Abstract
Background:
Emerging literature suggests that fine particulate matter [with aerodynamic diameter ()] air pollution and its components are linked to various neurodevelopmental outcomes. However, few studies have evaluated how component mixtures from distinct sources relate to cognitive outcomes in children.
Objectives:
This cross-sectional study investigated how ambient concentrations of component mixtures relate to neurocognitive performance in 9- to 10-year-old children, as well as explored potential source-specific effects of these associations, across the US.
Methods:
Using spatiotemporal hybrid models, annual concentrations of 15 chemical components of were estimated based on the residential address of child participants from the Adolescent Brain Cognitive Development (ABCD) Study. General cognitive ability, executive function, and learning/memory scores were derived from the NIH Toolbox. We applied positive matrix factorization to identify six major sources based on the 15 components, which included crustal, ammonium sulfate, biomass burning, traffic, ammonium nitrate, and industrial/residual fuel burning. We then utilized weighted quantile sum (WQS) and linear regression models to investigate associations between components’ mixture, their potential sources, and children’s cognitive scores.
Results:
Mixture modeling revealed associations between cumulative exposure and worse cognitive performance across all three outcome domains, including shared overlap in detrimental effects driven by ammonium nitrates, silicon, and calcium. Using the identified six sources of exposure, source-specific negative associations were identified between ammonium nitrates and learning & memory, traffic and executive function, and crustal and industrial mixtures and general cognitive ability. Unexpected positive associations were also seen between traffic and general ability as well as biomass burning and executive function.
Discussion:
This work suggests nuanced associations between outdoor exposure and childhood cognitive performance, including important differences in cognition related both to individual chemicals as well as to specific sources of these exposures. https://doi.org/10.1289/EHP14418
Introduction
Ambient particulate matter (PM) air pollution is considered to be one of the greatest environmental threats to human health due to its near ubiquity and widespread effects.1 In particular, PM with aerodynamic diameter () comprises chemicals with documented neurotoxic effects.2–4 As such, ambient exposure poses serious risks to brain health, which is likely exacerbated in children.5 As children have higher respiratory rates than adults, have developing lung and immune systems, are more active, and spend more time outdoors than adults, their exposure to outdoor air pollution is likely higher than that of adults.6 Beyond increased exposure and risk in early childhood, children may also be susceptible to longer-term effects of air pollution when it affects their ongoing development. The brain, in particular, follows a protracted course of growth and continues into the third decade of life,7–9 providing a large window of vulnerability for air pollution to impact neurodevelopment.10,11 Moreover, the transition from late childhood to early adolescence marks a dynamic period of brain plasticity that ultimately allows for the development of more complex thinking and reasoning skills, also known as executive functions, including the ability to focus, hold, and manipulate information.12–14 These cognitive abilities are central to healthy development and life course outcomes, as they are associated concurrently and prospectively with academic achievement,15–17 as well as physical health and financial wellbeing.18,19 Thus, potential neurotoxic effects of PM exposure on cognitive development during the transition from childhood to adolescence may have long-term consequences for growing youth.
Literature over the past 10 years suggests a link between PM exposure and various cognitive and behavioral impairments in both epidemiologic and animal inhalation studies.20 However, several knowledge gaps exist. Previous studies focused on air pollution and child and adolescent cognition have found mixed results,21–28 but few of these studies focused on the potential neurotoxic effects of PM during the window of exposure in late childhood and early adolescence.29,30 Moreover, most of these previous studies on neurobehavioral effects of PM came from small studies conducted in limited geographical locations. Geographic variability, in particular, is important for a comprehensive assessment of PM effects, as itself is a mixture of various organic and inorganic components, and its chemical composition and sources can vary significantly geographically,31 with differing effects on human health.32,33 To our knowledge, no study has examined how mixtures relate to cognitive outcomes, let alone during early adolescence. This work is important as mapping neurotoxic effects of components and source mixtures may not only facilitate a clearer picture of its neurodevelopmental impacts, but also provide actionable insights for policymakers that may help inform and facilitate source-specific mitigation strategies to improve air quality.
is a complex aerosol mixture, including carbons, metals, and trace elements.34–36 For example, traffic related includes primary emissions from fuel combustion and wear and abrasion of brakes, tires, etc. from light and heavy-duty vehicles,37,38 as well as secondarily formed particles from reactions of gaseous and particulate precursors.39–41 Larger abrasion/wear particles are typically deposited onto or near the road and mixed with crustal and mineral dust particles and corrosion of road materials.39,41 Fuel and oil combustion contributes to through burning of wood, coal, and gas for cooking or heating of the home as well as from industrial sources (i.e., coal burning power plants). Natural sources contributing to include crustal soil and dust and marine sea salt aerosols, which can be suspended and resuspended by winds. Secondary can also form because of chemical reactions between its precursor aerosols and gasses under the right conditions. For example, secondary organic and inorganic secondary particles can form through reactions with gasses, including nitrogen dioxide (), ammonia (), sulfur dioxide (), and/or volatile organic compounds.42 Moreover, not only do global differences exist in the relative contributions of these sources to ,35 but even within the US, regional and urban–rural differences exist in source contributions to the mixture.43 Many epidemiological studies to date have shown that health effects of can vary tremendously depending on its chemical components and major contributing sources.44–47 Therefore, understanding which specific chemical components and sources of the mixture are contributing to neurotoxic effects during key developmental life stages is of critical importance to reduce health burden.
Given this emerging literature suggesting the potential harmful neurological effects of and its components, the goal of the current study was to investigate how mixtures of components and their sources relate to neurocognitive performance in 9- to 10-year-old children across the US. We employed novel, machine learning–based exposure prediction models46,48 to estimate annual concentrations of 15 components [i.e., zinc (Zn), vanadium (V), silicon (Si), lead (Pb), nickel (Ni), potassium (K), iron (Fe), copper (Cu), calcium (Ca), bromine (Br), sulfate (), nitrate (), ammonium (), organic carbon (OC), and elemental carbon (EC)] at the residence of each child from the nationwide Adolescent Brain Cognitive Development Study (ABCD Study). Using positive matrix factorization (PMF), we also derived six major sources of these 15 components. Next, we used a series of weighted quantile sum (WQS) regression analyses to assess the associations between components and their sources on cognitive function derived from NIH Toolbox cognitive battery when children were 9–10 years old. The strength of WQS is that it is a supervised framework that treats multiple pollutants as a mixture to better capture the health effects of simultaneous co-exposure49–51 as seen in the real-world. WQS generates a single index (called weighted quantile sum index) summarizing the overall exposure effect of the mixture. The index, which gives exposures with weaker effect a lower weight, is utilized in a multivariate regression model to test the association of the overall mixture effect on the health outcome. Using WQS, we first examined the mixture effects of the 15 individual components on cognitive functioning. Next, we implemented a grouped WQS regression as well as a series of complementary multiple linear regressions using the six identified sources to examine how sources of exposures relate to cognitive performance. While the linear regression modeling allowed us to examine the overall association of each source with differences in cognition performance, the grouped weighted quantile sum regression model helped determine the potential unique mixture effects of each source group on children’s cognition.
Methods
Study Population
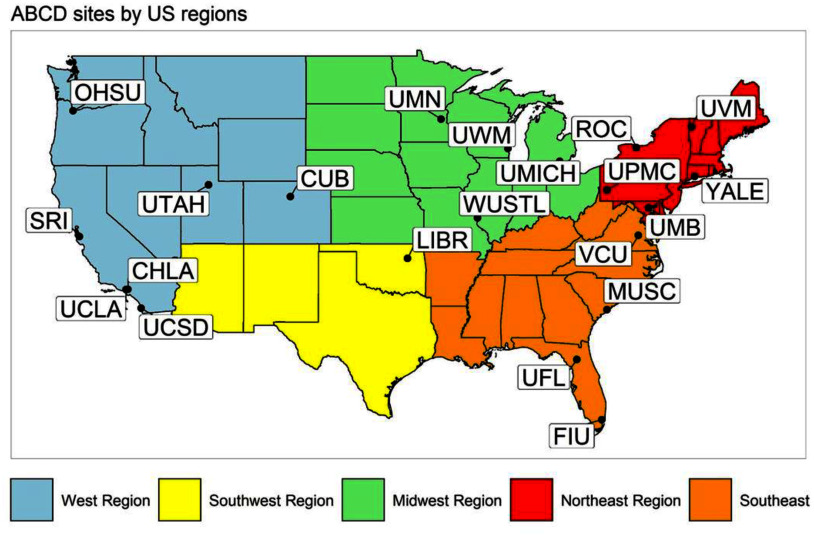
The ABCD Study is the largest long-term study to date on brain and behavior in children across the US.52–54 The study sampling was designed to capture nationwide sociodemographic diversity by recruiting participants from elementary schools (private, public, and charter schools) at 21 study sites across the US52 (Figure 1). Centralized institutional review board (IRB) approval was obtained from the University of California, San Diego. Study sites obtained approval from their local IRB. Each parent or caregiver provided written informed consent and each child provided assent. All ethical regulations were followed during data collection and analysis. For more information, see Garavan et al.52 and Volkow et al.54
Figure 1.
Geographic distribution of the 21 ABCD Study sites across the US. Map created using R depicting study sites included in the ABCD Study within five US regions. Note: CHLA, Children’s Hospital of Los Angeles; CUB, University of Colorado Boulder; FIU, Florida International University; LIBR, Laureate Institute for Brain Research; MUSC, Medical University of South Carolina; OHSU, Oregon Health and Science University; ROC, University of Rochester; SRI, SRI International; UCLA, University of California, Los Angeles; UCSD, UC San Diego; UFL, University of Florida; UMB, University of Maryland Baltimore; UMICH, University of Michigan; UMN, University of Minnesota; UPMC, University of Pittsburgh; UTAH, University of Utah; UVM, University of Vermont; UWM, University of Wisconsin—Milwaukee; VCU, Virginia Commonwealth University; WUSTL, Washington University in St. Louis; YALE, Yale University. Numeric data for Figure 1 can be found in Excel Table S1.
Data for the current analysis is a subset of this ABCD Study (sociodemographic and behavior data from NDA 4.0 data release 2021 and residential address exposure data from NDA 5.0 data release in 2023 can be found at https://doi.org/10.15154/8873-zj65 and https://doi.org/10.15154/1523041, respectively). Primary inclusion criteria for ABCD Study participants included age (9.0 to 10.99 years at baseline visit), fluency in English, and the ability to complete the baseline visit magnetic resonance imaging (MRI). The larger ABCD Study cohort is comprised of 11,876 participants 9.0 to 10.99 years of age at baseline visit. The current study excluded participants without a primary address for the baseline timepoint to assess mass and component exposures, leading to a sample size of for the source apportionment analysis. For the exposure and outcome analyses, additional subjects were excluded to minimize the number of hierarchical levels of study design by randomly selecting one participant per family. This resulted in a sample size of participants (Table 1) who had complete information and were included in the WQS regression analyses. For one participant, the source contributions were not available, leading to a final sample of for the multiple regression analyses based on source contributions.
Table 1.
Baseline study sample characteristics of the current sample of 9- to 10-year-old participants from the ABCD study cohort, 2016–2018.
| Variable | Study sample () |
|---|---|
| Age (months) | |
| 119 (7.4) | |
| Sex at birth | |
| Female | 4,086 (47.6%) |
| Male | 4,503 (52.4%) |
| Race/ethnicity | |
| Asian | 208 (2.4%) |
| Black | 1,252 (14.6%) |
| Hispanic | 1,822 (21.2%) |
| Other racea | 891 (10.4%) |
| White | 4,416 (51.4%) |
| Total household income | |
| 2,338 (27.2%) | |
| & | 2,233 (26%) |
| 3,289 (38.3%) | |
| Don’t know or refuse | 729 (8.5%) |
| Urbanicity | |
| Urbanized area | 7,633 (88.9%) |
| Urban cluster | 259 (3%) |
| Rural | 697 (8.1%) |
| Perceived neighborhood safety | |
| 3.87 (0.97) | |
| Physical activity (hours/week) | |
| 3.5 (2.3) | |
| Screen times (hours/day) | |
| 2.94 (2.21) | |
| Study site | |
| CHLA | 274 (3.2%) |
| CUB | 286 (3.3%) |
| FIU | 511 (5.9%) |
| LIBR | 568 (6.6%) |
| MUSC | 301 (3.5%) |
| OHSU | 486 (5.7%) |
| ROC | 275 (3.2%) |
| SRI | 290 (3.4%) |
| UCLA | 358 (4.2%) |
| UCSD | 590 (6.9%) |
| UFL | 368 (4.3%) |
| UMB | 466 (5.4%) |
| UMICH | 578 (6.7%) |
| UMN | 352 (4.1%) |
| UPMC | 347 (4.0%) |
| UTAH | 761 (8.9%) |
| UVM | 457 (5.3%) |
| UWM | 345 (4.0%) |
| VCU | 191 (2.2%) |
| WUSTL | 396 (4.6%) |
| YALE | 389 (4.5%) |
Note: Values shown are either mean (standard deviation) or (% frequency). All characteristics were reported by the child’s caregiver except for urbanicity, which was based on 2010 Census data. CHLA, Children’s Hospital of Los Angeles; CUB, University of Colorado Boulder; FIU, Florida International University; LIBR, Laureate Institute for Brain Research; MUSC, Medical University of South Carolina; OHSU, Oregon Health and Science University; ROC, University of Rochester; SD, standard deviation; SRI, SRI International; UCLA, University of California, Los Angeles; UCSD, UC San Diego; UFL, University of Florida; UMB, University of Maryland Baltimore; UMICH, University of Michigan; UMN, University of Minnesota; UPMC, University of Pittsburgh; UTAH, University of Utah; UVM, University of Vermont; UWM, University of Wisconsin—Milwaukee; VCU, Virginia Commonwealth University; WUSTL, Washington University in St. Louis; YALE, Yale University.
