Abstract
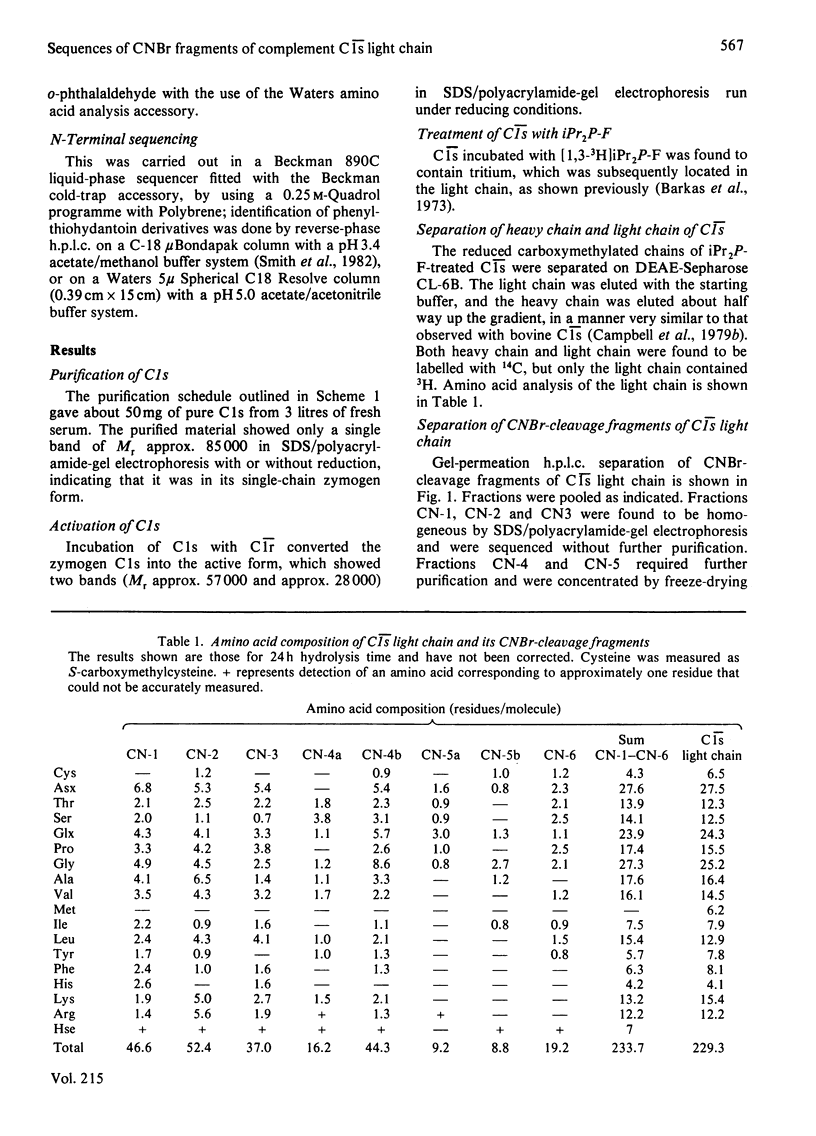
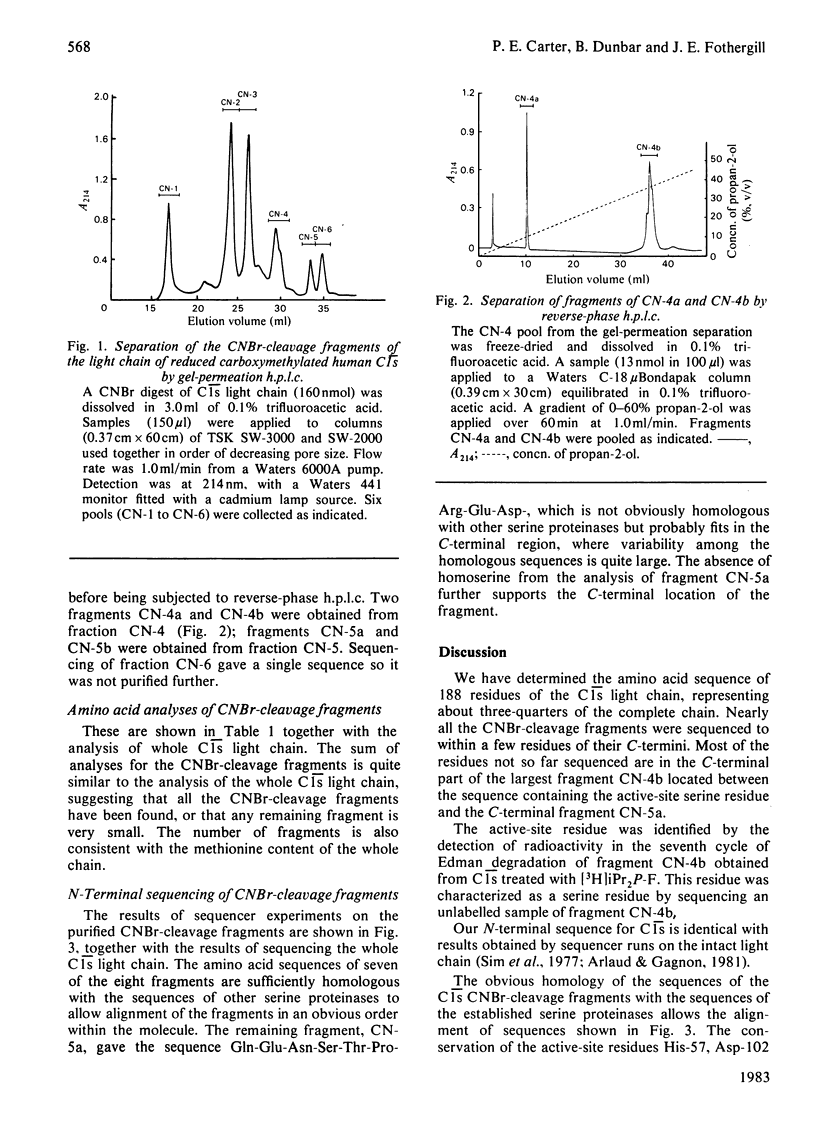
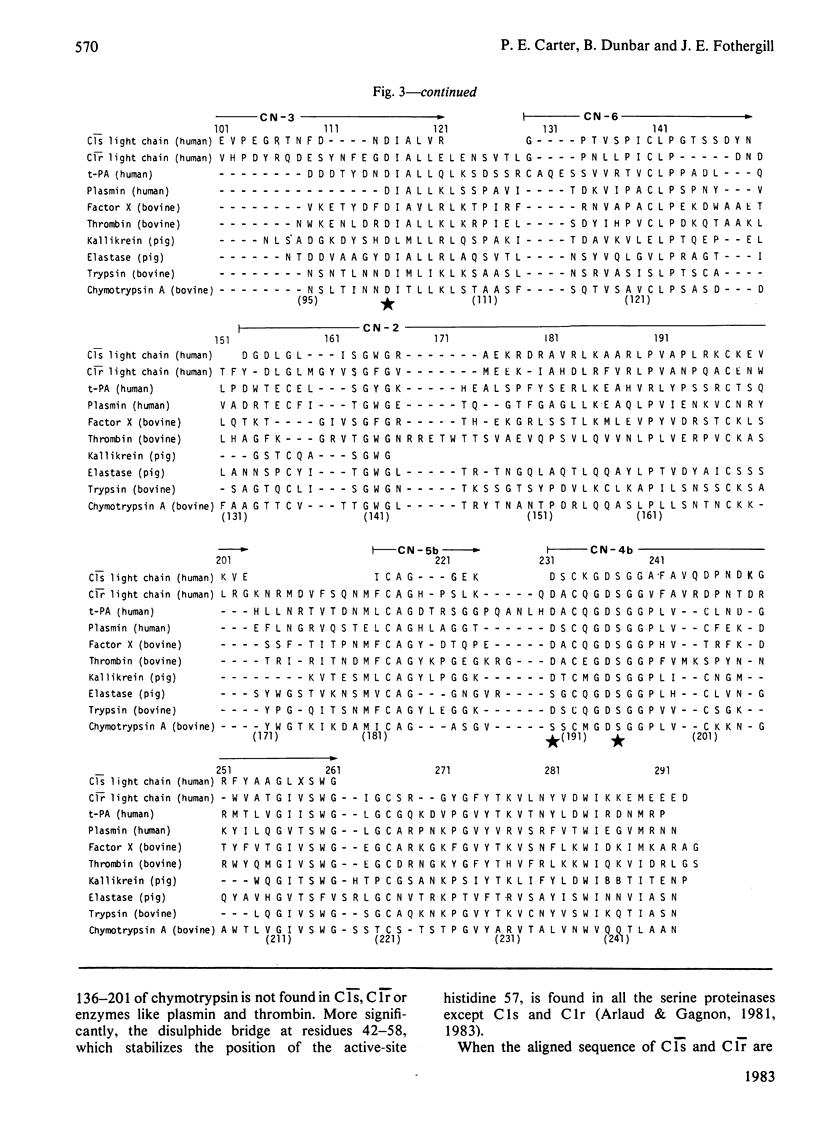
Human complement component C1s was purified from fresh blood by conventional methods of precipitation and chromatography. The single-chain zymogen form was activated by treatment with C1r. Reduction and carboxymethylation then allowed the light chain and heavy chain to be separated on DEAE-Sepharose CL-6B in 8 M-urea. Liquid-phase sequencing of the light chain determined 50 residues from the N-terminus. CNBr-cleavage fragments of the light chain were separated by high-pressure liquid chromatography on gel-permeation and reverse-phase columns. N-Terminal sequencing of these fragments determined the order of a further 138 residues, giving a total of 188 residues or about 75% of the light chain. Seven of these eight sequences could be readily aligned with the amino acid sequences of other serine proteinases. The typical serine proteinase active-site residues are clearly conserved in C1s, and the specificity-related side chain of the substrate-binding pocket is aspartic acid, as in trypsin, consistent with the proteolytic action of C1s on C4 at an arginine residue. Somewhat surprisingly, when the C1s sequence is compared with that of complement subcomponent C1r, the percentage difference (59%) is approximately the same as that found between the other mammalian serine proteinases (56-71%).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlaud G. J., Gagnon J. Clr and Cls subcomponents of human complement: two serine proteinases lacking the 'histidine-loop' disulphide bridge. Biosci Rep. 1981 Oct;1(10):779–784. doi: 10.1007/BF01114800. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Gagnon J. Complete amino acid sequence of the catalytic chain of human complement subcomponent C1-r. Biochemistry. 1983 Apr 12;22(8):1758–1764. doi: 10.1021/bi00277a003. