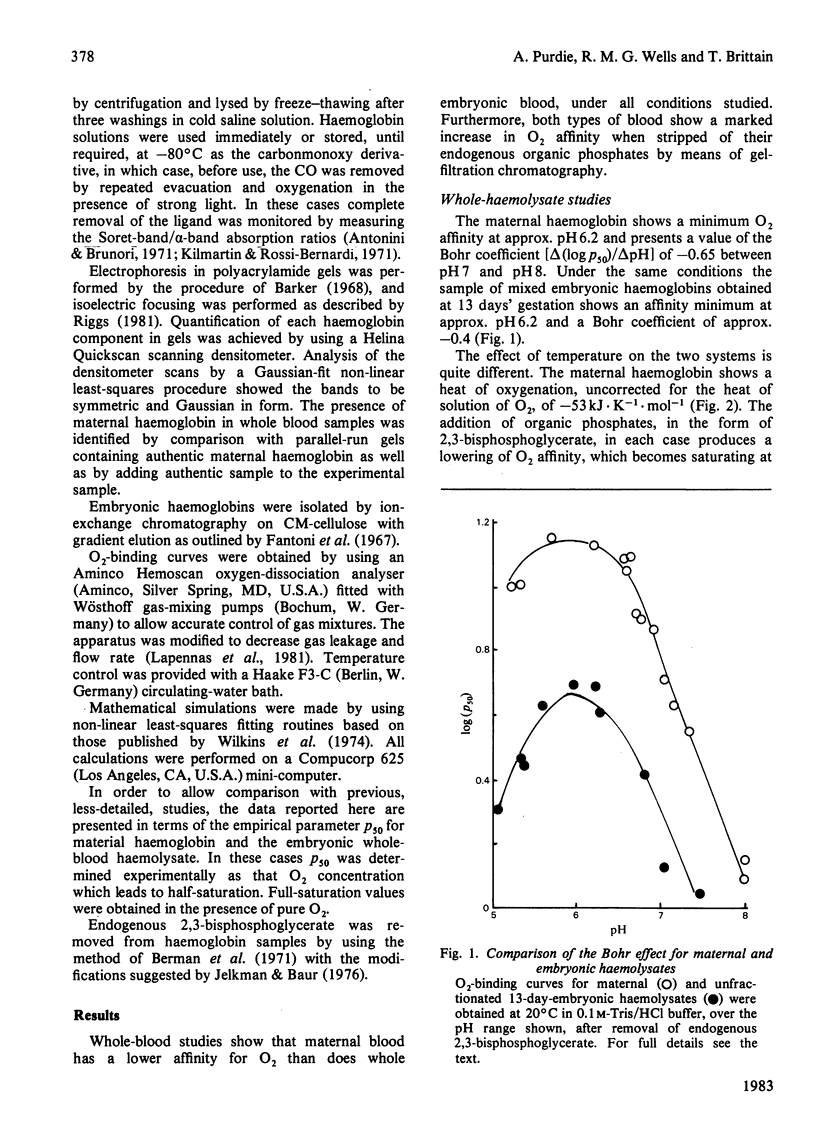
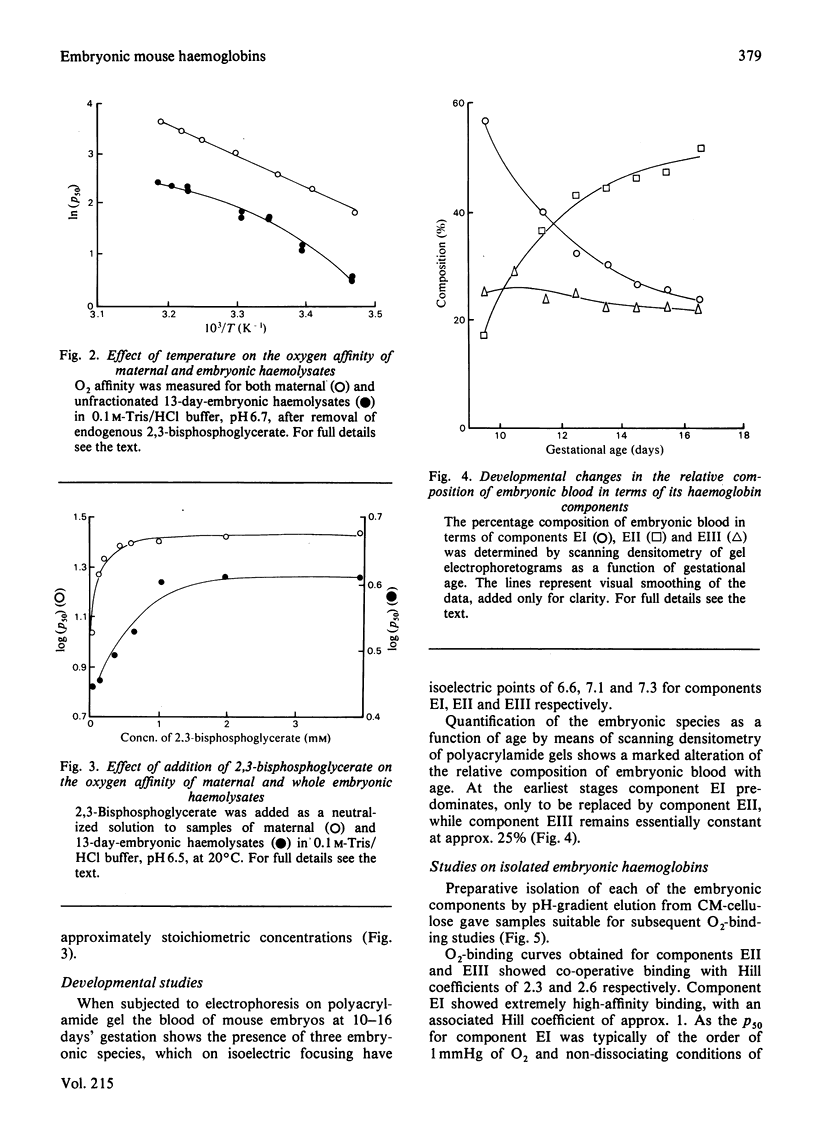
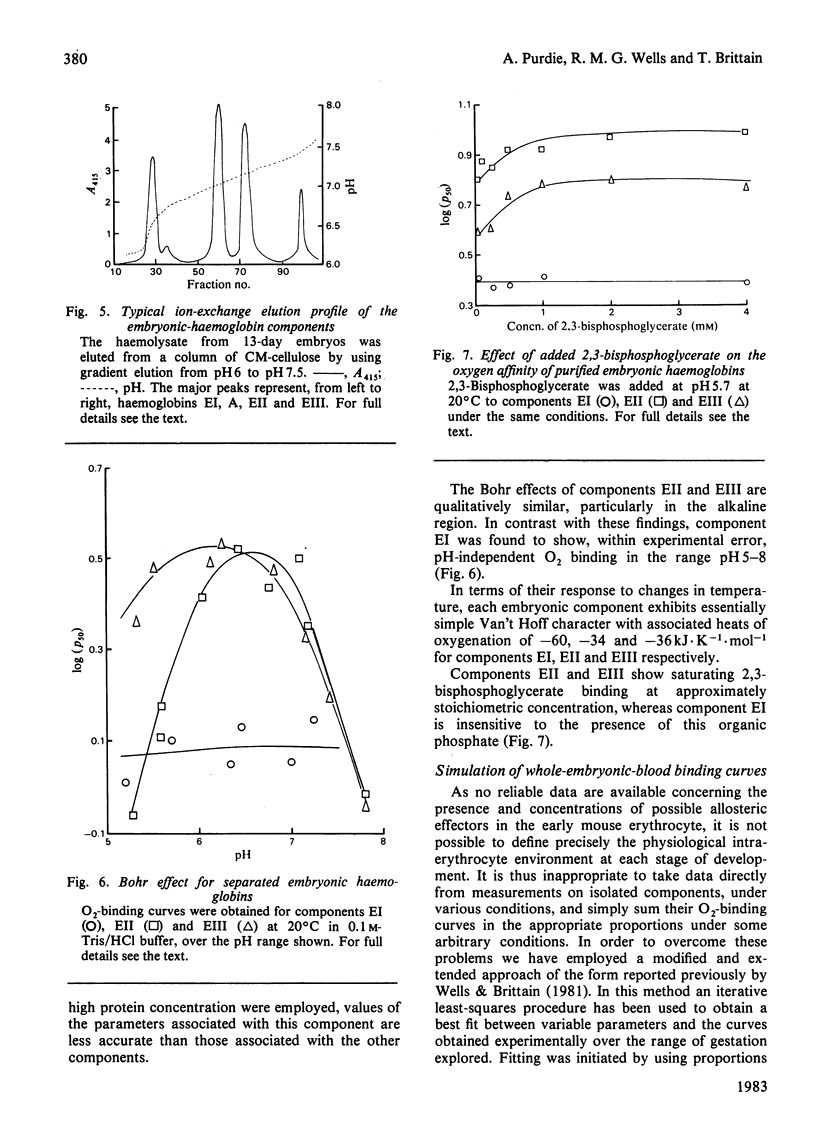
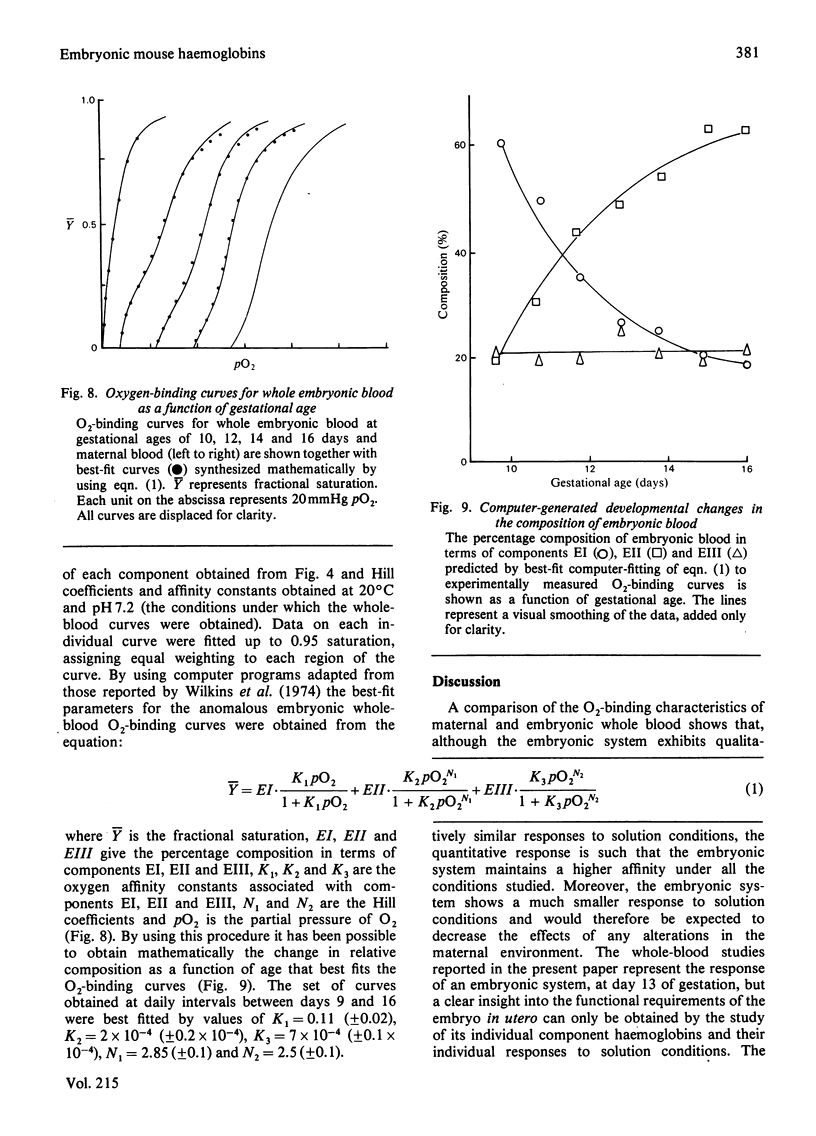
Abstract
Embryos from C57BL/6J mice between the gestational ages of 9 and 16 days possess three embryonic haemoglobins EI, EII and EIII, the proportions of which change as a function of gestational age. Component EI, originally present at approx. 65% at day 9, decreases to approx. 20% by day 16, while component EII increases in an inverse manner to that of component EI. During this period component EIII remains essentially constant at approx. 25%. Separation of these species by ion-exchange chromatography has allowed the characterization of the Hill coefficient, Bohr effect, heat of oxygenation and binding of allosterically active organic phosphates for each component. The three components show marked functional heterogeneity and also differ from maternal haemoglobin. Oxygenation curves for whole embryonic blood show distinct deviations from simple binding behaviour. The presence of a high-affinity component within the blood samples may be accounted for by the presence of haemoglobin EI. By using parameters obtained from the study of the isolated components it has been possible to synthesize mathematically the O2-binding