3.0%) utilized the 16-item MBI [66]. The median timeframe of interventions is 6 months (IQR, 3 to 12 months).
Risk of bias in studies
According to Cochrane RoB2 [33], all randomized studies were rated as a high risk of bias (See Supplementary Appendix 5.1, Additional File 1). This bias primarily stemmed from the fourth domain, concerning subjective participant-reported outcomes without blinding. Moreover, with the exception of one study [60] (91.0%), there were issues with defining sequence generation and allocation concealment, resulting in a rating of some concerns regarding the first domain. Also, 8 studies [45, 51, 52, 57, 63, 64, 74], comprising 72.7% of the total, were categorized as high risk of bias in the second or third domain due to naive per protocol analysis from complete cases at the end of the studies.
All non-randomized studies were evaluated to be at high risk of bias using Cochrane ROBINS-I [34] (See Supplementary Appendix 5.2, Additional File 1), primarily due to inadequate confounder control, with historical control studies in particular. All studies were also susceptible to a high risk of bias arising from subjective participant-reported outcomes without blinding.
Leave-one-out sensitivity analyses demonstrated robustness across all outcome domains (See Supplementary Appendix 3.1 to 3.6, Additional File 1), with no suspected publication bias indicated by the funnel plots (See Supplementary Appendix 4.1 to 4.6, Additional File 1).
Meta-analysis of individual intervention studies
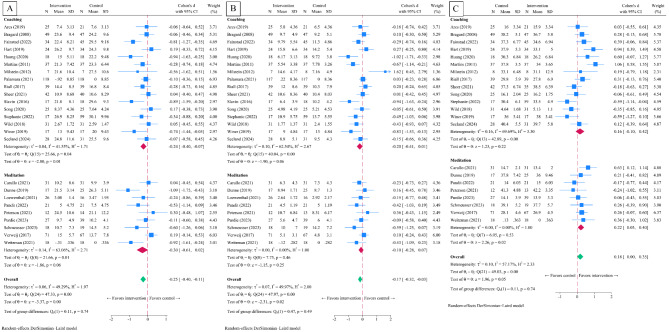
Comparison of the intervention group with the control group in individual intervention studies revealed a significant post-intervention Cohen’s d SMD in EE (-0.25, 95% CI -0.40 to -0.11, p < 0.001, I2 = 49.3%) (Fig. 2A) and DP (-0.18, 95% CI -0.32 to -0.03, p = 0.02, I2 = 50.0%) (Fig. 2B). However, there was no significant difference observed in PA (0.18, 95% CI 0.00 to 0.35, p = 0.05, I2 = 57.2%) (Fig. 2C).
Fig. 2.
Post-intervention standardized mean difference in twenty-five individual interventions included in the systematic review and meta-analyses. Legends: panel A, emotional exhaustion; panel B, depersonalization; panel C, personal accomplishment
Subgroup analyses of coaching intervention [59–72, 74, 75] demonstrated a post-intervention Cohen’s d SMD in EE (-0.24, 95% CI -0.40 to -0.07, p = 0.04, I2 = 41.6%). Nevertheless, non-significant differences were found for DP (-0.20, 95% CI -0.41 to 0.01, p = 0.07, I2 = 62.5%) and PA (0.16, 95% CI -0.10 to 0.42, p = 0.22, I2 = 69.7%). In the subgroup of meditation intervention studies [50–58], the Cohen’s d SMD was found to be non-statistically significant in EE (-0.30, 95% CI -0.61 to 0.02, p = 0.25, I2 = 63.1%) and DP (-0.10, 95% CI -0.28 to 0.07, p = 0.25, I2 = 0%), but statistically significant in PA (0.22, 95% CI 0.03 to 0.40, p = 0.02, I2 = 0%). Subgroup analyses for interventions with less than 6 months in timeframe yielded EE -0.32 (95% CI -0.61 to -0.03, p = 0.03, I2 = 58.3%), DP -0.12 (95% CI -0.40 to 0.15, p = 0.38, I2 = 56.2%), and PA (0.35, 95% CI 0.08 to 0.62, p = 0.01, I2 = 50.5%). Whereas in interventions with timeframe equals to 6 months and longer demonstrated EE -0.23 (95% CI -0.40 to -0.11, p = 0.01, I2 = 46.7%), and DP -0.19 (95% CI -0.38 to -0.02, p = 0.03, I2 = 49.5%), and PA (0.08, 95% CI -0.14 to 0.30, p = 0.47, I2 = 56.7%). (See Supplementary Appendix 2.1 to 2.3, Additional File 1).
Meta-analysis of organizational intervention studies
In organizational intervention studies [44, 45, 47–49, 73, 76], pooling of post-intervention intervention Cohen’s d SMD yielded non-statistically significant resulted in all outcomes, EE (-0.22, 95% CI -0.47 to 0.04, p = 0.10, I2 = 62.6%) (Fig. 3A), DP (-0.15, 95% CI -0.38 to 0.08, p = 0.21, I2 = 53.0%) (Fig. 3B), and PA (0.12, 95% CI -0.01 to 0.25, p = 0.07; I2 = 0%) (Fig. 3C).
Fig. 3.