Other race/ethnicity category includes subjects who were parent-identified as American Indian/Native American, Alaska Native, Native Hawaiian, Guamanian, Samoan, other Pacific Islander, Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese, other Asian, or other race (participants that were identified in more than one category or multiracial).
Air Pollution Exposure
Estimates of 2016 annual mean residential air pollution exposure, corresponding to when the children were 9–10 years of age, are included in the ABCD Study’s Linked External Data (LED), which is compiled and linked by the LED workgroup.55 We obtained annual mean air pollution predictions from high resolution, machine learning–based spatiotemporal models. These hybrid models combined satellite-based aerosol optical depth remote sensing retrievals, land-use regression, and chemical transport model outputs to predict, using an ensemble of machine learners, daily fine particulate matter (i.e., ) exposure in at resolution56 and daily 8-h ozone () in ppb at resolution.57 Daily predicted mass concentrations were then averaged into annual values for 2016, the first year of baseline data collection. Annual concentrations of 15 chemical components in across the US were also obtained from similar machine learning–based models with a spatial grid resolution. In PM component models, 166 predictors were used, including but not limited to time and geography information, satellite observation data (i.e., vegetation, water index, nighttime lights, aerosol optical depth, etc.), meteorological data (i.e., temperature, humidity, wind, etc.), and emissions or surrogates of emission sources (distance to power plants, distance to highways, traffic counts, etc.).46,48 These models were cross-validated with EPA monitoring data across the US and had very high performance accuracy [ mass root mean square error ; Supplemental Table S2].46,48,56 These 1-year annual averages of ambient air pollutants were then linked to the geocodes of the child’s primary residential addresses, which were collected in person from each participant’s caregiver at the baseline study visit (October 2016–October 2018).
Cognitive Data from NIH Toolbox
Participants completed a cognitive battery, which included Cognitive Battery of the NIH Toolbox (version 2) as well as additional cognitive tests,58 at the same visit the addresses were collected, when the children were 9–10 years old (i.e., baseline study visit). The cognitive battery entailed the following: Picture Vocabulary Test (theta score: , ), Oral Reading Recognition Test (theta score: , ), Flanker Task (score range: 0–10), List Sorting Working Memory Test (score range: 0–26), Dimensional Change Card Sort (score range: 0–10), Pattern Comparison Processing Speed Test (score range: 0–130), the Picture Sequence Memory Test (theta score: , ), the Little Man task (percent correct: range 0–1), and Rey Auditory Verbal Learning Test (RAVLT) (total correct score range: 0–45). All tests were administered using an iPad with one-on-one monitoring by a research assistant. As previously published, Bayesian probabilistic principal components analysis along with varimax rotation was implemented to obtain the principal components of the ABCD Baseline Cognitive Battery.59 The three derived PCA factor scores, known as general cognitive ability, executive function, and learning & memory, for each participant at 9–10 years of age were included in the analysis.59 The general cognitive ability included strongest loadings of Toolbox Picture Vocabulary and Oral Reading Test followed by the List Sorting Working Memory and Little Man Task performance. Learning & memory included the strongest loadings from the Toolbox Picture Sequence Memory Task and the RAVLT total number correct, as well as the NIH Toolbox List Sort Working Memory Task.59 Executive function included strongest loadings from the Toolbox Flanker Task, the Toolbox Dimensional Change Card Sort Task, and the Toolbox Pattern Comparison Processing Speed Task.59
Confounders and Covariates
We used a directed acyclic graph (DAG) to define, a priori, potential confounding variables (i.e., those variables known to both predict the outcomes of interest and also likely to influence where people live, and thus their exposure to ambient air pollutants estimated at the residence).60 This approach helped us to identify a minimally sufficient adjustment set of variables for our primary analyses (Figure S1). We considered both race/ethnicity and socioeconomic factors as potential confounders, given that certain types of pollution are higher in minority communities and for those from disadvantaged social status backgrounds61,62 due to structural racism and class bias increasing the likely proximity of these communities to major sources of pollution in the US.63,64 Questionnaire data on caregiver’s sociodemographic were self-reported at the baseline visit using REDCap (Research Electronic Data Capture), which is a secure, web-based software platform.65,66 These sociodemographic variables included age of child at the time of visit (in months), sex at birth, caregiver reported race/ethnicity of the child (non-Hispanic black, non-Hispanic white, Hispanic, Asian, other race), total household income in US dollars (, , , , or Do not Know/Refuse), average days per week of physical activity,67 and average hours of screen time use per day.68,69 Beyond sociodemographic and/or lifestyle factors, we also considered several available additional environmental exposures as potential confounding variables. These included perceived neighborhood safety (average score across three items including: “I feel safe walking in my neighborhood, day or night,” “Violence is not a problem in my neighborhood,” “My neighborhood is safe from crime”) as assessed by caregiver, derived from three questions of the ABCD Parent Neighborhood Quality/Crime Survey Modified from PhenX (NSC),70,71 as well as geospatially derived characterization of urbanicity (based on 2010 Census definitions of urban, urbanized area, and rural areas) within the primary residential census tract at baseline.55 Lastly, given the ABCD study design, we also adjusted all analyses for study site ().
Statistical Analysis
source apportionment analysis.
To identify major sources of exposure, we utilized the 15 components and conducted a positive matrix factorization (PMF) analysis using the Environmental Protection Agency (EPA) PMF software tool version 5.0.72 PMF is a multivariate factor analysis method, which aims to identify the optimal number of sources and apportion or quantify their contributions to (in this case).73 When PMF is applied to the concentration matrix of components, factor contributions and factor profiles can be obtained. Factor contributions quantify the contribution of the source factor to exposure and factor profiles describe the loading or chemical fingerprint that serves to identify them, which is the relative amount of each component in these derived source factors (hereafter called sources for simplicity) for a given number of specified sources. Some advantages of PMF over other source apportionment methods are a) its approach with inherent, physically realistic nonnegative constraints on decomposition of component concentrations into factors and contributions74 and b) its ability to down-weight individual data points by their uncertainties, minimizing the influence of noisy samples or species on the overall model solution. The EPA version 5.0 PMF model also includes advanced rotational functions,75 which allow rotational ambiguity to be minimized to derive more realistic and optimally separated sources. A detailed description of the EPA version 5.0 PMF model can be found elsewhere72,73,76; however, we outline the details of these equations as applied to the current study here.
As mentioned, when PMF is applied to the concentration matrix of components, it decomposes it into two matrices: factor contributions (G) quantifying the contribution of the source factor to exposure and factor profiles (F) describing the loading or relative amount of each component in these derived sources for a given number of sources. The F matrix serves to identify sources by their characteristic species or “chemical fingerprints,” while the G matrix serves to quantify their contributions (amounts) to the sample. Specifically,
| (1) |
where represents the component concentrations, represents the contributions of sources, represents the factor profiles, and represents the residuals. The subscript i corresponds to participants (samples), j to the components, and to the optimal number of factors selected by the analyst. G and F are derived by finding the best fit through a least squares optimization algorithm that iteratively minimizes the objective function Q, defined as the sum of the squared residuals weighted by their respective uncertainties:
| (2) |
where represents the sample specific uncertainty and with the nonnegativity constraint that G and F are positive (or not significantly negative) matrices. To estimate the sample specific uncertainties for this model, the EPA PMF 5.0 model guidelines72 provide an equation for calculating uncertainties that rely on a laboratory-provided method detection limit (MDL) values for each species. Since this analysis is using predicted component concentrations from ensemble learning–based spatiotemporal models, the model performance metric of root mean square error (RMSE) was utilized instead of MDL to correspond to the average prediction error (in concentration units) for each species. Therefore, sample- and species-specific uncertainties were calculated as follows, using 0.1 as the error fraction (EF):
| (3) |
Then, this matrix of uncertainties () corresponding to the component concentration matrix () was supplied as input to PMF and utilized in Equation 2 when minimizing Q. Since the component spatiotemporal models calculated RMSEs for urban and nonurban regions separately, these were used in Equation 3 based on the site’s urbanicity classification. The urban and urban cluster levels of the categorical variable urbanicity were grouped together and assigned the RMSE values for urban, while the rural level was assigned the RMSE value for nonurban, which were then used in Equation 3 to obtain the uncertainties. In addition, species were designated as “Strong” (S/N ) or “Weak” (S/N ) in the analysis based on their PMF calculated signal-to-noise ratio (S/N), which results in down-weighting species set as “Weak” by a factor of 2.
Evaluation of our results was performed using uncertainty evaluation and comparing Fpeak rotation results.77 We conducted an uncertainty evaluation to examine variability and error estimation using the displacement (DISP) analysis method, which helps to examine the selected solution intricately, including its sensitivity to small changes. We also examined Fpeak rotations, which allows for transforming a given solution space by rotating it and evaluating how the rotated results fill the solution space. Positive Fpeak values tend to sharpen the F matrix, and negative values tend to sharpen the G matrix. The optimal Fpeak value for solution rotation was chosen based on the comparison of the rotational runs and interpretability of profiles. G space plots were also evaluated for rotational ambiguity and correlations between factor contributions. We also evaluated the range of the objective function Q values to confirm that the selected solution was a global minimum rather than a local minimum. Q is a critical parameter for PMF, and there exists two versions of Q, namely, and for each model run. As per the PMF EPA guidelines72: is the goodness-of-fit parameter calculated including all points, whereas is the goodness-of-fit parameter calculated excluding points not fit by the model, defined as samples for which the uncertainty-scaled residual is . The convergent solution with the lowest value was selected per EPA PMF guidelines. Further, to examine the impact of outliers, the values were compared to values.
Only participants with complete components and their respective calculated uncertainties were included in the analysis (). To identify the optimal number of factors to retain, multiple model runs were performed with factors ranging from 4 to 8. Model solutions were evaluated for best separation of sources into physically interpretable, realistic factors. The sources were identified based on their loading profiles (F matrix) in relation to well-established literature and expert knowledge of their characteristic tracers. For the final chosen number of sources, 100 base runs were completed, and the convergent solution was selected per EPA PMF guidelines.72
Exposure and Outcome Analyses
To examine how mixtures of components and their sources influence cognitive functioning in children 9–10 years of age, we conducted three sets of analyses. First, we used a series of weighted quantile sum (WQS) regressions to assess the associations between the mixture of the 15 components and cognition. Second, using the identified six sources from PMF to guide the groupings, we implemented a grouped WQS regression to study how these chemical components groups (representing the six sources) simultaneously are linked with cognitive performance. Lastly, as a complementary approach to the grouped WQS, we also conducted multiple linear regressions examining how each of the six sources’ PMF-derived contributions separately relate to cognition.
WQS regression with 15 components and cognition.
WQS regression analysis fits a generalized regression model49–51 that estimates the overall mixture effect of all component exposures on the outcome(s) of interest (i.e., general ability, executive function and learning & memory) by constructing a weighted index (i.e., which includes all of the PM components together), wherein the weights determine the relevant contribution of each component to the relationship between this constructed index and the outcome.
The generalized WQS regression expression takes the following form:
| (4) |
where i iterates through the 15 components, is the weight for the ith component concentration, and is the quantile score for that component concentration for that observation and z, indicating the covariates included in the model. We performed this analysis in two stages using a training (40%) and validation (60%) sample per common practice using the ‘gwqs’ R package.78 First, WQS uses the training data to fit a generalized regression model with the quantile component exposures along with the covariates to adjust for in the model. Empirical weights are estimated from 1,000 bootstrapped samples via a maximum likelihood optimization function, which constrains the weights to sum to 1. By averaging across the weights from each bootstrap, we then obtain the WQS index which is a composite index inclusive of all the 15 component exposures, represented as . Second, the constructed WQS index from the training step is used to estimate the overall mixture effect on the outcome by obtaining the regression coefficient for the index and testing for significance of the WQS index using the remaining validation dataset. WQS has an advantage over other mixture analysis methods in that it can minimize the impact of collinearity between the components or highly correlated components while identifying cumulative associations.49,51 Additionally, WQS categorizes the mixture components into quantile scores such that extreme values of component exposures do not overpower the weight estimation.