curves, obtained experimentally, throughout the gestational period. The characteristics of the isolated haemoglobin components of embryonic mouse blood are discussed in terms of the changing demands for O2 likely to be encountered by the developing embryo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. E. Development of the mouse hematopoietic system. I. Types of hemoglobin produced in embryonic yolk sac and liver. Dev Biol. 1968 Jul;18(1):14–29. doi: 10.1016/0012-1606(68)90020-1. [DOI] [PubMed] [Google Scholar]
- Bauer C., Tamm R., Petschow D., Bartels R., Bartels H. Oxygen affinity and allosteric effects of embryonic mouse haemolglobins. Nature. 1975 Sep 25;257(5524):333–334. doi: 10.1038/257333a0. [DOI] [PubMed] [Google Scholar]
- Berman M., Benesch R., Benesch R. E. The removal of organic phosphates from hemoglobin. Arch Biochem Biophys. 1971 Jul;145(1):236–239. doi: 10.1016/0003-9861(71)90031-2. [DOI] [PubMed] [Google Scholar]
- Brotherton T. W., Chui D. H., Gauldie J., Patterson M. Hemoglobin ontogeny during normal mouse fetal development. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2853–2857. doi: 10.1073/pnas.76.6.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton T. W., Chui D. H., McFarland E. C., Russell E. S. Fetal erythropoiesis and hemoglobin ontogeny in tail-short (Ts/+) mutant mice. Blood. 1979 Sep;54(3):673–683. [PubMed] [Google Scholar]