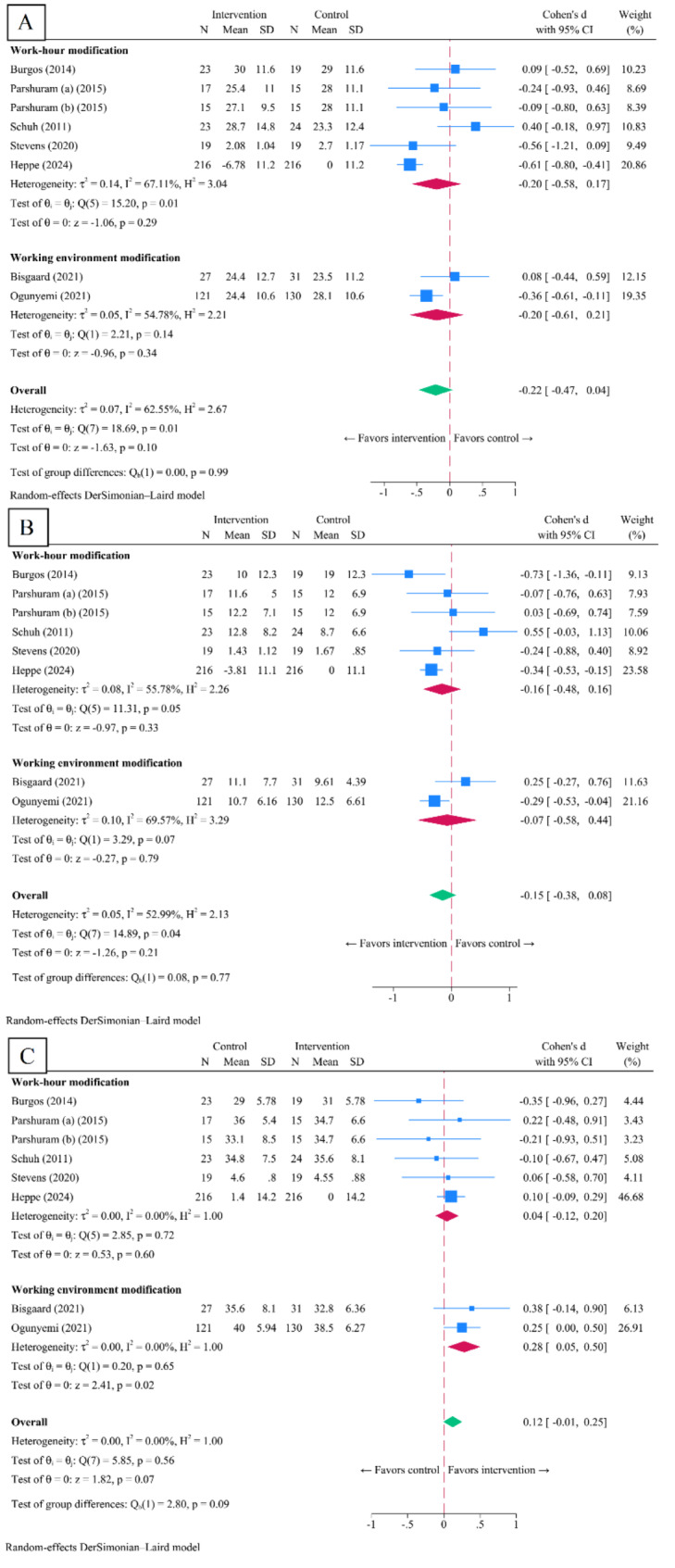
Post-intervention standardized mean score difference in eight organizational interventions included in the systematic review and meta-analyses. Legends: panel A, emotional exhaustion; panel B, depersonalization; panel C, personal accomplishment
Subgroup analyses revealed no post-intervention Cohen’s d SMD in work-hour interventions [44, 45, 47, 73, 76] across all outcome domains: EE (-0.20, 95% CI -0.58 to 0.17, p = 0.29, I2 = 67.1%), DP (-0.16, 95% CI -0.48 to 0.16, p = 0.33, I2 = 55.8%), and PA (0.04, 95% CI -0.12 to 0.20, p = 0.60, I2 = 0%). Moreover, the subgroup of improved learning environment interventions [48, 49] showed non-statistically significant post-intervention mean score differences in EE (-0.20, 95% CI -0.61 to 0.21, p = 0.74, I2 = 54.8%) and DP (-0.07, 95% CI -0.58 to 0.44, p = 0.79, I2 = 69.6%), but a significant difference in PA (0.28, 95% CI 0.05 to 0.50, p = 0.02, I2 = 0%). Subgroup analyses for interventions with less than 6 months in timeframe yielded EE (0.07, 95% CI -0.32 to 0.46, p = 0.71, I2 = 6.9%), DP (0.22, 95% CI -0.18 to 0.61, p = 0.28, I2 = 8.2%), and PA (-0.04, 95% CI -0.42 to 0.34, p = 0.84, I2 = 0%). Whereas in interventions with timeframe equals to 6 months and longer demonstrated EE (-0.33, 95% CI -0.60 to -0.07, p = 0.01, I2 = 60.8%), DP -0.28 (95% CI -0.48 to -0.08, p = 0.01, I2 = 36.9%), and PA (0.14, 95% CI -0.01 to 0.29, p = 0.06, I2 = 6.8%). (See Supplementary Appendix 2.4 to 2.6, Additional File 1).
GRADE evidence profile
All studies across different domains were predominantly non-randomized. Consequently, according to the GRADE evidence profile, we initially established low quality of evidence. However, due to the high risk of bias, we downgraded the quality assessment further, resulting in all studies providing very low quality of evidence (Table 2).
Table 2.
GRADE evidence profile of thirty-three eligible studies
| Outcomes | GRADE evidence profile* |
Number of participants (studies) | Effect size (Cohen’s d) | Quality of the evidence (GRADE) | ||||
|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Control group | Intervention group | |||
| Individual coaching intervention compared to no intervention | ||||||||
|
Population: Resident physicians Setting: Training center Intervention: Individual coaching intervention Comparison: No intervention | ||||||||
|
Emotional exhaustion assessed with • 22-item MBI, with score ranging from 0 (low EE) to 54 (high EE) (n = 13) • 16-item MBI, with score ranging from 0 (low EE) to 30 (high EE) (n = 1) • 9-item aMBI, with score ranging from 0 (low EE) to 18 (high EE) (n = 2) |
Serious | Serious | Not serious | Not serious | 587 (5 historical-control, 6 self-control, 5 RCT studies) | 528 (5 historical-control, 6 self-control, 5 RCT studies) | 0.24 lower (0.40 lower to 0.07 lower) |
Very low ⊕⊖⊖⊖ |
|
Depersonalization assessed with • 22-item MBI, with score ranging from 0 (low DP) to 30 (high DP) (n = 13) • 16-item MBI, with score ranging from 0 (low DP) to 30 (high DP) (n = 1) • 9-item aMBI, with score ranging from 0 (low DP) to 18 (high DP) (n = 2) |
Serious | Serious | Not serious | Not serious | 587 (5 historical-control, 6 self-control, 5 RCT studies) | 528 (5 historical-control, 6 self-control, 5 RCT studies) | 0.20 lower (0.41 lower to 0.01 higher) |
Very low ⊕⊖⊖⊖ |
| Individual coaching intervention compared to no intervention, continued | ||||||||
|
Personal accomplishment assessed with • 22-item MBI, with score ranging from 0 (low PA) to 48 (high PA) (n = 11) • 16-item MBI, with score ranging from 0 (low PA) to 36 (high PA) (n = 1) • 9-item aMBI, with score ranging from 0 (low PA) to 18 (high PA) (n = 2) |
Serious | Serious | Not serious | Not serious | 451 (4 historical-control, 5 self-control, 5 RCT studies) | 394 (4 historical-control, 5 self-control, 5 RCT studies) | 0.16 higher (0.10 lower to 0.42 higher) |
Very low ⊕⊖⊖⊖ |
| Individual meditation intervention compared to no intervention | ||||||||
|
Population: Resident physicians Setting: Training center Intervention: Individual meditation intervention Comparison: No intervention | ||||||||
|
Emotional exhaustion assessed with • 22-item MBI, with score ranging from 0 (low EE) to 54 (high EE) (n = 5) • 20-item MBI with score ranging from 0 (low EE) to 48 (high EE) (n = 1) • 9-item aMBI, with score ranging from 0 (low EE) to 18 (high EE) (n = 3) |
Serious | Not serious | Not serious | Not serious | 250 (1 historical-control, 4 self-control, 4 RCT studies) | 241 (1 historical-control, 4 self-control, 4 RCT studies) | 0.33 lower (0.59 lower to 0.08 lower) |