For each of our three cognitive outcomes of interest (i.e., general cognitive ability, executive function, and learning & memory), a separate WQS regression model was fit. To prevent imbalance between the 21 different study sites, stratified randomization by site was performed to generate the training and validation samples. We then scored these values into deciles (to capture most of the components’ nonnormal distribution variability) to make sure that the extreme values have less impact on the weight estimation. One thousand bootstrapped samples were included as an ensemble step to perform weight estimations of the exposure components in the mixture. We expected only the negative or null associations between components and cognition. However, due to the presence of convoluted correlation structures between air pollutants and components, we also allowed for the model to predict positive associations for completeness. All results presented were adjusted for the previously mentioned minimally sufficient set of confounders (e.g., site, sex, age, race/ethnicity, overall household income, perceived neighborhood safety, urban area, physical activity, and daily screen average hours).
Source-relevant grouped WQS and cognition.
Next, to determine if the associations seen were due to the particular mix of components (which can have more than one source, but generally tend to co-occur or correlate within a source) vs. the actual contribution of the source itself, we performed grouped weighted quantile sum (GWQS) to investigate mixture effects of components within groups based on the PMF results, and without overlap, on the cognitive outcomes of interest. Specifically, GWQS79 was implemented by grouping the 15 components into six groups that correspond to the six sources from PMF with the constraint of no overlapping components as follows: ammonium nitrates (), industrial (Pb, Ni, Zn), traffic (EC, Cu, Fe), coal-burning power plants (, V), biomass burning (OC, K, Br), and crustal (Ca, Si). Within these groups, the components are scored into deciles that can be plausibly combined into an index and are assigned a weight. The index weights in each source group are empirically estimated and constrained to be between 0 and 1 and sum to 1, which helps reduce potential issues with collinearity and can reduce dimensionality through zero or near-zero weights.79
Grouped WQS uses a mixture data with C components (15 component exposures) split between ,…, J groups (six source groups) with components in the jth group. The components are scored into quantiles (e.g., quartiles 0,1,2,3) within each J group, which can be combined into an index and are assigned a weight.
For continuous outcome, the general GWQS regression model is as follows:
where g() is any monotonic and differentiable link that relates to the mean and the predictor variables as in generalized linear models, represents the weight for the ith chemical component , and the summation represents a weighted index for the set of chemicals of interest within group j. The vector z is a vector of covariates for which to adjust.
Unlike the WQS, which treats all the mixture elements belonging to one single index as having the same direction of association with the outcome,51 the GWQS allows for grouping the mixture elements within the model such that different magnitudes and directions of associations are possible for each group.79 In addition to estimating exposure effects on outcomes for multiple component groups that reflect the PMF-derived sources, this method also identifies important components within each group contributing to the effect. These analyses were also adjusted for the previously mentioned minimally sufficient set of confounders (e.g., site, sex, age, race ethnicity, overall household income, perceived neighborhood safety, urban area, physical activity, and daily screen average hours).
sources and cognition associations.
The previously mentioned grouped WQS analysis explored how the mixture of components that tend to correlate with each other highly because they can be emitted or formed from a common source or common process are associated with children’s cognition. However, to complement this approach and understand whether it is the source contribution itself driving these associations, we also examined the potential unique (or independent) association between each PMF source and children’s cognition. Thus, we implemented multiple linear regression models using each source’s contributions as the exposure of interest separately, while adjusting for all identified confounding and precision covariates, as well as the fixed effect of site. To address the issue of increased type I due to multiple comparisons for each outcome, we applied the Bonferroni correction (; ).
Results
The final sample characteristics for the current study are described in Table 1. A comparison between the current analytic sample and the larger ABCD study cohort can be found in Table S1.
Table 2 summarizes the 1-year annual 15 component concentrations across the ABCD Study participants at 9–10 years of age. The Spearman correlations between components ranged from to 0.84 (Figure S2). Higher correlations were seen among several component groupings, including and ; trace elements Ni, Zn, and Pb; OC, EC, Cu, and Fe; and also Si and Ca (Figure S2). OC had the highest mean annual concentration of 1.89 followed by , , and EC with mean annual concentrations of , , and , respectively. Notable differences were seen in the concentrations of each component across the 21 sites (Figure S3). Participants from the two study sites within Los Angeles, CA [Children's Hospital Los Angeles (CHLA) and University of California Los Angeles (UCLA)] had the highest concentrations of Br, Fe, and OC, whereas the highest levels of Zn, Ni, and Pb were seen among participants from the study site located in Pittsburgh, PA [University of Pittsburgh (UPMC)]. Ca concentrations were higher among participants seen at the Utah [University of Utah (UTAH)] and Oklahoma [Laureate Institute for Brain Research (LIBR)] study sites. Cu, EC, and concentrations were also found to be higher among participants from the study sites within Los Angeles, CA and Pittsburgh, PA (CHLA/UCLA/UPMC). concentrations were larger among study sites in the West and Midwest regions. V concentrations were highest for most of the participants from the southeastern study site locations. concentrations were largest for participants from the Los Angeles, CA and Pittsburgh, PA study sites as well as sites within the Midwest and southeastern US. K concentrations were highest for participants from the two Florida study sites [Florida International University (FIU) and University of Florida (UFL)] as well as many of the study sites from the western regions. Si concentrations were highest for participants from the Colorado [University of Colorado Boulder (CUB)] and Utah (UTAH) sites.
Table 2.
Annual average component estimates at the child’s residence for 9- to 10-year-old participants from the ABCD Study cohort (), 2016–2018.
| components | Mean | SD | IQR |
|---|---|---|---|
| Carbons and ions () | |||
| OC | 1.90 | 0.46 | 0.57 |
| EC | 0.53 | 0.16 | 0.19 |
| 0.92 | 0.35 | 0.56 | |
| 0.92 | 0.28 | 0.42 | |
| 0.30 | 0.12 | 0.19 | |
| Trace elements () | |||
| Br | 2.58 | 0.59 | 0.57 |
| Ca | 47.93 | 21.08 | 31.82 |
| Cu | 4.65 | 1.67 | 2.28 |
| Fe | 65.83 | 24.51 | 30.79 |
| K | 63.08 | 9.77 | 13.34 |
| Ni | 0.84 | 0.26 | 0.26 |
| Pb | 4.54 | 1.24 | 1.36 |
| Si | 82.86 | 37.32 | 61.22 |
| V | 0.37 | 0.2 | 0.21 |
| Zn | 9.34 | 4.1 | 4.13 |
Note: Br, bromine; Ca, calcium; Cu, copper; EC, elemental carbon; Fe, iron; IQR, interquartile range; K, potassium; , ammonium; Ni, nickel; , nitrate; OC, organic carbon; Pb, lead; , fine particulate matter with aerodynamic diameter ; SD, standard deviation; Si, silicon; , sulfate; V, vanadium; Zn, zinc.
The range for the general cognitive ability score was to 3.04 (, , and ), the range for the learning & memory score was to 2.22 (, , and ), and the range for the executive function score was to 2.61 (, , and ).
Chemical Profiles of Sources across the Nationwide ABCD Study
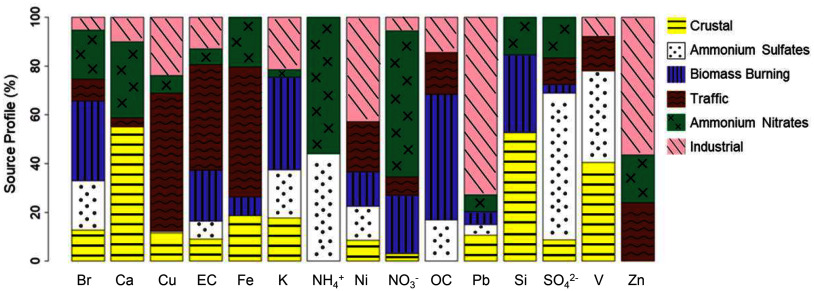
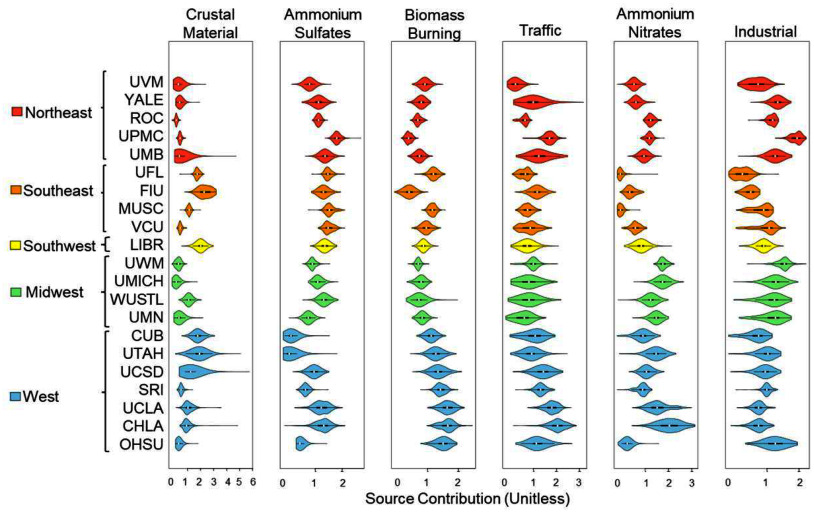
PMF analysis decomposed the 15 components into six factors, which were conceptualized into potential sources based on the components that largely loaded onto those factors and based on the geographic trends in their contributions. Specifically, the identified six major sources of based on the PMF analysis were crustal, ammonium sulfates, biomass burning, traffic, ammonium nitrates, and industrial (Figure 2). Figure 3 provides the estimated source profiles, indicating the relative contribution of each component to each source as well as the factor contribution distribution (quantities) for each of the 21 study sites. The first factor was deemed crustal because of high loadings of Ca and Si, which occur due to resuspended dust particles in the air known to originate from earth’s crust and soils.80 Moreover, the source contributions of crustal were highest for coastal study sites as well as portions of the study sites within the Southwest and western US. The second factor, ammonium sulfates, was characterized by high loadings of and , which are secondary inorganic aerosols typically formed from photochemical reactions occurring with long transport and aging of air masses, from precursors including gas commonly emitted by coal combustion and to a lesser extent diesel and other heavy fuel burning.81–83 Moreover, the ammonium sulfates source contributions were highest for eastern and Midwest study sites, areas known to be impacted by long range transport of emissions from Clean Air Act grandfathered coal power plants in the Midwest. The third factor had high loadings of OC, K, and Br and was labeled biomass burning, which corresponds with combustion sources such as residential wood burning, forest fires, and open burning of agricultural residues.84,85 The source contributions to biomass burning were especially high for West Coast study sites. The fourth factor was consistent with traffic with high loadings of EC and OC and trace elements like Fe and Cu that are known to originate from both exhaust and nonexhaust emissions like tire wear, brake wear,86,87 and tailpipe.39 Traffic source contributions were highest for the two Los Angeles study sites (CHLA, UCLA). The fifth factor was ammonium nitrates, given the high loadings of and , and it corresponds with secondary inorganic aerosol formation from reactions occurring between precursors emitted primarily from agricultural or farming activities (ammonium)88 as well as nonagricultural vehicular or other emissions (i.e., nitrogen oxides, etc.) under cooler temperatures.89 The largest contributions for ammonium nitrates were seen at study sites near farming and agricultural areas across the eastern, Midwest, and portions of the West, with higher contributions seen in the West as compared to the eastern US. Lastly, the sixth factor was deemed industrial/residual fuel oil burning, as it was characterized by high loadings of the trace elements Pb, Zn, and Ni that are emitted from industrial processes (Pb, Zn) and heavy residual fuel oil combustion (Ni).90,91 In addition, the contribution was highest for the Pittsburgh study site, which is a known industrial city.
Figure 2.
Profiles of the six source factors identified based on component exposures at the residences of 9- to 10-year-old participants from the ABCD Study cohort ( = 11,156) from 2016 to 2018. Results from source apportionment analysis using positive matrix factorization. Graph depicts the relative contribution of each component in percentages (values ranging from 0 to 100) to each of the identified six source factors as shown by stacked segments in each bar. Six source factors include the following: crustal (Ca, Si), ammonium sulfates (, ), biomass burning (OC, K, Br), traffic (EC, OC, Fe, Cu), ammonium nitrates (, ), and industrial/residual fuel oil burning (Pb, Zn, Ni). Note: Br, bromine; Ca, calcium; Cu, copper; EC, elemental carbon; Fe, iron; K, potassium; , ammonium; Ni, nickel; , nitrate; OC, organic carbon; Pb, lead; , fine particulate matter with aerodynamic diameter ; Si, silicon; , sulfate; V, vanadium; Zn, zinc. Numeric data for Figure 2 can be found in Excel Tables S2.