- CRAIG M. L., RUSSELL E. S. A DEVELOPMENTAL CHANGE IN HEMOGLOBINS CORRELATED WITH AN EMBRYONIC RED CELL POPULATION IN THE MOUSE. Dev Biol. 1964 Oct;10:191–201. doi: 10.1016/0012-1606(64)90040-5. [DOI] [PubMed] [Google Scholar]
- Fantoni A., Bank A., Marks P. A. Globin composition and synthesis of hemoglobins in developing fetal mice erythroid cells. Science. 1967 Sep 15;157(3794):1327–1329. doi: 10.1126/science.157.3794.1327. [DOI] [PubMed] [Google Scholar]
- Fantoni A., De la Chapelle A., Marks P. A. Synthesis of embryonic hemoglobins during erythroid cell development in fetal mice. J Biol Chem. 1969 Feb 25;244(4):675–681. [PubMed] [Google Scholar]
- Fantoni A., De la Chapelle A., Rifkind R. A., Marks P. A. Erythroid cell-development in fetal mice: synthetic capacity for different proteins. J Mol Biol. 1968 Apr 14;33(1):79–91. doi: 10.1016/0022-2836(68)90282-9. [DOI] [PubMed] [Google Scholar]
- Fantoni A., Farace M. G., Gambari R. Embryonic hemoglobins in man and other mammals. Blood. 1981 Apr;57(4):623–633. [PubMed] [Google Scholar]
- Farkas W., Marks P. A. Partial purification and properties of a ribonuclease from rabbit reticulocytes. J Biol Chem. 1968 Dec 25;243(24):6464–6473. [PubMed] [Google Scholar]