Very low ⊕⊖⊖⊖ |
| Individual meditation intervention compared to no intervention, continued | ||||||||
|
Depersonalization assessed with • 22-item MBI, with score ranging from 0 (low DP) to 30 (high DP) (n = 5) • 20-item MBI with score ranging from 0 (low DP) to 30 (high DP) (n = 1) • 9-item aMBI, with score ranging from 0 (low DP) to 18 (high DP) (n = 3) |
Serious | Not serious | Not serious | Not serious | 249 (1 historical-control, 4 self-control, 4 RCT studies) | 241 (1 historical-control, 4 self-control, 4 RCT studies) | 0.11 lower (0.34 lower to 0.11 higher) |
Very low ⊕⊖⊖⊖ |
|
Personal accomplishment assessed with • 22-item MBI, with score ranging from 0 (low PA) to 48 (high PA) (n = 4) • 20-item MBI, with score ranging from 0 (low PA) to 42 (high PA) (n = 1) • 9-item aMBI, with score ranging from 0 (low PA) to 18 (high PA) (n = 3) |
Serious | Not serious | Not serious | Not serious | 233 (1 historical-control, 4 self-control, 3 RCT studies) | 215 (1 historical-control, 4 self-control, 3 RCT studies) | 0.21 higher (0.03 higher to 0.40 higher) |
Very low ⊕⊖⊖⊖ |
| Organizational work-hour intervention compared to no intervention | ||||||||
|
Population: Resident physicians Setting: Training center Intervention: Organizational work-hour intervention Comparison: No intervention | ||||||||
|
Emotional exhaustion assessed with • 22-item MBI, with score ranging from 0 (low EE) to 54 (high EE) (n = 6) |
Serious | Serious | Not serious | Serious | 308 (2 historical-control, 2 self-control, 2 RCT studies) | 313 (2 historical-control, 2 self-control, 2 RCT studies) | 0.20 lower (0.58 lower to 0.17 higher) |
Very low ⊕⊖⊖⊖ |
| Organizational work-hour modification intervention compared to no intervention, continued | ||||||||
|
Depersonalization assessed with • 22-item MBI, with score ranging from 0 (low DP) to 30 (high DP) (n = 6) |
Serious | Serious | Not serious | Serious | 308 (2 historical-control, 2 self-control, 2 RCT studies) | 313 (2 historical-control, 2 self-control, 2 RCT studies) | 0.16 lower (0.49 lower to 0.16 higher) |
Very low ⊕⊖⊖⊖ |
|
Personal accomplishment assessed with • 22-item MBI, with score ranging from 0 (low PA) to 48 (high PA) (n = 6) |
Serious | Not serious | Not serious | Not serious | 308 (2 historical-control, 2 self-control, 2 RCT studies) | 313 (2 historical-control, 2 self-control, 2 RCT studies) | 0.04 lower (0.12 lower to 0.20 higher) |
Very low ⊕⊖⊖⊖ |
| Organizational improved learning environment compared to no intervention | ||||||||
|
Population: Resident physicians Setting: Training center Intervention: Organizational improved learning environment intervention Comparison: No intervention | ||||||||
|
Emotional exhaustion assessed with • 22-item MBI, with score ranging from 0 (low EE) to 54 (high EE) (n = 2) |
Serious | Serious | Not serious | Serious | 161 (2 historical-control studies) | 148 (2 historical-control studies) | 0.20 lower (0.61 lower to 0.21 higher) |
Very low ⊕⊖⊖⊖ |
|
Depersonalization assessed with • 22-item MBI, with score ranging from 0 (low DP) to 30 (high DP) (n = 2) |
Serious | Serious | Not serious | Serious | 161 (2 historical-control studies) | 148 (2 historical-control studies) | 0.07 lower (0.58 lower to 0.44 higher) |
Very low ⊕⊖⊖⊖ |
|
Personal accomplishment assessed with • 22-item MBI, with score ranging from 0 (low PA) to 48 (high PA) (n = 2) |
Serious | Not serious | Not serious | Not serious | 161 (2 historical-control studies) | 148 (2 historical-control studies) | 0.28 higher (0.05 higher to 0.50 higher) |
Very low ⊕⊖⊖⊖ |
*No studies fulfil the upward rating of evidence in large magnitude of an effect, dose-response gradient, and the effect of plausible residual confounding; no publication bias was found in all outcomes
Discussion
To our knowledge, this systematic review and meta-analysis examined the effectiveness of interventions reducing burnout aimed at resident physicians, both at the individual and organizational level. Our findings indicate that individual interventions were significantly associated with reduced EE and DP scores, as measure by Cohen’s d SMD, compared with no interventions. However, it is important to note that according to the Cochrane Handbook of Meta-analysis [77], although statistically significant, the effect sizes observed were considered to have small practical significant. Furthermore, organizational interventions did not show any significant association with any domain of burnout.