Figure 3.
Factor contributions for the six source factors for 9- to 10-year-old participants from each of the 21 ABCD Study sites ( = 11,156) from 2016 to 2018. Plots display the distributions of participant factor contribution scores by study site for each of the six source factors (i.e., crustal, ammonium sulfates, biomass burning, traffic, ammonium nitrates, and industrial/residual fuel oil burning). Study sites are ordered and color coded by geographical region. Note: CHLA, Children’s Hospital of Los Angeles; CUB, University of Colorado Boulder; FIU, Florida International University; LIBR, Laureate Institute for Brain Research; MUSC, Medical University of South Carolina; OHSU, Oregon Health and Science University; ROC, University of Rochester; SRI, SRI International; UCLA, University of California, Los Angeles; UCSD, UC San Diego; UFL, University of Florida; UMB, University of Maryland Baltimore; UMICH, University of Michigan; UMN, University of Minnesota; UPMC, University of Pittsburgh; UTAH, University of Utah; UVM, University of Vermont; UWM, University of Wisconsin—Milwaukee; VCU, Virginia Commonwealth University; WUSTL, Washington University in St. Louis; YALE, Yale University. Numeric data for Figure 3 can be found in Excel Tables S3.
Component Mixture Associations with Cognition
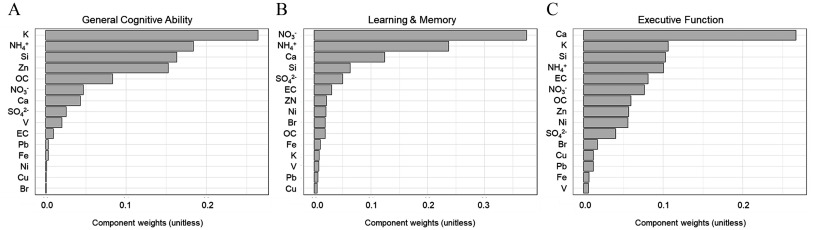
The mixture of 15 components was negatively associated with neurocognitive performance at ages 9–10 years old (Table 3). Weights of each component contributing toward this cumulative negative association with each of the three outcomes are shown in Figure 4. For general cognitive ability, we observed a significant cumulative negative association (indicating the cumulative effect of the mixture of 15 components) with larger weight contributions from K, , Si, and Zn. For learning & memory, , , and Ca were the major contributors to the negative association observed. The mixture effect for executive function showed a cumulative negative association driven by Ca, K, Si, and . There were no significant positive mixture effects seen for any measures of neurocognitive performance (all ).
Table 3.
Weighted quantile sum (WQS) regression results examining the mixture of 15 components on neurocognitive performance in 9- to 10-year-old participants from the ABCD Study cohort (), 2016–2018.
| Outcome | Estimatea | Standard error | -Value |
|---|---|---|---|
| General cognitive ability | 0.003** | ||
| Learning & memory | 0.001** | ||
| Executive function | 0.031* |
Note: PM, particulate matter.
Estimates are per 1-unit increase in the WQS index, reflected as an decile increase in all PM components. WQS regression models of the 15 PM components, adjusting for age, sex, race/ethnicity, overall household income, perceived neighborhood safety, urbanicity, physical activity, and daily screen average hours and site. Significance levels: *indicates < 0.05; **indicates < 0.01.
Figure 4.
Individual component weights from the mixture effects on neurocognitive performance in 9- to 10-year-old participants from the ABCD Study cohort () from 2016 to 2018. Bar charts indicate weights of components contributing to the overall WQS mixture index for the three cognitive outcomes: (A) general cognitive ability, (B) learning & memory, and (C) executive function. Results from WQS regression models of the 15 components, adjusting for age, sex, race/ethnicity, overall household income, perceived neighborhood safety, urbanicity, physical activity, and daily screen average hours and site. Note: Br, bromine; Ca, calcium; Cu, copper; EC, elemental carbon; Fe, iron; K, potassium; , ammonium; Ni, nickel; , nitrate; OC, organic carbon; Pb, lead; Si, silicon; , sulfate; V, vanadium; WQS, weighted quantile sum; Zn, zinc. Numeric data for Figure 4 can be found in Excel Tables S4–S6.
Mixture Effects of Component Groups Related to Major Sources on Cognition
Using the PMF identified six sources (i.e., crustal, ammonium sulfates, biomass burning, traffic, ammonium nitrates, and industrial) and its loadings, we grouped the components here without overlap to investigate mixture effects of components within each source group on the cognitive outcomes of interest. The results derived from grouped WQS regression are shown in Table 4. The corresponding weight contributions of each component toward each significant grouped WQS index is reported in Figure S4. We found a significant negative association between general ability and the mixtures of components related to both crustal and industrial sources, which were mainly driven by Si and Zn, respectively (Figure S4A). Surprisingly, we also found a positive association between the mixture of traffic-related components and general ability with Cu loading the highest. A significant negative association was also found between components related to ammonium nitrates with learning & memory, and these were driven by (Figure S4B). In terms of executive function, a negative association was observed between traffic-related groups of components and executive functioning, with the largest effects driven by Cu followed by Fe and EC, respectively (Figure S4C). In addition, an unexpected significant positive association was found between the mixture of components related to biomass burning and executive functioning performance, with the strongest effects driven by OC followed by K and Br, respectively (Figure S4C).
Table 4.
Grouped weighted quantile sum (WQS) results showing cumulative associations between groups of components, based on six identified source factors, and neurocognitive performance in 9- to 10-year-old participants from the ABCD study cohort (), 2016–2018.
| Outcome | WQS mixture group membership | Estimatea | Standard error | -Value |
|---|---|---|---|---|
| General cognitive ability | Ca, Si (crustal) | 0.0327* | ||
| , V (portion of ammonium sulfatesb) | 0.8663 | |||
| OC, K, Br (biomass burning) | 0.6471 | |||
| EC, Cu, Fe (traffic) | 0.0013** | |||
| , (ammonium nitrates) | 0.2798 | |||
| Pb, Ni, Zn (industrial) | 0.0318* | |||
| Learning & memory | Ca, Si (crustal) | 0.2075 | ||
| , V (portion of ammonium sulfatesb) | 0.3026 | |||
| OC, K, Br (biomass burning) | 0.3357 | |||
| EC, Cu, Fe (traffic) | 0.6599 | |||
| , (ammonium nitrates) | 0.0025** | |||
| Pb, Ni, Zn (industrial) | 0.5062 | |||
| Executive function | Ca, Si (crustal) | 0.1488 | ||
| , V (portion of ammonium sulfatesb) | 0.9263 | |||
| OC, K, Br (biomass burning) | 0.0108* | |||
| EC, Cu, Fe (traffic) | 0.0397* | |||
| , (ammonium nitrates) | 0.1686 | |||
| Pb, Ni, Zn (industrial) | 0.9639 |
Note: Br, bromine; Ca, calcium; Cu, copper; EC, elemental carbon; Fe, iron; K, potassium; , ammonium; Ni, nickel; , nitrate; OC, organic carbon; Pb, lead; , fine particulate matter with aerodynamic diameter ; PMF, positive matrix factorization; Si, silicon; , sulfate; V, vanadium; Zn, zinc.
Estimates are per 1-unit increase in the grouped WQS index, interpreted as an decile increase in source factor groups. Results from grouped WQS regression models wherein the 15 PM components grouped into six source factors, adjusting for age, sex, race/ethnicity, overall household income, perceived neighborhood safety, urbanicity, physical activity, and daily screen average hours and site.
PMF-identified ammonium sulfates components (, V, and ) were reduced to , V (likely reflecting coal-burning power plants) to reduce overlap of in grouped QWS analysis. Significance levels: *indicates < 0.05; **indicates < 0.01.
Sources and Cognition Associations
To complement quantifying mixture effects of source groupings, linear modeling aimed to further quantify the independent association between each source derived from PMF and children’s cognitive performance. We found a significant negative association between ammonium nitrates and learning & memory performance (Table 5). Exposure to higher crustal and traffic sources were also found related to poorer executive function, whereas higher exposure to ammonium sulfates was positively associated with executive functioning; albeit these did not pass multiple comparison correction.
Table 5.
Associations between each source factor and cognitive outcome in 9- to 10-year-old participants from the ABCD Study cohort (), 2016–2018.
| General cognitive ability | Learning & memory | Executive function | |||||||
|---|---|---|---|---|---|---|---|---|---|
| source factor | Standard error | -Value | Standard error | -Value | Standard error | -Value | |||
| Crustal | 0.3202 | 0.8947 | 0.0570 | ||||||
| Ammonium sulfates | 0.8932 | 0.1459 | 0.0237 | ||||||
| Biomass burning | 0.8599 | 0.6586 | 0.5937 | ||||||
| Traffic | 0.6272 | 0.2193 | 0.0458 | ||||||
| Ammonium nitrates | 0.1324 | 0.0002* | 0.9570 | ||||||
| Industrial | 0.8381 | 0.5484 | 0.3575 | ||||||
Note: Linear regression models, adjusted for age, sex, race/ethnicity, overall household income, neighborhood safety, urbanicity, physical activity, daily screentime average hours, and site. Estimates include unstandardized beta coefficients (b), standard errors, and -values. Asterisk sign(*) reflected models passing Bonferroni correction (). , fine particulate matter with aerodynamic diameter .
Sensitivity Analyses
Two of the six sources that we identified in the PMF analysis are considered secondary, meaning they are formed by chemical reactions between primary emissions (precursors) under specific weather conditions and with long range transport and aging of the air pollution plume. Since these same conditions can lead to ozone gas formation through photochemical reactions, we conducted a sensitivity analysis for our source factor related models, where we additionally adjusted for annual average ozone to test whether our findings were sensitive to potential confounding by ozone exposure.92 Our results remained the same (Tables S3 and S4). Lastly, our analytic approach used an a priori DAG to determine a minimally sufficient set of variables to minimize potential confounding, which included the socioeconomic variable of total household income. Nonetheless, to reduce concern of additional residual confounding of potential socioeconomic impacts on our results, we also conducted an additional set of sensitivity analyses that included our minimally sufficient set as well as further adjusting for highest parental education. The sample size for this sensitivity analysis was given parental education information was missing for nine participants (including the one subject in which source contributions were not available). Our results were nearly identical in direction and magnitude (Tables S5–S7), with the exception of the WQS effects of the 15 components (WQS index of as compared to ) as well as grouped WQS effects of industrial-based components (i.e., Pb, Ni, Zn) (WQS index of as compared to ) on general cognitive ability, which were reduced and no longer deemed significant.
Discussion
The current study incorporated mixture modeling and source apportionment to examine how annual exposure to 15 components and their major contributing sources is related to individual differences in cognitive performance in more than 8,000 children ages 9–10 years old from a nationwide cohort across the US. Mixture modeling revealed cumulative negative associations between chemical components of and children’s cognitive performance across all three cognitive domains, with the greatest contributions from ammonium, nitrates, potassium, silicon, and calcium (Figure 5). We then performed data-driven source apportionment on these 15 chemical components into source factors via positive matrix factorization, revealing six sources: crustal, ammonium sulfates, biomass burning, traffic, ammonium nitrates, and industrial sources. Next, we used two complementary approaches, including grouped mixture modeling (grouped components by source) and linear mixed effect modeling directly with the PM sources, to further understand how the six identified sources contribute to these overall mixture effects. Two main findings emerged that supported our interpretation of the PM sources driving the initial mixture effects: Higher exposure to mixtures of crustal and industrial components were related to poorer general cognitive ability, whereas exposures to ammonium nitrates were associated with worse learning & memory scores (Figure 5). Both traffic and biomass source mixtures were related to general cognitive ability and executive function, albeit these findings were less robust to analytic methods employed (Figure 5). Building upon a decade of research suggesting that exposure is detrimental to cognition,93 this work links childhood exposure to source mixtures to individual differences in cognition at 9–10 years of age.
Figure 5.
