Abstract
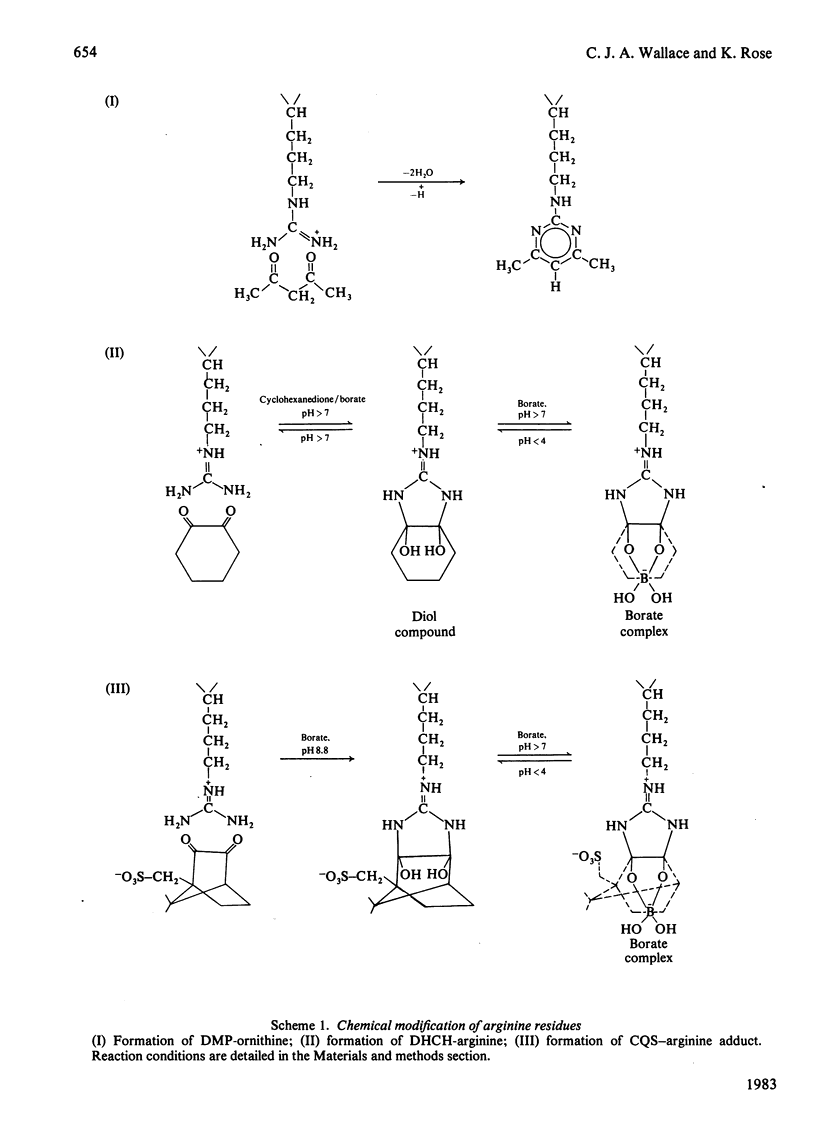
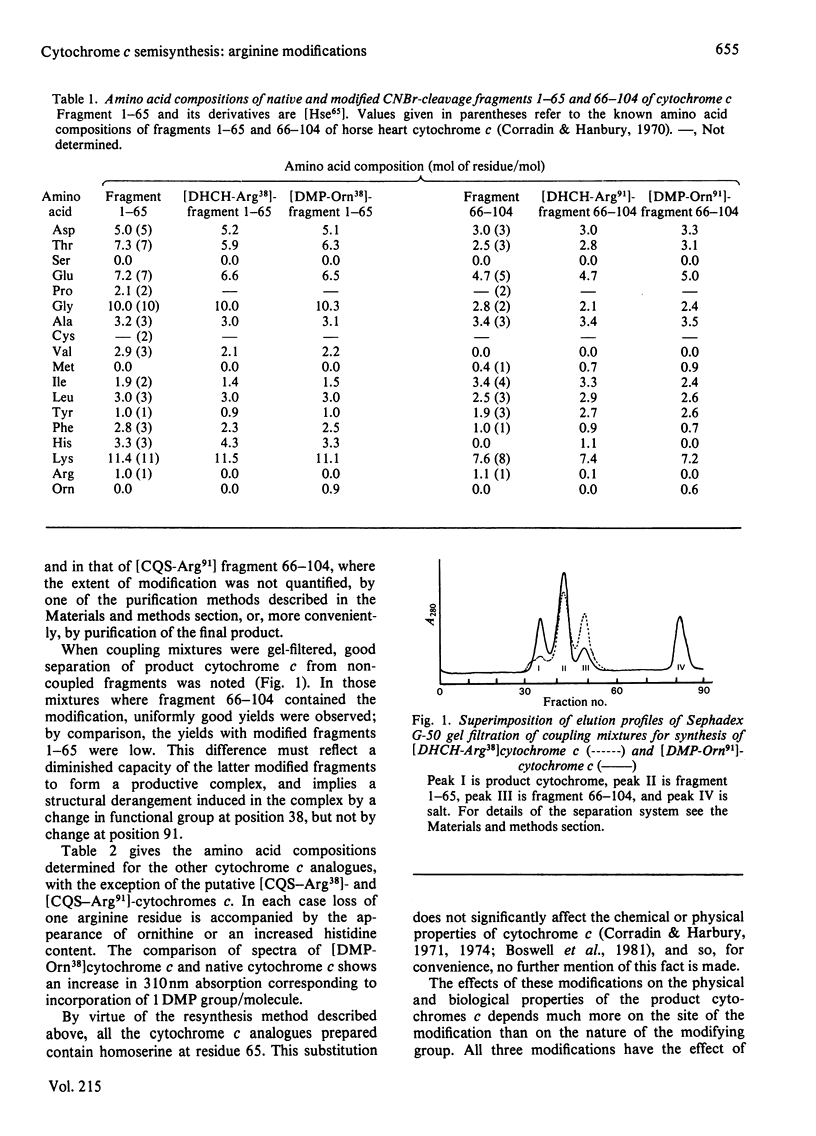
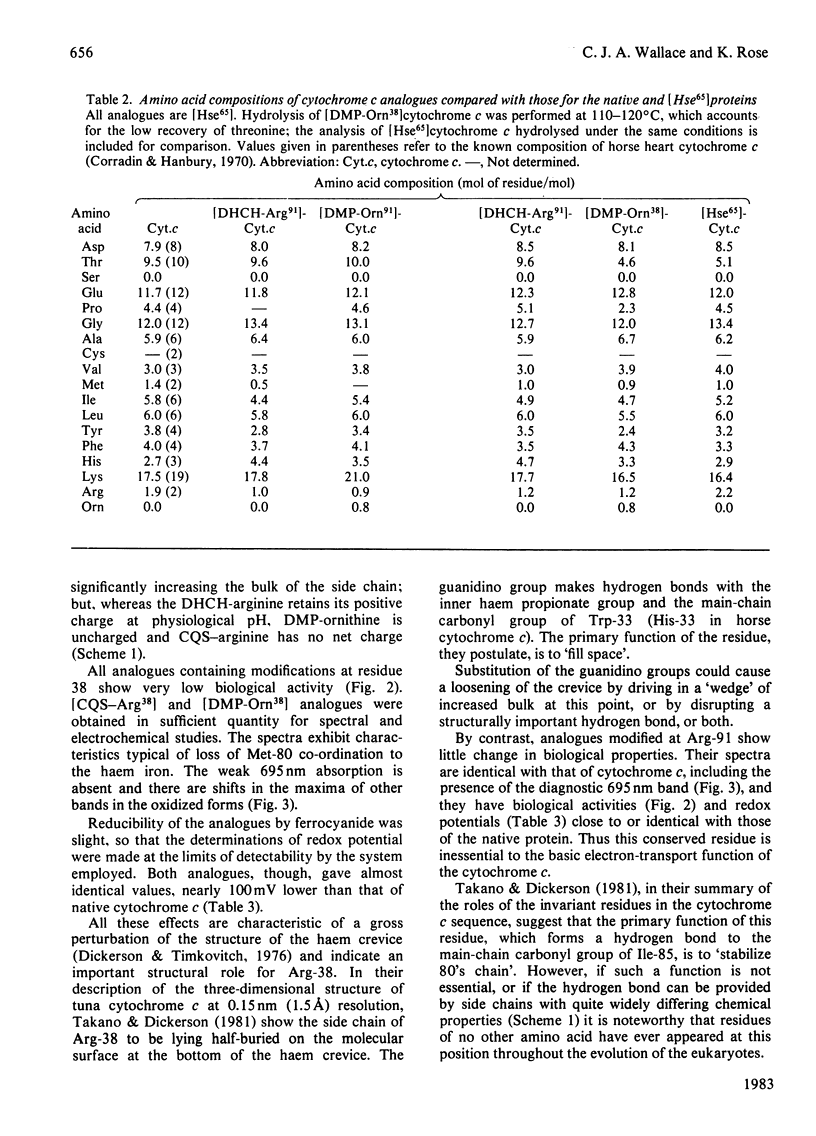
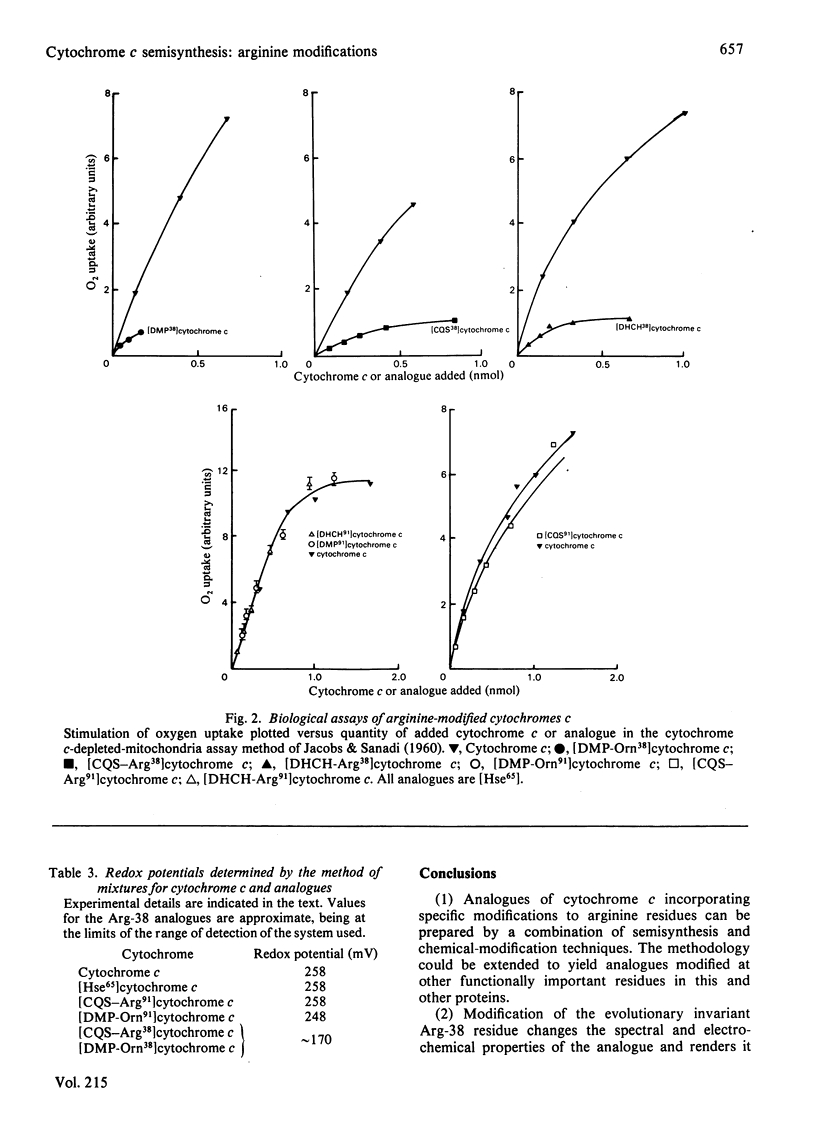
The arginine residues at positions 38 and 91 of horse cytochrome c are absolutely conserved throughout eukaryotic evolution. For studies of the functional roles of these residues, we have prepared, by semisynthetic techniques, analogues of cytochrome c in which one or the other of the arginine residues has been modified. The products of modification by adduct formation with pentane-2,4-dione were purified and extensively characterized. In biological tests, the arginine-91-modified cytochrome c showed little difference in behaviour from native horse cytochrome c. Modification of arginine-38, however, led to extensive changes in biological and chemical properties. We also prepared and tested adducts with cyclohexane-1,2-dione and camphorquinone-10-sulphonic acid. The same effects on biological properties were noted irrespective of the nature of the modifying group. We suggest reasons for the differences in sensitivity of the two