Previous systematic reviews conducted on general practitioners (GP) and other health personnel yielded similar results to our findings. EE scores consistently reduced across all reporting studies [19, 78]. Some studies also showed a trend towards reduced DP scores [26], with a few demonstrating statistically significant results [19, 78]. However, the inconsistent in reduction in DP only reached statistically significance when pooling all individual interventions. This increased significance was due to the inclusion of additional studies in the last two years [53–56, 60, 65, 70], enhancing the statistical power and precision, thus establishing small effect sizes. Conversely, the limited addition of new organizational studies during this period prevented the attainment of statistically significance in DP reduction [49]. PA scores were reported in only a few studies [26, 79], with significant improvements observed. However, our finding showed only a trend towards statistical significance, even with the inclusion of newer studies [53–56, 60, 65, 70].
Exploratory subgroup analyses revealed notable differences between the effects of individual coaching and individual meditation interventions. In the case of individual coaching, post-intervention Cohen’s d SMD in EE scores were statistically significant, although with a small practical significance. Conversely, for individual meditation interventions, statistically significant were observed in PA scores, also with a small practical significance. This suggests distinct outcomes for these two types of interventions reducing burnout. This finding aligns with a recent clustered randomized study [80] conducted among a similar group of physicians. We suspected that various factors such as the characteristics of interventions, participant preferences, and voluntariness [81] might have influenced these results. Meditation sessions, focusing on breath and posture, differed significantly from the interactive, contemporary psychological techniques offered by coaching interventions in addressing day-to-day clinical demands. Consequently, they may have targeted distinct domain of burnout [82, 83]. While some studies suggest that coaching can help individuals discover and reflect on their strengths [84, 85], this effect was not clearly observed in our population, possibly due to differences in the content of each coaching intervention’s curriculum. In summary, our findings suggest the influence of interventions characteristics on the observed outcomes, as well as emphasizing the potential benefits of combining mediation with coaching interventions, may lead to improvement in both EE and PA [62, 66, 86]. This highlights the potential synergy between these approaches in addressing mitigating burnout among resident physicians.
In studies focusing on organizational intervention, improvement in PA scores were pronounced in interventions targeting improved learning environment compared to those addressing work hours. This difference may be attributed to the lessor disruption to personal schedules caused by interventions such as healthy food catering and workflow streamlining, as opposed to modifications to work hours. Changes in work hours can pose challenges to the continuity of patient care and shift transitions [45, 73, 87, 88]. Additionally, abrupt mandatory changes imposed by overseeing organizations may be perceived negatively by resident physicians, who may see them as a reduction in their already limited autonomy over work hours [73, 89]. This perception is supported by other systematic reviews on resident physicians and work-hour restrictions [90]. In summary, modifying work processes appears to better meet the needs of resident physicians compared to extensive changes to work hours [91]. This finding can help clarify the reasons behind the observed differences in PA score improvements between various organizational interventions. It emphasizes the potential challenges associated with modifying work hours and underscores the importance of considering resident physicians’ autonomy and needs when implementing interventions.
This review demonstrated several methodology strengths and adherence to recommendation guidelines outlined by Cochrane [77] and PRISMA [32] for a systematic review and meta-analysis. We utilized standardized quality assessment tools, namely RoB2 [33], ROBINS-I [34], and GRADE [39–43] to comprehensively evaluate risk of bias and certainty of evidences. Also, apart from individual and organizational intervention, we provided subgroup analyses to find possible differences in effect sizes across different study attributes. The robustness of sensitivity analyses and low risk of publication bias provided us with reliability and impartiality of the synthesized results. Additionally, in employing SMD, enables us to assess both statistical and practical significance. However, it is crucial to interpret the findings cautiously due to described limitations. Firstly, we included in our search strategy only the MBI as diagnostic tool for burnout. Different tools are nowadays existing for evaluate burnout. Examples included Melamed Burnout Questionnaire (SMBQ) [92], Oldenburg Burnout Inventory (OLBI) [93], Copenhagen Burnout Inventory (CBI) [94] and School Burnout Inventory (SBI) [95]. Secondly, conducting pairwise meta-analyses necessitated assuming comparability between control and intervention types, leading to significant heterogeneity, possibly stemming from methodological differences among intervention and control groups [37, 96, 97]. Population heterogeneity, including specialty types and cultural contexts, may also influence intervention effectiveness and compliance. High heterogeneity in outcome domains, often observed in other meta-analyses [18, 24, 26, 78, 98], suggests a mix of healthcare professionals in the studies [24], complicating the interpretation. Limited intervention comparability further contributed to heterogeneity [24–27, 78]. Thirdly, the included studies’ risk of bias was high, consistent with previous assessments [18, 24, 78], due to subjective participant-reported outcomes without blinding and inadequate confounder control in non-randomized studies [33]. Fourthly, organizational interventions were limited in varieties and numbers, which may result in underpower in detecting the true effect sizes. Therefore, any reported in burnout scores should be cautiously interpreted [20, 23, 79].
The implications of this study for practice and policy are substantial, particularly within postgraduate medical education curricula. Individual coaching interventions exhibit promise in reducing EE, with the potential for even greater impact when combined with meditation interventions to enhance PA. Individual coaching intervention consisted of positive psychology workshop such as resilience, stress management, and also encompassed the individual-driven development of soft skill include teamwork and communication. The qualitative synthesis of intervention characteristics, along with the quantitative synthesis consisting of subgroup analyses by implementation timeframe, provided an insight to the optimal intervention duration of longer than 6 months. Regarding those individual coaching interventions, we suggest 1–2 h per week in frequency of 1–2 times per month, with sustained activity for 6–12 months in order to harness their effectiveness. Whereas meditation should be practiced 1–2 h per week in frequency of 1–2 times per month for 6–12 months to properly introduce participants to its concept. On the other hand, the organizational interventions, especially those centered on work-hour modifications, have demonstrated limited benefits, while interventions addressing improved learning environment have shown improvement in PA. In this case, we recommended that work-hour modifications included shift-length modification, work-hour limitation and day-of-rest after shift should be evaluated after the participant have been able to adjust to the new work schedule, optimally 2–4 months after initiation. However, for more complex organizational interventions to improve learning environment include workflow streamlining and healthy snacks delivery should be evaluated in longer timeframe, in terms of 1–2 years for their effectiveness. Nevertheless, a critical consideration for program coordinators before implementing interventions is participant compliance, which requires careful planning and solutions. Finally, qualitative syntheses suggest considering a mixed bundle of approaches to burnout prevention, incorporating both individual and organizational interventions for synergistic effectiveness [18, 22–24, 26, 27].