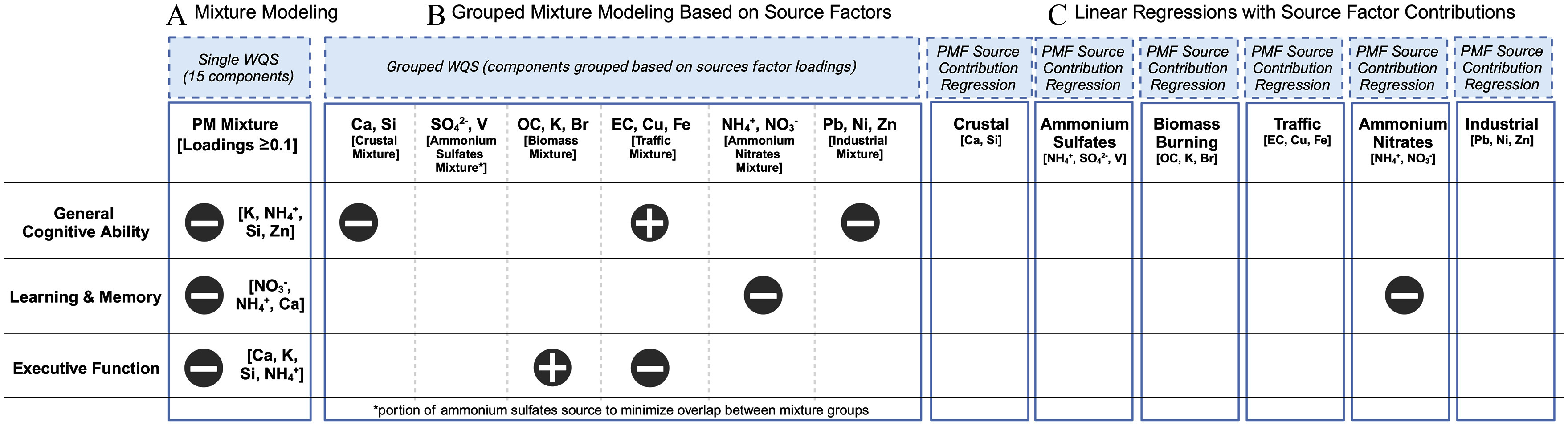
Visual synthesis of results using WQS, grouped WQS, and linear regression to quantify the associations between components, the six identified source factors, and neurocognitive outcomes in 9- to 10-year-old participants from the ABCD Study cohort () from 2016 to 2018. Created with Biorender.com. Numeric data for Figure 5 can be found in Excel Tables S7. Note: Br, bromine; Ca, calcium; Cu, copper; EC, elemental carbon; Fe, iron; K, potassium; , ammonium; Ni, nickel; , nitrate; OC, organic carbon; Pb, lead; PM, particulate matter; PMF, positive matrix factorization; Si, silicon; , sulfate; V, vanadium; WQS, weighted quantile sum; Zn, zinc. Due to unavailable source contribution data for one participant, regression models reflect .
Much of the existing literature concerning neurocognitive impacts of and other pollutants in children has focused on prenatal exposures.93–95 A few previous studies have assessed differences in cognition related to specific components of . In four European birth cohorts from Holland, Germany, Italy, and Spain, worse fine motor functioning in children 1–9 years of age () was linked to greater prenatal exposure to iron but not to seven other elemental components (i.e., Zn, V, Si, Cu, K, Ni, S).96 Further, attention problems have been linked to childhood copper exposure at 7–11 years of age (), depending on polymorphisms in a copper transporting gene,97 and to both childhood and prenatal elemental or black carbon exposure, which are characteristic of diesel and traffic emissions.24,28,98 However, air pollution effects on neurodevelopment during later childhood and adolescence is emerging as an imperative research area that warrants further study.11,93,99
Among studies of the cognitive impacts of childhood exposure to total mass, there is some inconsistency among findings, which could largely be due to the heterogeneity in composition across these studies.44 For example, several large-scale studies from Europe, the UK, and the US have found no association between total exposure and cognitive functioning during childhood,23,100 even in the current ABCD Study cohort of 9–10 year olds.22 Our current study highlights that the chemical composition of may play a vital role in the neurodevelopmental effects of air pollution, which may help to explain conflicting findings in the extant literature. Using mixture modeling to assess 15 chemical components of , we found that greater exposure to the overall mixture was linked to worse performance across the three cognitive domains (i.e., general cognitive ability, learning & memory, executive function). The negative association between mixture and general cognitive ability was largely driven by exposure to potassium, ammonium, silicon, and zinc, whereas the mixture effects on learning & memory was driven by exposure to nitrate, ammonium, and calcium. Similar components also contributed to the mixture effect seen with executive function, with highest contributions seen from calcium, potassium, silicon, and ammonium. Thus, it seems that these three aspects of cognition are differentially impacted by the chemical composition of , but exposure to ammonium (), silicon (Si), and calcium (Ca) contribute substantially to worse cognitive outcomes that span multiple domains. These findings suggest that mixture effects on cognition at 9–10 years old seem to be differentially impacted by the chemical composition of , building upon the known heterogeneity in health effects that are widely documented in the literature.47,101–103
Given the nature of the cognitive domain scores (i.e., principal components with , ranges: 4.44–6.21; unitless), it is useful to consider the mixture effects in context of the relative age-related differences seen in each model. A 1-month increase in age from 9–10 years old was associated with 0.24, 0.09, and 0.23 increases in general cognitive abilities, learning & memory, and executive functioning performance, accordingly. By using these expected age-related increases in cognition as context and comparing the relevant standardized coefficients from each model (i.e., ), mixture effects translate to approximately a one-third reduction of the estimated monthly age-related improvements in general ability (: ) and executive function (: ), as well as a 1.4-fold decrease in the monthly age-related improvements seen in learning & memory (: ) from ages 9–10 years old in the current study. Although these are relatively small individual-level effects from a 1-year annual exposure, it is feasible they could translate to a large effect on children’s cognition at a population level and/or at an individual-level if such exposure effects are cumulative over time.
Differences in composition are, in part, due to the many sources of outdoor , anthropogenic and otherwise, which may vary geographically across the US. For example, from windblown dust is more prevalent in the dry and dusty American West and Southwest, compared with from heavy industry, which is more prevalent in the American Midwest and Northeast. Thus, in addition to quantifying mixture effects, identifying which common source(s) are responsible for potential adverse childhood cognitive and health effects across the US may provide pertinent to helping guide decisions of both parents and policymakers. It can also inform additional source-specific emissions reduction regulations. In the current study, we used multisite, geographically diverse data to identify shared source categories of components across all participants from the 21 ABCD research sites to capture major source factors that have the most overall importance, and allow direct comparability of exposures, across the entire child population of interest here.104 This revealed six commonly shared major source factors across all 9- to 10-year-old participants within the ABCD Study cohort, including crustal (e.g., resuspended dust, soil; largely Ca and Si), ammonium sulfates (e.g., secondary inorganic aerosols; largely and ), biomass burning (e.g., from wood fires, restaurants, forest fires; largely OC, K, and Br), traffic (e.g., exhaust, tire/brake wear; largely EC, OC, Fe, and Cu), ammonium nitrates (e.g., secondary aerosols related to agricultural fertilizer; largely and ), and an industrial source factor (e.g., heavy fuel oil and combustion; largely with Pb, Zn, and Ni). After investigating the effects of the overall components as one mixture, we then implemented complementary analyses using the six identified source factors. First, we investigated potential unique mixture effects of each set of components related to a particular source on cognition using grouped weighted quantile sum regression (where the groups are guided by the sources). Second, we investigated the direct, specific association of each source with differences in cognition using linear mixed-effects modeling. These approaches detected that greater annual exposures to certain source categories were related to poorer neurocognitive performance at 9–10 years of age. Both approaches found greater exposure to ammonium nitrate, as a source and as its mixture of components, was associated with worse learning & memory performance. To our knowledge, this is the first study to link ammonium nitrates exposure in to differences in learning & memory performance in childhood. However, our findings are in line with prior aging literature linking ammonium and, to a more modest degree, nitrates to increased risk of dementia.105 Excessive ammonium levels have also been noted in brains of those affected by Alzheimer’s disease.106 Moreover, a study comparing different exposure sources found attributable to agriculture (i.e., including ammonium) and wildfires robustly associated with dementia risk in the US.107 In the same study, exposure to traffic and coal combustion were also related to dementia risk, although effects were sensitive to adjustment of co-exposure to other sources.107
Beyond ammonium nitrates, we found worse general cognitive abilities in late childhood were related to higher levels of mixtures of crustal and industrial-related pollutants (i.e., as seen in the WQS of 15 components and grouped WQS of source mixtures). These findings are congruent with previous studies linking silicon to poorer cognitive capabilities, decreased memory recognition, and cognitive decline in aging populations.108,109 Lastly, we found that exposure to traffic-related components, driven primarily by copper, was linked to worse executive function. These findings are similar to previous findings from the Spanish BREATHE cohort, which found greater exposure to traffic sources of at school (measured twice annually) related to reduced attention and working memory performance over time in children.110
While further study is needed to understand mechanisms underlying differential neurocognitive impacts of exposure to components and sources, extant literature can help contextualize these findings by elucidating the pathways and mechanisms by which exposure impacts the brain. Ambient air pollution is believed to directly impact the brain via the olfactory bulb and indirectly impact the brain by instigating peripheral inflammatory responses.111,112 In the brain, exposure can cause neuroinflammation, oxidative stress, and blood-brain-barrier damage, resulting in neuron loss, diminished synaptic function, impaired neurogenesis, metal dyshomeostasis, and neurodegenerative pathologies.3,45 We propose that our findings, linking ammonium nitrate exposure with worse learning and memory performance, may be explained, in part, by impacts of ammonium on the hippocampus and its central role in learning and memory. A recent study of chronic ammonium exposure in mice uncovered olfactory system damage and damage to the hippocampus,105 while another revealed that even short-term exposure to low levels of ammonium can depolarize hippocampal neurons with potential downstream neurotoxic effects.113 Conversely, both our component and source mixture models found that general cognitive ability was negatively associated with exposure to crustal materials and industrial fuel burning, whereas executive function was negatively associated with different components across the two mixture models (i.e., Ca, K, , vs. traffic). Here, general cognitive ability reflects reading, vocabulary, list-sorting, and spatial reasoning, whereas executive function includes inhibitory control, attention, and set shifting,59 all of which would engage regions from large-scaled systems throughout the brain. Such a decentralized neural substrate suggests more diffuse impacts of exposure, such as systemic inflammation and physiological stress.114 Moreover, postnatal inhalation exposure studies in animals show that concentrated ambient ultrafine particles, which include the elements that we identified, increase the levels of metals, such as calcium, in the developing brain, which may ultimately contribute to brain metal dyshomeostasis.115,116
Beyond the expected negative associations, the current study also identified a few unexpected positive associations with source mixtures and the studied outcomes, including exposure to traffic with general cognitive ability as well as biomass burning with executive function. Some sources may be inversely correlated with each other due to chemical reactions in the air (e.g., primary traffic emissions, which act as precursors for ozone, may decrease as secondary particle formation increases under conditions that promote ozone formation), potentially leading to these unexpected positive associations. Thus, we ran additional sensitivity analyses to adjust for ozone co-exposure. Further, we included parental education in these sensitivity analyses to examine potential residual confounding of socioeconomic effects. These analyses identified smaller negative effects between and source-based mixtures on general cognitive ability (i.e., highest loadings of Toolbox Picture Vocabulary and Oral Reading Test performance). This is likely due to parental education having a stronger effect on tests of crystalized intelligence as compared to fluid intelligence and other cognitive domains.117 However, all other results, including the positive associations, remained unchanged. As such, it is likely that atmospheric chemistry contributes to the counterintuitive positive effects identified here, due to anticorrelations between component concentrations as a result of how different sources are emitted or formed in the air.47,103,118 Furthermore, these unexpected positive associations and disparate findings between methods may be due to correlations between the sources themselves and/or the fact that each chemical component likely originates from more than one source. As grouped mixture modeling only included unique chemical components that loaded highly onto each source factor in separate groups, and all source factors were included in a single grouped WQS model, potentially spurious positive effects in these models may arise from anticorrelations between sources. Alternatively, the linear models captured the individual contributions of each source factor, each in its own model (i.e., their independent effects, not adjusted for other sources) and would be unaffected by anticorrelations or overlap in components between source factors. The linear source models also do not fully capture any given component’s impact on the outcome, but rather the total contribution of all the components as they exist within a source as it pertains to the outcome. Thus, linear modeling estimates the total association of each PM source factor with cognition (without adjusting for other sources), while grouped WQS estimates the mixture effects of source groupings of chemical components on cognition, while simultaneously accounting for all other mixture-cognition associations. Regardless of each analytical strength and weakness, both mixture models revealed the potential importance of crustal and industrial-related particles to general cognitive ability, whereas all approaches suggest higher ammonium nitrate levels might be harmful to learning and memory in late childhood.