- Jelkmann W., Baufer C. What is the best method to remove 2,3-diphosphoglycerate from hemoglobin? Anal Biochem. 1976 Oct;75(2):382–388. doi: 10.1016/0003-2697(76)90092-0. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. The binding of carbon dioxide by horse haemoglobin. Biochem J. 1971 Aug;124(1):31–45. doi: 10.1042/bj1240031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Woody J. N., Green N. Human T-cell clones recognize chemically synthesized peptides of influenza haemagglutinin. Nature. 1982 Nov 4;300(5887):66–69. doi: 10.1038/300066a0. [DOI] [PubMed] [Google Scholar]
- Lapennas G. N., Colacino J. M., Bonaventura J. Thin-layer methods for determination of oxygen binding curves of hemoglobin solutions and blood. Methods Enzymol. 1981;76:449–470. doi: 10.1016/0076-6879(81)76136-6. [DOI] [PubMed] [Google Scholar]
- Neville J. R. Altered haem--haem interaction and tissue-oxygen supply: a theoretical analysis. Br J Haematol. 1977 Mar;35(3):387–395. doi: 10.1111/j.1365-2141.1977.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Petschow R., Petschow D., Bartels R., Baumann R., Bartels H. Regulation of oxygen affinity in blood of fetal, newborn and adult mouse. Respir Physiol. 1978 Dec;35(3):271–282. doi: 10.1016/0034-5687(78)90003-8. [DOI] [PubMed] [Google Scholar]