For future studies, rigorous methodologies are essential to confirm the synthesized evidence. Randomized studies, such as preference-based trials [99], and non-randomized studies with targeted trial frameworks, incorporating adequate baseline and time-varying confounder control methods like regression and inverse probability weighting can enhance the effectiveness of outcomes [34, 100, 101]. Additionally, organizational interventions could be more efficiently using cluster parallel [102] or step-wedge design RCTs [103], which harness collective compliance within physician clusters in the same specialties. Alternatively, time-series designs may be suitable for organizational interventions [104] in institutes with active surveillance and consistent data collection of burnout, allowing for the assessment of long-term population-level changes in MBI scores [105, 106].
Conclusions
A diverse array of interventions, both individual and organizational interventions, have been implemented among resident physicians. Individual coaching intervention led to a small yet significant improvement in EE, while individual meditation interventions were associated with a similar small but significant enhancement in PA. Organizational intervention, primarily focused on improved learning environment, resulted in small but significant enhancements in PA. However, the strength of these recommendations is relatively limited due risk of bias and inconsistency in the data. Further studies should prioritize a combined approach, integrating both individual and organizational interventions, with a rigorous methodology aimed at generating credible evidence for a synergistic approach to prevention burnout in post-graduate medical education.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Faculty of Medicine, Chiang Mai University, Chiang Mai, 50200, Thailand.
Abbreviations
- CBI
Copenhagen Burnout Inventory
- DP
Depersonalization
- EE
Emotional exhaustion
- GP
General practitioners
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluations
- HSE
Health and Safety Executive
- MBI
Maslach Burnout Inventory
- MeSH
Medical Subject Headings
- OLBI
Oldenburg Burnout Inventory
- PA
Personal accomplishment
- PICO
Participant, intervention, control, outcome
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
- RCT
Randomized-controlled trial
- RoB2
Cochrane Risk-of-Bias Tool for Randomized Trials
- ROBINS-I
Cochrane Risk Of Bias In Non-randomized Studies - of Interventions
- SBI
School Burnout Inventory
- SMBQ
Shirom-Melamed Burnout Questionnaire
- SMD
Standardized mean difference
- SMIs
Stress Management Interventions
- WHO
World Health Organization
Author contributions
W.K. registered the study protocol on PROSPERO, planned the methodology, assessed quality and risk of bias, synthesized quantitative results, prepared draft manuscript and uploaded the data repository files. V.S. planned the methodology, assessed quality and risk of bias, and corrected the final manuscript. W.S. provided detailed guidance and practice on systematic review and meta-analysis and rechecked quantitative results.
Funding
No funding had been received for this manuscript.
Data availability
The datasets generated and analyzed during the current study are available in the Open Science Framework (OSF) repository; DOI: 10.17605/OSF.IO/3T5RB.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Burn-out an occupational phenomenon: International Classification of Diseases 2019. https://www.who.int/news/item/28-05-2019-burn-out-an-occupational-phenomenon-international-classification-of-diseases
- 2.Demerouti E, Bakker AB, Peeters MCW, Breevaart K. New directions in burnout research. Eur J Work Organizational Psychol. 2021;30(5):686–91. [Google Scholar]
- 3.Han S, Shanafelt TD, Sinsky CA, Awad KM, Dyrbye LN, Fiscus LC, et al. Estimating the attributable cost of Physician Burnout in the United States. Ann Intern Med. 2019;170(11):784–90. [DOI] [PubMed] [Google Scholar]
- 4.Belfi LM, Chetlen A, Frigini A, Jay A, Methratta ST, Robbins J, et al. Recovering Joy in the Workplace requires P.R.A.C.T.I.C.E. Acad Radiol. 2023;30(3):536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low ZX, Yeo KA, Sharma VK, Leung GK, McIntyre RS, Guerrero A et al. Prevalence of Burnout in Medical and Surgical residents: a Meta-analysis. Int J Environ Res Public Health. 2019;16(9). [DOI] [PMC free article] [PubMed]
- 6.Rotenstein LS, Torre M, Ramos MA, Rosales RC, Guille C, Sen S, et al. Prevalence of Burnout among Physicians: a systematic review. JAMA. 2018;320(11):1131–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyrbye LN, West CP, Satele D, Boone S, Tan L, Sloan J, et al. Burnout among U.S. medical students, residents, and early career physicians relative to the general U.S. population. Acad Med. 2014;89(3):443–51. [DOI] [PubMed] [Google Scholar]
- 8.Maslach C, Schaufeli WB, Leiter MP. Job burnout. Annu Rev Psychol. 2001;52:397–422. [DOI] [PubMed] [Google Scholar]
- 9.Maslach C, Leiter MP. Early predictors of job burnout and engagement. J Appl Psychol. 2008;93(3):498–512. [DOI] [PubMed] [Google Scholar]
- 10.Maslach C, Leiter MP. Understanding the burnout experience: recent research and its implications for psychiatry. World Psychiatry. 2016;15(2):103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel RS, Bachu R, Adikey A, Malik M, Shah M. Factors related to Physician Burnout and its consequences: a review. Behav Sci (Basel). 2018;8(11). [DOI] [PMC free article] [PubMed]
- 12.West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516–29. [DOI] [PubMed] [Google Scholar]