sites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boon P. J., Tesser G. I., Nivard R. J. Semisynthetic horse heart [65-homoserine]cytochrome c from three fragments. Proc Natl Acad Sci U S A. 1979 Jan;76(1):61–65. doi: 10.1073/pnas.76.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell A. P., Moore G. R., Williams R. J., Wallace C. J., Boon P. J., Nivard R. J., Tesser G. I. Structural studies of eukaryotic cytochrome c modified at methionine-65. Biochem J. 1981 Feb 1;193(2):493–502. doi: 10.1042/bj1930493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradin G., Harbury H. A. Cleavage of cytochrome c with cyanogen bromide. Biochim Biophys Acta. 1970 Dec 22;221(3):489–496. doi: 10.1016/0005-2795(70)90219-9. [DOI] [PubMed] [Google Scholar]
- Corradin G., Harbury H. A. Reconstitution of horse heart cytochrome c: interaction of the components obtained upon cleavage of the peptide bond following methionine residue 65. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3036–3039. doi: 10.1073/pnas.68.12.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradin G., Harbury H. A. Reconstitution of horse heart cytochrome c: reformation of the peptide bond linking residues 65 and 66. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1400–1406. doi: 10.1016/s0006-291x(74)80439-0. [DOI] [PubMed] [Google Scholar]
- DAVENPORT H. E., HILL R. The preparation and some properties of cytochrome f. Proc R Soc Lond B Biol Sci. 1952 Apr 24;139(896):327–345. doi: 10.1098/rspb.1952.0016. [DOI] [PubMed] [Google Scholar]
- Harris D. E., Offord R. E. A functioning complex between tryptic fragments of cytochrome c. A route to the production of semisynthetic analogues. Biochem J. 1977 Jan 1;161(1):21–25. doi: 10.1042/bj1610021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS E. E., SANADI D. R. The reversible removal of cytochrome c from mitochondria. J Biol Chem. 1960 Feb;235:531–534. [PubMed] [Google Scholar]
- Morris H. R., Dickinson R. J., Williams D. H. Studies towards the complete sequence determination of proteins by mass spectrometry: derivatisation of methionine, cysteine and arginine containing peptides. Biochem Biophys Res Commun. 1973 Mar 5;51(1):247–255. doi: 10.1016/0006-291x(73)90535-4. [DOI] [PubMed] [Google Scholar]