Strengths and Limitations
This work contributes vital information, from a large and geographically diverse cohort, regarding cognitive impacts of outdoor source-specific exposure. Specifically, we made use of a rare and rich data resource (i.e., nationwide spatiotemporally resolved exposure estimates for 15 chemical components of ) to derive source factors of outdoor air pollution, which provides directly actionable policy evidence on these poorly understood neurocognitive and developmental phenomena. Importantly, our six identified source factors reaffirm prior source apportionment studies, such as those identifying and quantifying sources of in the Atlanta, GA US metropolitan area from 1998 to 2002,33,119 and a more recent US-wide source apportionment study using data from 2001 to 2014.120 This alignment of our identified source factors with the literature is especially important, as it suggests our source-based findings may have greater external validity in capturing shared and prominent source-category impacts on child development in the US. By focusing both on chemical components and source factors of , we provide detailed insight to inform future, mechanistic studies of the neurobiology underlying exposure–cognition associations, as well as actionable insight for policymakers to regulate, where possible, the anthropogenic sources that contribute to . Further, addressing sources of is likely more interpretable and actionable for caregivers investigating how they can best avoid pollution that may impair their children’s cognitive function and development.
Several limitations should also be noted about the current study. Source apportionment in the current study was not based on measured concentrations of outdoor components but rather on annual average predictions assigned to residential addresses using novel hybrid spatiotemporal models. While models may not be able to perfectly reproduce the variability of observed measurements, these are state-of-the-art, sophisticated models that provide complete spatial coverage of the US, especially where measurements lack since speciation monitoring stations are relatively sparse nationwide. Another consideration is our use of geographically diverse multisite data to conduct the source apportionment (i.e., analyzing all sites in the ABCD Study representing most of the US in one factor analysis), which has both strengths and weaknesses.104 This multisite approach intrinsically detects or derives common sources that contributed in more major ways to air pollution levels at all 21 sites and assumes their source profiles are common across sites when there could be variations (within a source). As such, our findings here reflect the cognitive effects of the common and average source factors seen across the ABCD Study population. While this approach will not be able to confidently resolve more minor sources that could be impacting fewer sites, deriving common exposure sources allows us to compare their impacts across the entire study population. Thus, with this approach, we can further investigate the effects of air pollution from specific source categories on children recruited nationwide for the largest long-term study of adolescent development conducted in the US (i.e., the ABCD Study).104 Since we also adjusted for study site as a fixed effect in our analyses as an important design variable, and some of the sources we identified have large, regional spatial gradients across the US (especially the secondary ones, ammonium sulfates and ammonium nitrates), it is feasible that the site adjustment could be capturing or diluting some of these specific sources’ effects.
It is also important to acknowledge that the source names assigned to each factor are not based on certainty. That is, while extensive information exists in the air quality literature to date on the typical spatial patterns and chemical profiles of many emission sources of (e.g., biomass burning), the chemical profiles for the same “source” can also be widely variable across areas and over time.104,121,122 As such, expert knowledge combined with literature review of earlier work were used to label the statistically derived factors from PMF as specific “source factors” of air pollution based on widely agreed upon naming conventions.40 However, a certain degree of subjectivity remains in naming these factors, and it is important to recognize that a “biomass burning” source identified in our study for example may chemically resemble but not be identical to a “biomass burning” source identified in another study. Both will share similar chemical profiles and characteristics and will generally represent this category of sources emitting , but they may be capturing impacts of different types of biomass or different woods burning in each study or area.
Another limitation of the current study is that it did not assess exposure estimates for locations where children may spend their time outside of the home, such as schools. The data explored in this study were cross-sectional (i.e., average annual exposure in 2016 and cognitive performance at 9–10 years of age), limiting this work’s capacity to make causal inferences or to assess developments in exposure–cognition associations over time or investigate more acute effects. Although we aimed to be mindful about both choosing and adjusting for key confounders and used high-resolution daily spatiotemporal models, we cannot rule out the possibility of residual confounding. The selection of 1-year average concentrations examined here were based on residential data availability of participants in the ABCD Study55 and the spatiotemporal resolution of the exposure models.48 However, these initial geospatial linkages of air pollution have laid the groundwork for future possibilities of understanding how air pollution impacts adolescent health in the US. While component exposure data are only currently available for the ABCD consortium as a 1-year estimate at this single wave of data collection, future data releases from the ABCD Study are expected to contain full lifetime histories of air pollution exposure. This will eventually allow us to build upon this foundational work to determine the longitudinal impacts of air pollution on cognitive development. Moreover, given that the ABCD Study is an ongoing, 10-year longitudinal study, we hope to build on the findings reported here and eventually assess temporality and cumulative effects of exposure from childhood into early adulthood.
Conclusions
Using a nationwide cohort with residential estimates of annual exposure to 15 chemical components and six derived source factors of and cognitive assessments from more than 8,500 youths 9–10 years of age, we identified several source factors and overall component mixture effects on cognition. Our findings imply different profiles of exposure–cognition associations that shared overlap in detrimental effects of ammonium, nitrates, silicon, calcium, and zinc. Regardless of analytic approach, the most robust finding was that higher levels of ammonium nitrates were linked to worse learning & memory performance.
Supplementary Material
Acknowledgments
Kirthana Sukamaran: Project Administration, Formal Analysis, Interpretation, Writing - Original Draft and Editing, Visualization. Katherine Botternhorn: Interpretation, Writing - Original Draft and Editing, Visualization. Joel Schwartz: Conceptualization, Methodology, Data Curation, Resources, Writing - Review & Editing. Jim Gauderman: Methodology, Writing - Review & Editing. Carlos Cardenas-Iniguez: Interpretation, Writing - Review & Editing. Rob McConnell: Methodology, Writing - Review & Editing. Daniel A. Hackman: Interpretation, Writing - Review & Editing. Kiros Berhane: Methodology, Writing - Review & Editing. Hedyeh Ahmadi: Statistical Supervision, Writing - Review & Editing. Shermaine Abad: Methodology, Data Curation, Writing - Review & Editing. Rima Habre: Conceptualization, Methodology, Supervision, Interpretation, Writing - Original Draft and Editing. Megan M. Herting: Funding Acquisition, Conceptualization, Methodology, Supervision, Project Administration, Writing - Original Draft and Editing.
Research described in this article was supported by the National Institutes of Health (NIEHS R01ES032295, R01ES031074, P30ES007048) and EPA grants (RD 83587201, RD 83544101). C.C.-I. would like to acknowledge scholars involved in NSP (R25 NS089462), BRAINS (R25 NS094094), and Diversifying CNS (R25 NS117356), as well as R25MH125545 and R25MH120869 for creating a supportive network of ABCD Study users.
A special thank you to all participants and their families for their participation in the ABCD Study.
ABCD Acknowledgements: Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children 9–10 years of age and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from https://doi.org/10.15154/8873-zj65 and https://doi.org/10.15154/1523041. Additional support for this work was made possible from NIEHS R01-ES032295 and R01-ES031074.
Conclusions and opinions are those of the individual authors and do not necessarily reflect the policies or views of EHP Publishing or the National Institute of Environmental Health Sciences.
References
- 1.Fuller R, Landrigan PJ, Balakrishnan K, Bathan G, Bose-O’Reilly S, Brauer M, et al. . 2022. Pollution and health: a progress update. Lancet Planet Health 6(6):e535–e547, PMID: 35594895, 10.1016/S2542-5196(22)00090-0. [DOI] [PubMed] [Google Scholar]
- 2.Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen J-C, et al. . 2012. The outdoor air pollution and brain health workshop. Neurotoxicology 33(5):972–984, PMID: 22981845, 10.1016/j.neuro.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P. 2014. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. BioMed Res Int 2014:e736385, 10.1155/2014/736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genc S, Zadeoglulari Z, Fuss SH, Genc K. 2012. The adverse effects of air pollution on the nervous system. J Toxicol 2012:e782462, 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brumberg HL, Karr CJ, Council on Environmental Health. 2021. Ambient air pollution: health hazards to children. Pediatrics 147(6):e2021051484, PMID: 34001642, 10.1542/peds.2021-051484. [DOI] [PubMed] [Google Scholar]
- 6.Bateson TF, Schwartz J. 2008. Children’s response to air pollutants. J Toxicol Environ Health A 71(3):238–243, PMID: 18097949, 10.1080/15287390701598234. [DOI] [PubMed] [Google Scholar]
- 7.Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, et al. . 2022. Brain charts for the human lifespan. Nature 604(7906):525–533, PMID: 35388223, 10.1038/s41586-022-04554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herting MM, Kim R, Uban KA, Kan E, Binley A, Sowell ER. 2017. Longitudinal changes in pubertal maturation and white matter microstructure. Psychoneuroendocrinology 81:70–79, PMID: 28419914, 10.1016/j.psyneuen.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herting MM, Johnson C, Mills KL, Vijayakumar N, Dennison M, Liu C, et al. . 2018. Development of subcortical volumes across adolescence in males and females: a multisample study of longitudinal changes. Neuroimage 172:194–205, PMID: 29353072, 10.1016/j.neuroimage.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brockmeyer S, D’Angiulli A. 2016. How air pollution alters brain development: the role of neuroinflammation. Transl Neurosci 7(1):24–30, PMID: 28123818, 10.1515/tnsci-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herting MM, Bottenhorn KL, Cotter DL. 2024. Outdoor air pollution and brain development in childhood and adolescence. Trends in Neurosciences 47(8):593–607, 10.1016/j.tins.2024.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crone EA. 2009. Executive functions in adolescence: inferences from brain and behavior. Dev Sci 12(6):825–830, PMID: 19840037, 10.1111/j.1467-7687.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- 13.Galván A. 2021. Adolescent brain development and contextual influences: a decade in review. J Res Adolesc 31(4):843–869, PMID: 34820955, 10.1111/jora.12687. [DOI] [PubMed] [Google Scholar]
- 14.Selemon LD. 2013. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry 3(3):e238, PMID: 23462989, 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alloway TP, Alloway RG. 2010. Investigating the predictive roles of working memory and IQ in academic attainment. J Exp Child Psychol 106(1):20–29, PMID: 20018296, 10.1016/j.jecp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 16.St Clair-Thompson HL, Gathercole SE. 2006. Executive functions and achievements in school: shifting, updating, inhibition, and working memory. Q J Exp Psychol (Hove) 59(4):745–759, PMID: 16707360, 10.1080/17470210500162854. [DOI] [PubMed] [Google Scholar]