- 13.Pantenburg B, Luppa M, König HH, Riedel-Heller SG. Burnout among young physicians and its association with physicians’ wishes to leave: results of a survey in Saxony, Germany. J Occup Med Toxicol. 2016;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou AY, Panagioti M, Esmail A, Agius R, Van Tongeren M, Bower P. Factors Associated with burnout and stress in Trainee Physicians: a systematic review and Meta-analysis. JAMA Netw Open. 2020;3(8):e2013761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merlo G, Rippe J. Physician burnout: a Lifestyle Medicine Perspective. Am J Lifestyle Med. 2021;15(2):148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attenello FJ, Buchanan IA, Wen T, Donoho DA, McCartney S, Cen SY, et al. Factors associated with burnout among US neurosurgery residents: a nationwide survey. J Neurosurg. 2018;129(5):1349–63. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues H, Cobucci R, Oliveira A, Cabral JV, Medeiros L, Gurgel K, et al. Burnout syndrome among medical residents: a systematic review and meta-analysis. PLoS ONE. 2018;13(11):e0206840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aryankhesal A, Mohammadibakhsh R, Hamidi Y, Alidoost S, Behzadifar M, Sohrabi R, et al. Interventions on reducing burnout in physicians and nurses: a systematic review. Med J Islam Repub Iran. 2019;33:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busireddy KR, Miller JA, Ellison K, Ren V, Qayyum R, Panda M. Efficacy of interventions to reduce Resident Physician Burnout: a systematic review. J Grad Med Educ. 2017;9(3):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalani SD, Azadfallah P, Oreyzi H, Adibi P. Interventions for Physician Burnout: a systematic review of systematic reviews. Int J Prev Med. 2018;9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panagioti M, Panagopoulou E, Bower P, Lewith G, Kontopantelis E, Chew-Graham C, et al. Controlled interventions to reduce burnout in Physicians: a systematic review and Meta-analysis. JAMA Intern Med. 2017;177(2):195–205. [DOI] [PubMed] [Google Scholar]
- 22.Rothenberger DA. Physician burnout and Well-Being: a systematic review and Framework for Action. Dis Colon Rectum. 2017;60(6):567–76. [DOI] [PubMed] [Google Scholar]
- 23.Wiederhold BK, Cipresso P, Pizzioli D, Wiederhold M, Riva G. Intervention for Physician Burnout: a systematic review. Open Med (Wars). 2018;13:253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang XJ, Song Y, Jiang T, Ding N, Shi TY. Interventions to reduce burnout of physicians and nurses: an overview of systematic reviews and meta-analyses. Med (Baltim). 2020;99(26):e20992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tement S, Ketiš ZK, Miroševič Š, Selič-Zupančič P. The impact of psychological interventions with elements of mindfulness (PIM) on Empathy, Well-Being, and reduction of Burnout in Physicians: a systematic review. Int J Environ Res Public Health. 2021;18(21). [DOI] [PMC free article] [PubMed]
- 26.De Simone S, Vargas M, Servillo G. Organizational strategies to reduce physician burnout: a systematic review and meta-analysis. Aging Clin Exp Res. 2021;33(4):883–94. [DOI] [PubMed] [Google Scholar]
- 27.DeChant PF, Acs A, Rhee KB, Boulanger TS, Snowdon JL, Tutty MA, et al. Effect of Organization-Directed Workplace interventions on Physician Burnout: a systematic review. Mayo Clin Proc Innov Qual Outcomes. 2019;3(4):384–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery A, Panagopoulou E, Esmail A, Richards T, Maslach C. Burnout in healthcare: the case for organisational change. BMJ. 2019;366:l4774. [DOI] [PubMed] [Google Scholar]
- 29.Jordan JG, Tinline E, Giga G, Faragher S, Cooper B. In: HaSE HSE, editor. C.;. Beacons of excellence in stress prevention. Manchester, United Kingdom: Health and Safety Executive (HSE); 2003. p. 7. [Google Scholar]
- 30.Medicine NL. Medical Subject Headings. 2022.
- 31.Library C. MeSH browser 2022. https://www.cochranelibrary.com/advanced-search/mesh
- 32.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 34.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CJ; C. Entering data with the RevMan calculator: Cochrane; 2015. https://training.cochrane.org/resource/entering-data-revman-calculator
- 36.E DAB, RevMan Calculator C. 2015. https://training.cochrane.org/resource/revman-calculator
- 37.Sedgwick P. Meta-analyses: what is heterogeneity? BMJ. BMJ. 2015;350:h1435. [DOI] [PubMed] [Google Scholar]
- 38.Jackson D, Bowden J, Baker R. How does the DerSimonian and Laird procedure for random effects meta-analysis compare with its more efficient but harder to compute counterparts? J Stat Plann Inference. 2010;140(4):961–70. [Google Scholar]
- 39.Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–35. [DOI] [PubMed] [Google Scholar]
- 40.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64(12):1294–302. [DOI] [PubMed] [Google Scholar]
- 41.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol. 2011;64(12):1303–10. [DOI] [PubMed] [Google Scholar]
- 42.Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol. 2011;64(12):1283–93. [DOI] [PubMed] [Google Scholar]
- 43.Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol. 2011;64(12):1277–82. [DOI] [PubMed] [Google Scholar]
- 44.Burgos L, Battioni L, Costabel J, Alves de Lima A. Evaluation of Burnout Syndrome in Medical residents following a Rest after Shift intervention. Revista Argentina De Cardiologia. 2018;86:123–6. [Google Scholar]
- 45.Parshuram CS, Amaral AC, Ferguson ND, Baker GR, Etchells EE, Flintoft V, et al. Patient safety, resident well-being and continuity of care with different resident duty schedules in the intensive care unit: a randomized trial. CMAJ. 2015;187(5):321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevens K, Davey C, Lassig AA. Association of Weekly Protected Nonclinical Time with Resident Physician Burnout and Well-being. JAMA Otolaryngol Head Neck Surg. 2020;146(2):168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens K, Davey C, Lassig AA. Association of Weekly Protected Nonclinical Time with Resident Physician Burnout and Well-being. JAMA otolaryngology– head neck Surg. 2020;146(2):168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bisgaard E, Clark A, Hester C, Napier R, Grant J, Scielzo S, et al. Resident Engagement in a Wellness Program in a large academic residency: a Follow-Up after two years of Wellness. J Surg Educ. 2021;78(5):1430–7. [DOI] [PubMed] [Google Scholar]
- 49.Ogunyemi D, Darwish AG, Young G, Cyr E, Lee C, Arabian S, et al. Graduate medical education-led continuous assessment of burnout and learning environments to improve residents’ wellbeing. BMC Med Educ. 2022;22(1):292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carullo PC, Ungerman EA, Metro DG, Adams PS. The impact of a smartphone meditation application on anesthesia trainee well-being. J Clin Anesth. 2021;75. [DOI] [PubMed]
- 51.Dunne PJ, Lynch J, Prihodova L, O’Leary C, Ghoreyshi A, Basdeo SA, et al. Burnout in the emergency department: Randomized controlled trial of an attention-based training program. J Integr Med. 2019;17(3):173–80. [DOI] [PubMed] [Google Scholar]