- Pande C. S., Pelzig M., Glass J. D. Camphorquinone-10-sulfonic acid and derivatives: convenient reagents for reversible modification of arginine residues. Proc Natl Acad Sci U S A. 1980 Feb;77(2):895–899. doi: 10.1073/pnas.77.2.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthy L., Smith E. L. Reversible modification of arginine residues. Application to sequence studies by restriction of tryptic hydrolysis to lysine residues. J Biol Chem. 1975 Jan 25;250(2):557–564. [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. I. Ferrocytochrome c structure refined at 1.5 A resolution. J Mol Biol. 1981 Nov 25;153(1):79–94. doi: 10.1016/0022-2836(81)90528-3. [DOI] [PubMed] [Google Scholar]
- Vetter-Diechtl H., Vetter W., Richter W., Biemann K. Ein für Massenspektrometrie und Gaschromatographie geeignetes Argininderivat. Experientia. 1968 Apr 15;24(4):340–341. doi: 10.1007/BF02140808. [DOI] [PubMed] [Google Scholar]
- Wallace C. J., Offord R. E. The semisynthesis of fragments corresponding to residues 66-104 of horse heart cytochrome c. Biochem J. 1979 Apr 1;179(1):169–182. doi: 10.1042/bj1790169. [DOI] [PMC free article] [PubMed] [Google Scholar]