- Popp R. A., Marsh C. L., Skow L. C. Expression of embryonic hemoglobin genes in mice heterozygous for alpha-thalassemia or beta-duplication traits and in mice heterozygous for both traits. Dev Biol. 1981 Jul 15;85(1):123–128. doi: 10.1016/0012-1606(81)90241-4. [DOI] [PubMed] [Google Scholar]
- Rifkind R. A., Cantor L. N., Cooper M., Levy J., Maniatis G. M., Bank A., Marks P. A. Ontogeny of erythropoiesis in the fetal mouse. Ann N Y Acad Sci. 1974 Nov 29;241(0):113–118. doi: 10.1111/j.1749-6632.1974.tb21871.x. [DOI] [PubMed] [Google Scholar]
- Riggs A. Preparation of blood hemoglobins of vertebrates. Methods Enzymol. 1981;76:5–29. doi: 10.1016/0076-6879(81)76111-1. [DOI] [PubMed] [Google Scholar]
- WHITTEN W. K. Modification of the oestrous cycle of the mouse by external stimuli associated with the male; changes in the oestrous cycle determined by vaginal smears. J Endocrinol. 1958 Sep;17(3):307–313. doi: 10.1677/joe.0.0170307. [DOI] [PubMed] [Google Scholar]
- Weber G. Asymmetric ligand binding by haemoglobin. Nature. 1982 Dec 16;300(5893):603–607. doi: 10.1038/300603a0. [DOI] [PubMed] [Google Scholar]
- Wells R. M., Brittain T. Transition to cooperative oxygen-binding by embryonic haemoglobin in mice. J Exp Biol. 1981 Feb;90:351–355. doi: 10.1242/jeb.90.1.351. [DOI] [PubMed] [Google Scholar]