- 52.Loewenthal J, Dyer NL, Lipsyc-Sharf M, Borden S, Mehta DH, Dusek JA et al. Evaluation of a yoga-based mind-body intervention for Resident Physicians: a Randomized Clinical Trial. Global Adv Health Med. 2021;10. [DOI] [PMC free article] [PubMed]
- 53.Pandit AS, Reka A, Layard Horsfall H, Marcus HJ. Mindfulness Training for Young neurosurgeons: a virtual multicenter prospective pilot study. World Neurosurg. 2022;164:e446–57. [DOI] [PubMed] [Google Scholar]
- 54.Peterson B, Fitzmaurice L, Boehm JK, Bendix B. Pilot study evaluating a 12-h mindfulness-based curriculum for OB/GYN residents. Complement Ther Clin Pract. 2022;48:101620. [DOI] [PubMed] [Google Scholar]
- 55.Purdie DR, Federman M, Chin A, Winston D, Bursch B, Olmstead R, et al. Hybrid delivery of mindfulness meditation and perceived stress in Pediatric Resident Physicians: a Randomized Clinical Trial of In-Person and Digital Mindfulness Meditation. J Clin Psychol Med Settings. 2023;30(2):425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmeusser B, Gauthier Z, Nagy K. Assessment of Resident Burnout After Formalization of Wellness Program. Military medicine. 2022. [DOI] [PubMed]
- 57.Verweij H, van Ravesteijn H, van Hooff MLM, Lagro-Janssen ALM, Speckens AEM. Mindfulness-based stress reduction for residents: a Randomized Controlled Trial. J Gen Intern Med. 2018;33(4):429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weitzman RE, Wong K, Worrall DM, Park C, McKee S, Tufts RE, et al. Incorporating virtual reality to improve Otolaryngology Resident Wellness: one Institution’s experience. Laryngoscope. 2021;131(9):1972–6. [DOI] [PubMed] [Google Scholar]
- 59.Ares WJ, Maroon JC, Jankowitz BT. Pursuit of balance: the UPMC Neurosurgery Wellness Initiative. World Neurosurg. 2019;132:e704–9. [DOI] [PubMed] [Google Scholar]
- 60.Fainstad T, Mann A, Suresh K, Shah P, Dieujuste N, Thurmon K, et al. Effect of a Novel Online Group-Coaching Program to Reduce Burnout in Female Resident Physicians: a Randomized Clinical Trial. JAMA Netw open. 2022;5(5):e2210752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hart D, Paetow G, Zarzar R. Does implementation of a corporate wellness initiative improve burnout? West J Emerg Med. 2019;20(1):138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang L, Harsh J, Cui H, Wu J, Thai J, Zhang X, et al. A Randomized Controlled Trial of Balint Groups to prevent burnout among residents in China. Front Psychiatry. 2019;10:957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martins AE, Davenport MC, Del Valle MP, Di Lalla S, Domínguez P, Ormando L, et al. Impact of a brief intervention on the burnout levels of pediatric residents. J Pediatr (Rio J). 2011;87(6):493–8. [DOI] [PubMed] [Google Scholar]
- 64.Milstein JM, Raingruber BJ, Bennett SH, Kon AA, Winn CA, Paterniti DA. Burnout assessment in house officers: evaluation of an intervention to reduce stress. Med Teach. 2009;31(4):338–41. [DOI] [PubMed] [Google Scholar]
- 65.Palamara K, Chu JT, Chang Y, Yu L, Cosco D, Higgins S, et al. Who benefits most? A Multisite Study of Coaching and Resident Well-being. J Gen Intern Med. 2022;37(3):539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riall TS, Teiman J, Chang M, Cole D, Leighn T, McClafferty H, et al. Maintaining the fire but avoiding burnout: implementation and evaluation of a Resident Well-Being Program. J Am Coll Surg. 2018;226(4):369–79. [DOI] [PubMed] [Google Scholar]
- 67.Sheer A, Estores I, Nickels R, Radhakrishnan N, Goede D, Mramba L et al. Improving burnout and well-being among medicine residents: impact of a grassroots intervention compared to a formal program curriculum. J Edu Health Promotion. 2021;10(1). [DOI] [PMC free article] [PubMed]
- 68.Slavin S, Shoss M, Broom MA. A program to prevent burnout, Depression, and anxiety in First-Year Pediatric residents. Acad Pediatr. 2017;17(4):456–8. [DOI] [PubMed] [Google Scholar]
- 69.Song Y, Swendiman RA, Shannon AB, Torres-Landa S, Khan FN, Williams NN, et al. Can we coach Resilience? An evaluation of Professional Resilience Coaching as a Well-Being Initiative for Surgical interns. J Surg Educ. 2020;77(6):1481–9. [DOI] [PubMed] [Google Scholar]
- 70.Tan-Lim CSC, Dumagay TE, Dahildahil RO, Del Castillo RT, Carasco MAAG. Assessing the impact of a Physician Resiliency and Wellness Program to physician burnout levels in a Pediatric Department of a Tertiary Hospital: a pilot study of the I-CARE program. Acta Med Philippina. 2022;56(6):17–26. [Google Scholar]
- 71.Wild D, Nawaz H, Ullah S, Via C, Vance W, Petraro P. Teaching residents to put patients first: Creation and evaluation of a comprehensive curriculum in patient-centered communication. BMC Med Educ. 2018;18(1). [DOI] [PMC free article] [PubMed]
- 72.Winer LK, Cortez AR, Kassam A-F, Quillin RC, Goodman MD, Makley AT, et al. The impact of a Comprehensive Resident Curriculum and required participation in this week in SCORE on general surgery ABSITE performance and well-being. J Surg Educ. 2019;76(6):e102–9. [DOI] [PubMed] [Google Scholar]
- 73.Schuh LA, Khan MA, Harle H, Southerland AM, Hicks WJ, Falchook A, et al. Pilot trial of IOM duty hour recommendations in neurology residency programs: unintended consequences. Neurology. 2011;77(9):883–7. [DOI] [PubMed] [Google Scholar]
- 74.Bragard I, Etienne Am Fau - Merckaert I, Merckaert I, Fau - Libert Y, Libert Y, Fau - Razavi D, Razavi D. Efficacy of a communication and stress management training on medical residents’ self-efficacy, stress to communicate and burnout: a randomized controlled study. (1461–7277 (Electronic)). [DOI] [PubMed]
- 75.Seeland GR, Williams BM, Yadav M, et al. Implementation and evaluation of a Comprehensive Resident Wellness Curriculum during the COVID-19 pandemic. J Surg Educ. 2024;81(3):397–403. 10.1016/j.jsurg.2023.11.014. [DOI] [PubMed] [Google Scholar]
- 76.Heppe D, Baduashvili A, Limes JE, et al. Resident Burnout, Wellness, Professional Development, and Engagement before and after new training schedule implementation. JAMA Netw Open. 2024;7(2):e240037. 10.1001/jamanetworkopen.2024.0037. Published 2024 Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. (editors);. Cochrane Handbook for systematic reviews of interventions version 6.4. Cochrane; 2023.