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Gagnon J., Porter R. R. The catalytic chain of human complement subcomponent C1r. Purification and N-terminal amino acid sequences of the major cyanogen bromide-cleavage fragments. Biochem J. 1982 Jan 1;201(1):49–59. doi: 10.1042/bj2010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER E. L. Concerning the mechanism of complement action. I. Inhibition of complement activity by diisopropyl fluophosphate. J Immunol. 1956 Dec;77(6):462–468. [PubMed] [Google Scholar]
- Birktoft J. J., Blow D. M. Structure of crystalline -chymotrypsin. V. The atomic structure of tosyl- -chymotrypsin at 2 A resolution. J Mol Biol. 1972 Jul 21;68(2):187–240. doi: 10.1016/0022-2836(72)90210-0. [DOI] [PubMed] [Google Scholar]
- Booth N. A., Campbell R. D., Fothergill J. E. The purification and characterization of bovine C4, the fourth component of complement. Biochem J. 1979 Mar 1;177(3):959–965. doi: 10.1042/bj1770959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. D., Booth N. A., Fothergill J. E. Purification and characterization of subcomponent C1q of the first component of bovine complement. Biochem J. 1979 Feb 1;177(2):531–540. doi: 10.1042/bj1770531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. D., Booth N. A., Fothergill J. E. The purification and characterization of subcomponent C1s of the first component of bovine complement. Biochem J. 1979 Dec 1;183(3):579–588. doi: 10.1042/bj1830579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill J. E., Anderson W. H. A molecular approach to the complement system. Curr Top Cell Regul. 1978;13:259–311. doi: 10.1016/b978-0-12-152813-3.50012-4. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S. AMINO-ACID SEQUENCE OF BOVINE CHYMOTRYPSINOGEN-A. Nature. 1964 Mar 28;201:1284–1287. doi: 10.1038/2011284a0. [DOI] [PubMed] [Google Scholar]
- Hugli T. E., Müller-Eberhard H. J. Anaphylatoxins: C3a and C5a. Adv Immunol. 1978;26:1–53. doi: 10.1016/s0065-2776(08)60228-x. [DOI] [PubMed] [Google Scholar]