- 17.Graziano PA, Reavis RD, Keane SP, Calkins SD. 2007. The role of emotion regulation and children’s early academic success. J Sch Psychol 45(1):3–19, PMID: 21179384, 10.1016/j.jsp.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClelland MM, Cameron CE, Duncan R, Bowles RP, Acock AC, Miao A, et al. . 2014. Predictors of early growth in academic achievement: the head-toes-knees-shoulders task. Front Psychol 5:599, PMID: 25071619, 10.3389/fpsyg.2014.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, et al. . 2011. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci USA 108(7):2693–2698, PMID: 21262822, 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cory-Slechta DA, Sobolewski M, Marvin E, Conrad K, Merrill A, Anderson T, et al. . 2019. The impact of inhaled ambient ultrafine particulate matter on developing brain: potential importance of elemental contaminants. Toxicol Pathol 47(8):976–992, PMID: 31610749, 10.1177/0192623319878400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu Y-HM, Hsu H-HL, Coull BA, Bellinger DC, Kloog I, Schwartz J, et al. . 2016. Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environ Int 87:56–65, PMID: 26641520, 10.1016/j.envint.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cserbik D, Chen J-C, McConnell R, Berhane K, Sowell ER, Schwartz J, et al. . 2020. Fine particulate matter exposure during childhood relates to hemispheric-specific differences in brain structure. Environ Int 143:105933, PMID: 32659528, 10.1016/j.envint.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guxens M, Garcia-Esteban R, Giorgis-Allemand L, Forns J, Badaloni C, Ballester F, et al. . 2014. Air pollution during pregnancy and childhood cognitive and psychomotor development: six European birth cohorts. Epidemiology 25(5):636–647, PMID: 25036432, 10.1097/EDE.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 24.Harris MH, Gold DR, Rifas-Shiman SL, Melly SJ, Zanobetti A, Coull BA, et al. . 2015. Prenatal and childhood traffic-related pollution exposure and childhood cognition in the Project Viva cohort (Massachusetts, USA). Environ Health Perspect 123(10):1072–1078, PMID: 25839914, 10.1289/ehp.1408803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris MH, Gold DR, Rifas-Shiman SL, Melly SJ, Zanobetti A, Coull BA, et al. . 2016. Prenatal and childhood traffic-related air pollution exposure and childhood executive function and behavior. Neurotoxicol Teratol 57:60–70, PMID: 27350569, 10.1016/j.ntt.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lertxundi A, Andiarena A, Martínez MD, Ayerdi M, Murcia M, Estarlich M, et al. . 2019. Prenatal exposure to PM2.5 and NO2 and sex-dependent infant cognitive and motor development. Environ Res 174:114–121, PMID: 31055169, 10.1016/j.envres.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Porta D, Narduzzi S, Badaloni C, Bucci S, Cesaroni G, Colelli V, et al. . 2016. Air pollution and cognitive development at age 7 in a prospective Italian birth cohort. Epidemiology 27(2):228–236., PMID: 26426942, 10.1097/EDE.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 28.Saenen ND, Provost EB, Viaene MK, Vanpoucke C, Lefebvre W, Vrijens K, et al. . 2016. Recent versus chronic exposure to particulate matter air pollution in association with neurobehavioral performance in a panel study of primary schoolchildren. Environ Int 95:112–119, PMID: 27575366, 10.1016/j.envint.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Clifford A, Lang L, Chen R, Anstey KJ, Seaton A. 2016. Exposure to air pollution and cognitive functioning across the life course – a systematic literature review. Environ Res 147:383–398, PMID: 26945620, 10.1016/j.envres.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Kusters MSW, Essers E, Muetzel R, Ambrós A, Tiemeier H, Guxens M. 2022. Air pollution exposure during pregnancy and childhood, cognitive function, and emotional and behavioral problems in adolescents. Environ Res 214(pt 2):113891, PMID: 35839913, 10.1016/j.envres.2022.113891. [DOI] [PubMed] [Google Scholar]
- 31.Snider G, Weagle CL, Murdymootoo KK, Ring A, Ritchie Y, Stone E, et al. . 2016. Variation in global chemical composition of PM2.5: emerging results from SPARTAN. Atmos Chem Phys 16(15):9629–9653, 10.5194/acp-16-9629-2016. [DOI] [Google Scholar]
- 32.Holguin F. 2008. Traffic, outdoor air pollution, and asthma. Immunol Allergy Clin North Am 28(3):577–588, PMID: 18572108, 10.1016/j.iac.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Sarnat JA, Marmur A, Klein M, Kim E, Russell AG, Sarnat SE, et al. . 2008. Fine particle sources and cardiorespiratory morbidity: an application of chemical mass balance and factor analytical source-apportionment methods. Environ Health Perspect 116(4):459–466, PMID: 18414627, 10.1289/ehp.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. 2007. Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ Health Perspect 115(7):989–995, PMID: 17637911, 10.1289/ehp.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karagulian F, Belis CA, Dora CFC, Prüss-Ustün AM, Bonjour S, Adair-Rohani H, et al. . 2015. Contributions to cities’ ambient particulate matter (PM): a systematic review of local source contributions at global level. Atmos Environ 120:475–483, 10.1016/j.atmosenv.2015.08.087. [DOI] [Google Scholar]
- 36.Reff A, Bhave PV, Simon H, Pace TG, Pouliot GA, Mobley JD, et al. . 2009. Emissions inventory of PM2.5 trace elements across the United States. Environ Sci Technol 43(15):5790–5796, PMID: 19731678, 10.1021/es802930x. [DOI] [PubMed] [Google Scholar]
- 37.Fussell JC, Franklin M, Green DC, Gustafsson M, Harrison RM, Hicks W, et al. . 2022. A review of road traffic-derived non-exhaust particles: emissions, physicochemical characteristics, health risks, and mitigation measures. Environ Sci Technol 56(11):6813–6835, PMID: 35612468, 10.1021/acs.est.2c01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDuffie EE, Smith SJ, O’Rourke P, Tibrewal K, Venkataraman C, Marais EA, et al. . 2020. A global anthropogenic emission inventory of atmospheric pollutants from sector- and fuel-specific sources (1970–2017): an application of the community emissions data system (CEDS). Earth Syst Sci Data 12(4):3413–3442, 10.5194/essd-12-3413-2020. [DOI] [Google Scholar]
- 39.Habre R, Girguis M, Urman R, Fruin S, Lurmann F, Shafer M, et al. . 2021. Contribution of tailpipe and non-tailpipe traffic sources to quasi-ultrafine, fine and coarse particulate matter in southern California. J Air Waste Manag Assoc 71(2):209–230, PMID: 32990509, 10.1080/10962247.2020.1826366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hopke PK, Dai Q, Li L, Feng Y. 2020. Global review of recent source apportionments for airborne particulate matter. Sci Total Environ 740:140091, PMID: 32559544, 10.1016/j.scitotenv.2020.140091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seinfeld JH, Pandis SN. 2006. Atmospheric Chemistry and Physics: from Air Pollution to Climate Change. Hoboken, NJ: Wiley. [Google Scholar]
- 42.Maciejczyk P, Chen LC, Thurston G. 2021. The role of fossil fuel combustion metals in PM2.5 air pollution health associations. Atmosphere 12(9):1086, 10.3390/atmos12091086. [DOI] [Google Scholar]
- 43.Skyllakou K, Rivera PG, Dinkelacker B, Karnezi E, Kioutsioukis I, Hernandez C, et al. . 2021. Changes in PM2.5 concentrations and their sources in the US from 1990 to 2010. Atmos Chem Phys 21(22):17115–17132, 10.5194/acp-21-17115-2021. [DOI] [Google Scholar]
- 44.Hopke PK, Hidy G. 2022. Changing emissions results in changed PM2.5 composition and health impacts. Atmosphere 13(2):193, 10.3390/atmos13020193. [DOI] [Google Scholar]
- 45.Cory-Slechta DA, Merrill A, Sobolewski M. 2023. Air pollution–related neurotoxicity across the life span. Annu Rev Pharmacol Toxicol 63:143–163, PMID: 36028225, 10.1146/annurev-pharmtox-051921-020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin T, Amini H, Kosheleva A, Danesh Yazdi M, Wei Y, Castro E, et al. . 2022. Associations between long-term exposures to airborne PM2.5 components and mortality in Massachusetts: mixture analysis exploration. Environ Health 21(1):96, PMID: 36221093, 10.1186/s12940-022-00907-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krall JR, Anderson GB, Dominici F, Bell ML, Peng RD. 2013. Short-term exposure to particulate matter constituents and mortality in a national study of U.S. urban communities. Environ Health Perspect 121(10):1148–1153, PMID: 23912641, 10.1289/ehp.1206185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amini H, Danesh-Yazdi M, Di Q, Requia W, Wei Y, Abu-Awad Y, et al. . 2022. Hyperlocal super-learned PM2.5 components across the contiguous US. In Review. 10.21203/rs.3.rs-1745433/v2. [DOI]
- 49.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. 2015. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat 20(1):100–120, PMID: 30505142, 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gennings C, Carrico C, Factor-Litvak P, Krigbaum N, Cirillo PM, Cohn BA. 2013. A cohort study evaluation of maternal PCB exposure related to time to pregnancy in daughters. Environ Health 12(1):66, PMID: 23962309, 10.1186/1476-069X-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czarnota J, Gennings C, Wheeler DC. 2015. Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Inform 14(suppl 2):159–171, 10.4137/CIN.S17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, et al. . 2018. Recruiting the ABCD sample: design considerations and procedures. Dev Cogn Neurosci 32:16–22, PMID: 29703560, 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jernigan TL, Brown SA, Dowling GJ. 2018. The adolescent brain cognitive development study. J Res Adolesc 28(1):154–156, 10.1111/jora.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, et al. . 2018. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev Cogn Neurosci 32:4–7, PMID: 29051027, 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan CC, Marshall A, Smolker H, Gonzalez MR, Tapert SF, Barch DM, et al. . 2021. Adolescent Brain Cognitive Development (ABCD) study linked external data (LED): protocol and practices for geocoding and assignment of environmental data. Dev Cogn Neurosci 52:101030, PMID: 34891080, 10.1016/j.dcn.2021.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Q, Amini H, Shi L, Kloog I, Silvern R, Kelly J, et al. . 2019. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int 130:104909, PMID: 31272018, 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Requia WJ, Di Q, Silvern R, Kelly JT, Koutrakis P, Mickley LJ, et al. . 2020. An ensemble learning approach for estimating high spatiotemporal resolution of ground-level ozone in the contiguous United States. Environ Sci Technol 54(18):11037–11047, PMID: 32808786, 10.1021/acs.est.0c01791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, et al. . 2018. Adolescent neurocognitive development and impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci 32:67–79, PMID: 29525452, 10.1016/j.dcn.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson WK, Barch DM, Bjork JM, Gonzalez R, Nagel BJ, Nixon SJ, et al. . 2019. The structure of cognition in 9 and 10 year-old children and associations with problem behaviors: findings from the ABCD study’s baseline neurocognitive battery. Dev Cogn Neurosci 36:100606, PMID: 30595399, 10.1016/j.dcn.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA. 2002. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 155(2):176–184, PMID: 11790682, 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 61.Kodros JK, Bell ML, Dominici F, L’Orange C, Godri Pollitt KJ, Weichenthal S, et al. . 2022. Unequal airborne exposure to toxic metals associated with race, ethnicity, and segregation in the USA. Nat Commun 13(1):6329, PMID: 36319637, 10.1038/s41467-022-33372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tessum CW, Paolella DA, Chambliss SE, Apte JS, Hill JD, Marshall JD. 2021. PM2.5 polluters disproportionately and systemically affect people of color in the United States. Sci Adv 7(18):eabf4491, PMID: 33910895, 10.1126/sciadv.abf4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hajat A, Hsia C, O’Neill MS. 2015. Socioeconomic disparities and air pollution exposure: a global review. Curr Environ Health Rep 2(4):440–450, PMID: 26381684, 10.1007/s40572-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perry MJ, Arrington S, Freisthler MS, Ibe IN, McCray NL, Neumann LM, et al. . 2021. Pervasive structural racism in environmental epidemiology. Environ Health 20(1):119, PMID: 34784917, 10.1186/s12940-021-00801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381, PMID: 18929686, 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. . 2019. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95:103208, PMID: 31078660, 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, et al. . 2018. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: rationale and description. Dev Cogn Neurosci 32:55–66, PMID: 29113758, 10.1016/j.dcn.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paulich KN, Ross JM, Lessem JM, Hewitt JK. 2021. Screen time and early adolescent mental health, academic, and social outcomes in 9- and 10-year old children: utilizing the Adolescent Brain Cognitive DevelopmentSM (ABCD) study. PLoS One 16(9):e0256591, PMID: 34496002, 10.1371/journal.pone.0256591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paulus MP, Squeglia LM, Bagot K, Jacobus J, Kuplicki R, Breslin FJ, et al. . 2019. Screen media activity and brain structure in youth: evidence for diverse structural correlation networks from the ABCD study. NeuroImage 185:140–153, PMID: 30339913, 10.1016/j.neuroimage.2018.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Echeverria SE, Diez-Roux AV, Link BG. 2004. Reliability of self-reported neighborhood characteristics. J Urban Health 81(4):682–701, PMID: 15466849, 10.1093/jurban/jth151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan T. 2007. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol 165(8):858–867, PMID: 17329713, 10.1093/aje/kwm040. [DOI] [PubMed] [Google Scholar]
- 72.Norris G, Duvall R, Brown S, Bai S. 2014. Positive Matrix Factorization (PMF) 5.0 Fundamentals and User Guide. Washington, DC: EPA (US Environmental Protection Agency). [Google Scholar]
- 73.Paatero P, Tapper U. 1994. Positive matrix factorization: a non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 5(2):111–126, 10.1002/env.3170050203. [DOI] [Google Scholar]
- 74.Hopke PK. 2016. Review of receptor modeling methods for source apportionment. J Air Waste Manag Assoc 66(3):237–259, PMID: 26756961, 10.1080/10962247.2016.1140693. [DOI] [PubMed] [Google Scholar]
- 75.Paatero P, Hopke PK. 2009. Rotational tools for factor analytic models. J Chemometrics 23(2):91–100, 10.1002/cem.1197. [DOI] [Google Scholar]
- 76.Paatero P, Eberly S, Brown SG, Norris GA. 2014. Methods for estimating uncertainty in factor analytic solutions. Atmos Meas Tech 7(3):781–797, 10.5194/amt-7-781-2014. [DOI] [Google Scholar]
- 77.Brown SG, Eberly S, Paatero P, Norris GA. 2015. Methods for estimating uncertainty in PMF solutions: examples with ambient air and water quality data and guidance on reporting PMF results. Sci Total Environ 518–519:626–635, PMID: 25776202, 10.1016/j.scitotenv.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 78.Renzetti S, Curtin P, Just AC, Bello G, Gennings C. 2021. gWQS: Generalized Weighted Quantile Sum Regression. https://cran.r-project.org/web/packages/gWQS/index.html [accessed 8 November 2023].