- 78.West CP, Dyrbye LN, Erwin PJ, Shanafelt TD. Interventions to prevent and reduce physician burnout: a systematic review and meta-analysis. Lancet. 2016;388(10057):2272–81. [DOI] [PubMed] [Google Scholar]
- 79.Melnyk BM, Kelly SA, Stephens J, Dhakal K, McGovern C, Tucker S, et al. Interventions to Improve Mental Health, Well-Being, Physical Health, and Lifestyle Behaviors in Physicians and nurses: a systematic review. Am J Health Promot. 2020;34(8):929–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fraiman YS, Cheston CC, Cabral HJ, Allen C, Asnes AG, Barrett JT, et al. Effect of a Novel Mindfulness Curriculum on Burnout during Pediatric Internship: a Cluster Randomized Clinical Trial. JAMA Pediatr. 2022;176(4):365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scheepers RA, Emke H, Epstein RM, Lombarts K. The impact of mindfulness-based interventions on doctors’ well-being and performance: a systematic review. Med Educ. 2020;54(2):138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Odom A, Romain A, Phillips J. Using photovoice to explore Family Medicine residents’ burnout experiences and resiliency strategies. Fam Med. 2022;54(4):277–84. [DOI] [PubMed] [Google Scholar]
- 83.Nutting R, Nilsen K, Walling A, Level E. Origin storytelling in Faculty Well-being: a pilot study. PRiMER. 2021;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nalan P, Manning A. The juice is worth the squeeze: Psychiatry residents’ experience of Balint Group. Int J Psychiatry Med. 2022;57(6):508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nease DE Jr., Lichtenstein A, Pinho-Costa L, Hoedebecke K. Balint 2.0: a virtual Balint group for doctors around the world. Int J Psychiatry Med. 2018;53(3):115–25. [DOI] [PubMed] [Google Scholar]
- 86.Fainstad T, Mann A, Suresh K, Shah P, Dieujuste N, Thurmon K, et al. Effect of a Novel Online Group-Coaching Program to Reduce Burnout in Female Resident Physicians: a Randomized Clinical Trial. JAMA Netw Open. 2022;5(5):e2210752–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fang ZA, Hudson D. Resident Work Hour restrictions and Change Management: a cautionary tale. Healthc Q. 2015;18(2):50–1. [DOI] [PubMed] [Google Scholar]
- 88.Typpo KV, Tcharmtchi MH, Thomas EJ, Kelly PA, Castillo LD, Singh H. Impact of resident duty hour limits on safety in the intensive care unit: a national survey of pediatric and neonatal intensivists. Pediatr Crit Care Med. 2012;13(5):578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mookerjee A, Li B, Arora B, Surapaneni R, Rajput V, Van de Ridder M. Micromanagement during Clinical Supervision: solutions to the challenges. Cureus. 2022;14(3):e23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mauser NS, Michelson JD, Gissel H, Henderson C, Mauffrey C. Work-hour restrictions and orthopaedic resident education: a systematic review. Int Orthop. 2016;40(5):865–73. [DOI] [PubMed] [Google Scholar]
- 91.Harris JD, Staheli G, LeClere L, Andersone D, McCormick F. What effects have resident work-hour changes had on education, quality of life, and safety? A systematic review. Clin Orthop Relat Res. 2015;473(5):1600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lundgren-Nilsson Å, Jonsdottir IH, Pallant J, Ahlborg G. Internal construct validity of the Shirom-Melamed Burnout Questionnaire (SMBQ). BMC Public Health. 2012;12(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tipa RO, Tudose C, Pucarea VL. Measuring Burnout among Psychiatric residents using the Oldenburg Burnout Inventory (OLBI) Instrument. J Med Life. 2019;12(4):354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kristensen T, Borritz M, Villadsen E, Christensen K. The Copenhagen Burnout Inventory: a new tool for the assessment of burnout. Work Stress - WORK STRESS. 2005;19:192–207. [Google Scholar]
- 95.Carmona-Halty M, Mena-Chamorro P, Sepúlveda-Páez G, Ferrer-Urbina R. School Burnout Inventory: Factorial Validity, Reliability, and Measurement Invariance in a Chilean Sample of High School Students. 2022;12. [DOI] [PMC free article] [PubMed]
- 96.Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334(7584):94–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Imrey PB. Limitations of Meta-analyses of studies with high heterogeneity. JAMA Netw Open. 2020;3(1):e1919325–e. [DOI] [PubMed] [Google Scholar]
- 98.Bazargan-Hejazi S, Shirazi A, Wang A, Shlobin NA, Karunungan K, Shulman J, et al. Contribution of a positive psychology-based conceptual framework in reducing physician burnout and improving well-being: a systematic review. BMC Med Educ. 2021;21(1):593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Younge JO, Kouwenhoven-Pasmooij TA, Freak-Poli R, Roos-Hesselink JW, Hunink MM. Randomized study designs for lifestyle interventions: a tutorial. Int J Epidemiol. 2015;44(6):2006–19. [DOI] [PubMed] [Google Scholar]
- 100.Lousdal ML. An introduction to instrumental variable assumptions, validation and estimation. Emerg Themes Epidemiol. 2018;15(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Austin PC. An introduction to Propensity score methods for reducing the effects of confounding in Observational studies. Multivar Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hemming K, Eldridge S, Forbes G, Weijer C, Taljaard M. How to design efficient cluster randomised trials. BMJ. 2017;358:j3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ: Br Med J. 2015;350:h391. [DOI] [PubMed] [Google Scholar]
- 104.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2016;46(1):348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Korevaar E, Karahalios A, Turner SL, Forbes AB, Taljaard M, Cheng AC, et al. Methodological systematic review recommends improvements to conduct and reporting when meta-analyzing interrupted time series studies. J Clin Epidemiol. 2022;145:55–69. [DOI] [PubMed] [Google Scholar]
- 106.Hudson J, Fielding S, Ramsay CR. Methodology and reporting characteristics of studies using interrupted time series design in healthcare. BMC Med Res Methodol. 2019;19(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available in the Open Science Framework (OSF) repository; DOI: 10.17605/OSF.IO/3T5RB.