- LEPOW I. H., NAFF G. B., TODD E. W., PENSKY J., HINZ C. F. Chromatographic resolution of the first component of human complement into three activities. J Exp Med. 1963 Jun 1;117:983–1008. doi: 10.1084/jem.117.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J Biol Chem. 1980 Dec 10;255(23):11199–11203. [PubMed] [Google Scholar]
- Moon K. E., Gorski J. P., Hugli T. E. Complete primary structure of human C4a anaphylatoxin. J Biol Chem. 1981 Aug 25;256(16):8685–8692. [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. Subunit composition and structure of subcomponent C1q of the first component of human complement. Biochem J. 1976 Apr 1;155(1):19–23. doi: 10.1042/bj1550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Scott J. D., Fothergill J. E. A general method for affinity purification of complement component C3b using factor H-sepharose. Biochem J. 1982 Sep 1;205(3):575–580. doi: 10.1042/bj2050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., Porter R. R. Isolation and comparison of the proenzyme and activated forms of the human serum complement subcomponents C1r and C1s. Biochem Soc Trans. 1976;4(1):127–129. doi: 10.1042/bst0040127. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Porter R. R., Reid K. B., Gigli I. The structure and enzymic activities of the C1r and C1s subcomponents of C1, the first component of human serum complement. Biochem J. 1977 May 1;163(2):219–227. doi: 10.1042/bj1630219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Gerrie L. M., Dunbar B., Fothergill J. E. Primary structure of bovine complement activation fragment C4a, the third anaphylatoxin. Purification and complete amino acid sequence. Biochem J. 1982 Nov 1;207(2):253–260. doi: 10.1042/bj2070253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Physicochemical and functional characterization of the C1r subunit of the first complement component. J Immunol. 1976 Feb;116(2):496–503. [PubMed] [Google Scholar]