- 79.Wheeler DC, Rustom S, Carli M, Whitehead TP, Ward MH, Metayer C. 2021. Assessment of grouped weighted quantile sum regression for modeling chemical mixtures and cancer risk. Int J Environ Res Public Health 18(2):504, PMID: 33435473, 10.3390/ijerph18020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Belis CA, Karagulian F, Larsen BR, Hopke PK. 2013. Critical review and meta-analysis of ambient particulate matter source apportionment using receptor models in Europe. Atmos Environ 69:94–108, 10.1016/j.atmosenv.2012.11.009. [DOI] [Google Scholar]
- 81.Manousakas M, Papaefthymiou H, Diapouli E, Migliori A, Karydas AG, Bogdanovic-Radovic I, et al. . 2017. Assessment of PM2.5 sources and their corresponding level of uncertainty in a coastal urban area using EPA PMF 5.0 enhanced diagnostics. Sci Total Environ 574:155–164, PMID: 27631196, 10.1016/j.scitotenv.2016.09.047. [DOI] [PubMed] [Google Scholar]
- 82.Scerri MM, Kandler K, Weinbruch S, Yubero E, Galindo N, Prati P, et al. . 2018. Estimation of the contributions of the sources driving PM2.5 levels in a Central mediterranean coastal town. Chemosphere 211:465–481, PMID: 30081219, 10.1016/j.chemosphere.2018.07.104. [DOI] [PubMed] [Google Scholar]
- 83.Viana M, Kuhlbusch TAJ, Querol X, Alastuey A, Harrison RM, Hopke PK, et al. . 2008. Source apportionment of particulate matter in Europe: a review of methods and results. J Aerosol Sci 39(10):827–849, 10.1016/j.jaerosci.2008.05.007. [DOI] [Google Scholar]
- 84.Ikemori F, Uranishi K, Asakawa D, Nakatsubo R, Makino M, Kido M, et al. . 2021. Source apportionment in PM2.5 in Central Japan using positive matrix factorization focusing on small-scale local biomass burning. Atmos Pollut Res 12(3):162–172, 10.1016/j.apr.2021.01.006. [DOI] [Google Scholar]
- 85.Turpin BJ, Lim HJ. 2001. Species contributions to PM2.5 mass concentrations: revisiting common assumptions for estimating organic mass. Aerosol Sci Technol 35(1):602–610, 10.1080/02786820119445. [DOI] [Google Scholar]
- 86.Demir T, Karakaş D, Yenisoy-Karakaş S. 2022. Source identification of exhaust and non-exhaust traffic emissions through the elemental carbon fractions and positive matrix factorization method. Environ Res 204(pt D):112399, PMID: 34800531, 10.1016/j.envres.2021.112399. [DOI] [PubMed] [Google Scholar]
- 87.Weckwerth G. 2001. Verification of traffic emitted aerosol components in the ambient air of Cologne (Germany). Atmos Environ 35(32):5525–5536, 10.1016/S1352-2310(01)00234-5. [DOI] [Google Scholar]
- 88.McCulloch RB, Stephen Few G, Murray GC, Aneja VP. 1998. Analysis of ammonia, ammonium aerosols and acid gases in the atmosphere at a commercial hog farm in eastern North Carolina, USA. Environ Pollut 102(1):263–268, 10.1016/S0269-7491(98)80042-0. [DOI] [Google Scholar]
- 89.Chen Z-L, Song W, Hu C-C, Liu X-J, Chen G-Y, Walters WW, et al. . 2022. Significant contributions of combustion-related sources to ammonia emissions. Nat Commun 13(1):7710, PMID: 36513669, 10.1038/s41467-022-35381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen H, Yan Y, Hu D, Peng L, Wang C. 2023. High contribution of vehicular exhaust and coal combustion to PM2.5-bound Pb pollution in an industrial city in North China: an insight from isotope. Atmos Environ 294:119503, 10.1016/j.atmosenv.2022.119503. [DOI] [Google Scholar]
- 91.Corbin JC, Mensah AA, Pieber SM, Orasche J, Michalke B, Zanatta M, et al. . 2018. Trace metals in soot and PM2.5 from heavy-fuel-oil combustion in a marine engine. Environ Sci Technol 52(11):6714–6722, PMID: 29688717, 10.1021/acs.est.8b01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cakmak S, Hebbern C, Pinault L, Lavigne E, Vanos J, Crouse DL, et al. . 2018. Associations between long-term PM2.5 and ozone exposure and mortality in the Canadian Census Health and Environment Cohort (CANCHEC), by spatial synoptic classification zone. Environ Int 111:200–211, PMID: 29227849, 10.1016/j.envint.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 93.Lopuszanska U, Samardakiewicz M. 2020. The relationship between air pollution and cognitive functions in children and adolescents: a systematic review. Cogn Behav Neurol 33(3):157–178, PMID: 32889949, 10.1097/WNN.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 94.Castagna A, Mascheroni E, Fustinoni S, Montirosso R. 2022. Air pollution and neurodevelopmental skills in preschool- and school-aged children: a systematic review. Neurosci Biobehav Rev 136:104623, PMID: 35331818, 10.1016/j.neubiorev.2022.104623. [DOI] [PubMed] [Google Scholar]
- 95.Hamis AA, Shaharuddin MAA, Fauzi NAFA, Manaf MRA, Universiti Kebangsaan Malaysia. 2023. Prenatal PM2.5 exposure and its association with neurodevelopmental impairment in children: a narrative review. Int J Public Health Ref 13(2):1743–1755. [Google Scholar]
- 96.Lubczyńska MJ, Sunyer J, Tiemeier H, Porta D, Kasper-Sonnenberg M, Jaddoe VWV, et al. . 2017. Exposure to elemental composition of outdoor PM2.5 at birth and cognitive and psychomotor function in childhood in four European birth cohorts. Environ Int 109:170–180, PMID: 28988795, 10.1016/j.envint.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 97.Alemany S, Vilor-Tejedor N, Bustamante M, Álvarez-Pedrerol M, Rivas I, Forns J, et al. . 2017. Interaction between airborne copper exposure and ATP7B polymorphisms on inattentiveness in scholar children. Int J Hyg Environ Health 220(1):51–56, PMID: 28008856, 10.1016/j.ijheh.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 98.Alvarez-Pedrerol M, Rivas I, López-Vicente M, Suades-González E, Donaire-Gonzalez D, Cirach M, et al. . 2017. Impact of commuting exposure to traffic-related air pollution on cognitive development in children walking to school. Environ Pollut 231(pt 1):837–844, PMID: 28866425, 10.1016/j.envpol.2017.08.075. [DOI] [PubMed] [Google Scholar]
- 99.Herting MM, Younan D, Campbell CE, Chen JC. 2019. Outdoor air pollution and brain structure and function from across childhood to young adulthood: a methodological review of brain MRI studies. Front Public Health 7:332, PMID: 31867298, 10.3389/fpubh.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clark C, Crombie R, Head J, van Kamp I, van Kempen E, Stansfeld SA. 2012. Does traffic-related air pollution explain associations of aircraft and road traffic noise exposure on children’s health and cognition? A secondary analysis of the United Kingdom sample from the RANCH project. Am J Epidemiol 176(4):327–337, PMID: 22842719, 10.1093/aje/kws012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Franklin M, Koutrakis P, Schwartz J. 2008. The role of particle composition on the association between PM2.5 and mortality. Epidemiology 19(5):680–689, PMID: 18714438, 10.1097/EDE.0b013e3181812bb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kioumourtzoglou MA, Austin E, Koutrakis P, Dominici F, Schwartz J, Zanobetti A. 2015. PM2.5 and survival among older adults: effect modification by particulate composition. Epidemiology 26(3):321–327, PMID: 25738903, 10.1097/EDE.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Achilleos S, Kioumourtzoglou MA, Wu CD, Schwartz JD, Koutrakis P, Papatheodorou SI. 2017. Acute effects of fine particulate matter constituents on mortality: a systematic review and meta-regression analysis. Environ Int 109:89–100, PMID: 28988023, 10.1016/j.envint.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thurston GD, Ito K, Lall R. 2011. A source apportionment of U.S. fine particulate matter air pollution. Atmos Environ (1994) 45(24):3924–3936, PMID: 24634604, 10.1016/j.atmosenv.2011.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shi L, Zhu Q, Wang Y, Hao H, Zhang H, Schwartz J, et al. . 2023. Incident dementia and long-term exposure to constituents of fine particle air pollution: a national cohort study in the United States. Proc Natl Acad Sci USA 120(1):e2211282119, PMID: 36574646, 10.1073/pnas.2211282119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Adlimoghaddam A, Sabbir MG, Albensi BC. 2016. Ammonia as a potential neurotoxic factor in Alzheimer’s disease. Front Mol Neurosci 9:57, PMID: 27551259, 10.3389/fnmol.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang B, Weuve J, Langa KM, D’Souza J, Szpiro A, Faul J, et al. . 2023. Comparison of particulate air pollution from different emission sources and incident dementia in the US. JAMA Intern Med 183(10):1080–1089, PMID: 37578757, 10.1001/jamainternmed.2023.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pan R, Zhang Y, Xu Z, Yi W, Zhao F, Song J, et al. . 2022. Exposure to fine particulate matter constituents and cognitive function performance, potential mediation by sleep quality: a multicenter study among Chinese adults aged 40–89 years. Environ Int 170:107566, PMID: 36219911, 10.1016/j.envint.2022.107566. [DOI] [PubMed] [Google Scholar]
- 109.Wang M, Zhou XHA, Curl C, Fitzpatrick A, Vedal S, Kaufman J. 2023. Long-term exposure to ambient air pollution and cognitive function in older US adults. Environ Epidemiol 7(1):e242, PMID: 36777527, 10.1097/EE9.0000000000000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Basagaña X, Esnaola M, Rivas I, Amato F, Alvarez-Pedrerol M, Forns J, et al. . 2016. Neurodevelopmental deceleration by urban fine particles from different emission sources: a longitudinal observational study. Environ Health Perspect 124(10):1630–1636, PMID: 27128166, 10.1289/EHP209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. . 2004. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 16(6–7):437–445, PMID: 15204759, 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 112.Block ML, Calderón-Garcidueñas L. 2009. Air pollution: mechanisms of neuroinflammation & CNS disease. Trends Neurosci 32(9):506–516, PMID: 19716187, 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balakrishnan S, Mironov SL. 2020. Instant activation of TRP channels by NH4 + promotes neuronal bursting and glutamate spikes in CA1 neurons. Curr Res Physiol 3:20–29, PMID: 34746817, 10.1016/j.crphys.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu L, Urch B, Szyszkowicz M, Evans G, Speck M, Van Huang A, et al. . 2018. Metals and oxidative potential in urban particulate matter influence systemic inflammatory and neural biomarkers: a controlled exposure study. Environ Int 121(pt 2):1331–1340, PMID: 30420132, 10.1016/j.envint.2018.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cory-Slechta DA, Sobolewski M, Oberdörster G. 2020. Air pollution-related brain metal dyshomeostasis as a potential risk factor for neurodevelopmental disorders and neurodegenerative diseases. Atmosphere 11(10):1098, 10.3390/atmos11101098. [DOI] [Google Scholar]
- 116.Sobolewski M, Conrad K, Marvin E, Eckard M, Goeke CM, Merrill AK, et al. . 2022. The potential involvement of inhaled iron (Fe) in the neurotoxic effects of ultrafine particulate matter air pollution exposure on brain development in mice. Part Fibre Toxicol 19(1):56, PMID: 35945578, 10.1186/s12989-022-00496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rindermann H, Flores-Mendoza C, Mansur-Alves M. 2010. Reciprocal effects between fluid and crystallized intelligence and their dependence on parents’ socioeconomic status and education. Learning Individual Differences 20(5):544–548, 10.1016/j.lindif.2010.07.002. [DOI] [Google Scholar]
- 118.Habre R, Moshier E, Castro W, Nath A, Grunin A, Rohr A, et al. . 2014. The effects of PM2.5 and its components from indoor and outdoor sources on cough and wheeze symptoms in asthmatic children. J Expo Sci Environ Epidemiol 24(4):380–387, PMID: 24714073, 10.1038/jes.2014.21. [DOI] [PubMed] [Google Scholar]
- 119.Chung Y, Dominici F, Wang Y, Coull BA, Bell ML. 2015. Associations between long-term exposure to chemical constituents of fine particulate matter (PM2.5) and mortality in Medicare enrollees in the Eastern United States. Environ Health Perspect 123(5):467–474, , 10.1289/ehp.1307549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rahman MM, Thurston G. 2022. A hybrid satellite and land use regression model of source-specific PM2.5 and PM2.5 constituents. Environ Int 163:107233, PMID: 35429918, 10.1016/j.envint.2022.107233. [DOI] [PubMed] [Google Scholar]
- 121.Chen Y, Rich DQ, Hopke PK. 2024. Changes in source specific PM2.5 from 2010 to 2019 in New York and New Jersey identified by dispersion normalized PMF. Atmos Res 304:107353, 10.1016/j.atmosres.2024.107353. [DOI] [Google Scholar]
- 122.Stanimirova I, Rich DQ, Russell AG, Hopke PK. 2024. Spatial variability of pollution source contributions during two (2012–2013 and 2018–2019) sampling campaigns at ten sites in Los Angeles basin. Environ Pollut 354:124244, PMID: 38810681, 10.1016/j.envpol.2024.124244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.