Abstract
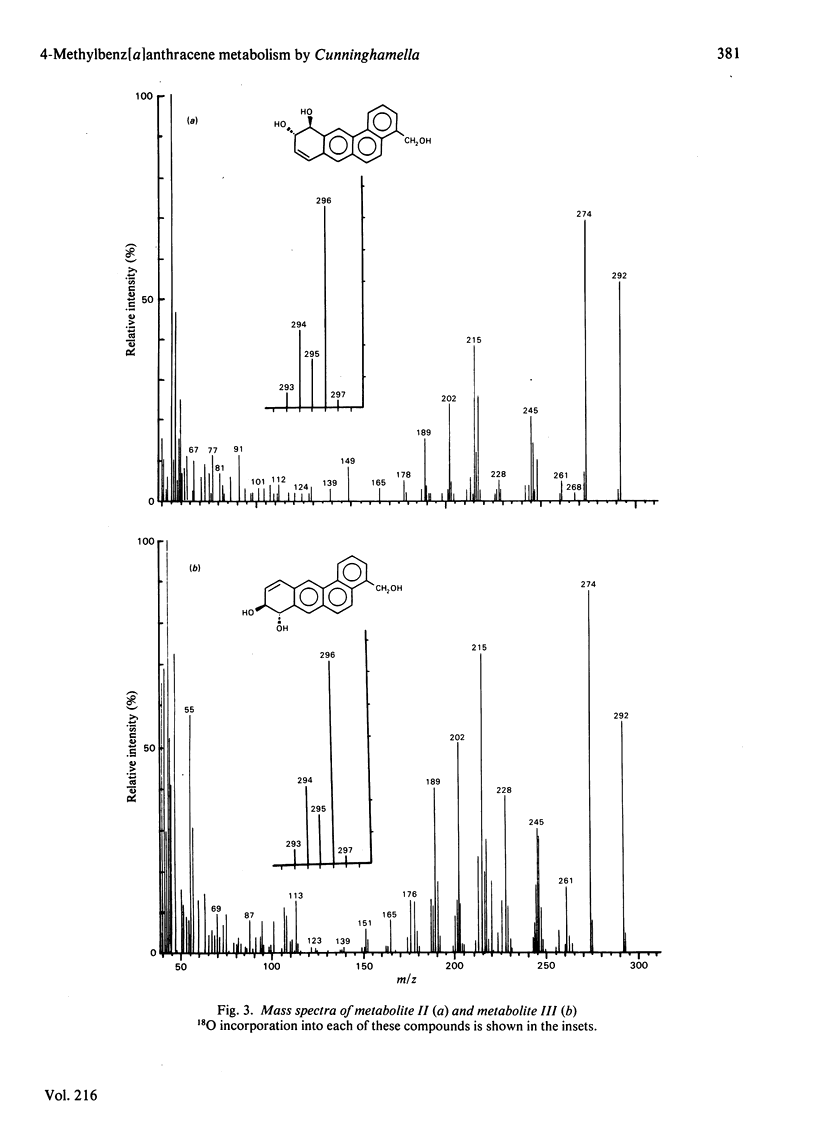
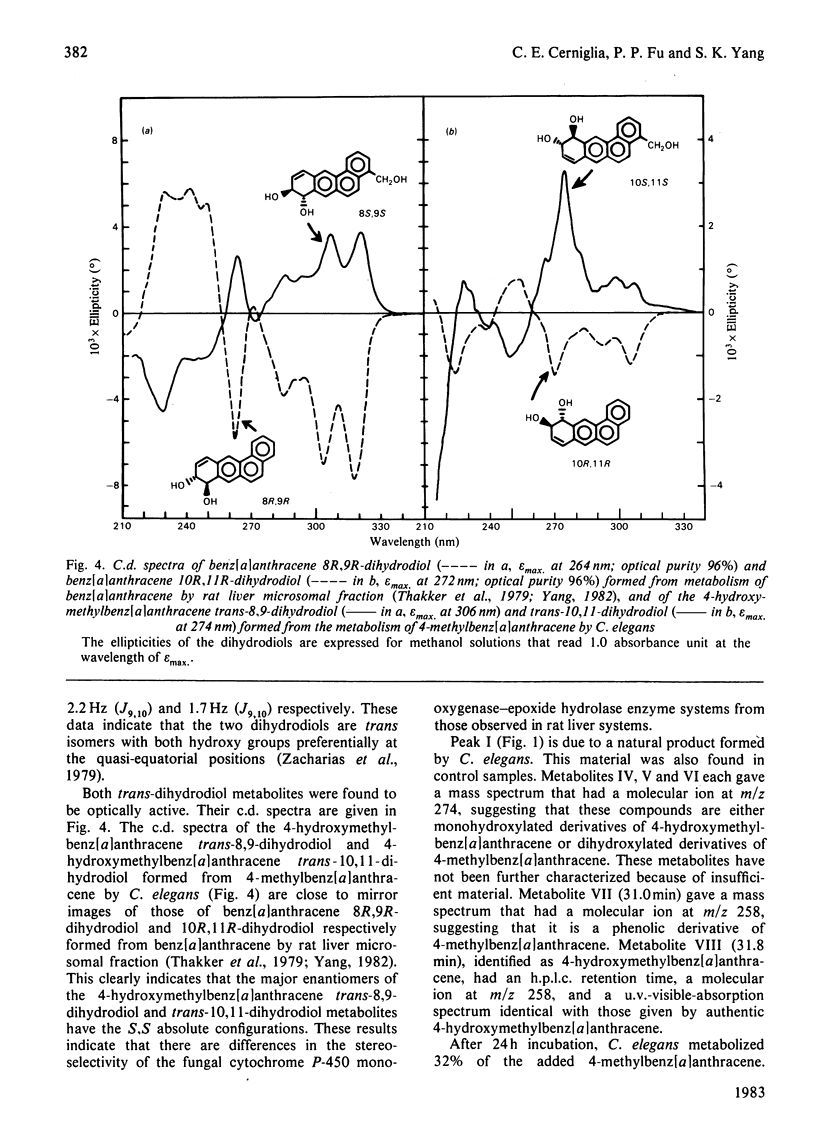
Metabolism of 4-methylbenz[a]anthracene by the fungus Cunninghamella elegans was studied. C. elegans metabolized 4-methylbenz[a]anthracene primarily at the methyl group, this being followed by further metabolism at the 8,9- and 10,11-positions to form trans-8,9-dihydro-8,9-dihydroxy-4-hydroxymethylbenz[a]anthracene and trans-10,11-dihydro-10,11-dihydroxy-4-hydroxymethylbenz[a]anthracene. There was no detectable trans-dihydrodiol formed at the methyl-substituted double bond (3,4-positions) or at the 'K' region (5,6-positions). The metabolites were isolated by reversed-phase high-pressure liquid chromatography and characterized by the application of u.v.-visible-absorption-, 1H-n.m.r.- and mass-spectral techniques. The 4-hydroxymethylbenz[a]anthracene trans-8,9- and -10,11-dihydrodiols were optically active. Comparison of the c.d. spectra of the trans-dihydrodiols formed from 4-methylbenz[a]anthracene by C. elegans with those of the corresponding benz[a]anthracene trans-dihydrodiols formed by rat liver microsomal fraction indicated that the major enantiomers of the 4-hydroxymethylbenz[a]anthracene trans-8,9-dihydrodiol and trans- 10,11-dihydrodiol formed by C. elegans have S,S absolute stereochemistries, which are opposite to those of the predominantly 8R,9R- and 10R,11R-dihydrodiols formed by the microsomal fraction. Incubation of C. elegans with 4-methylbenz[a]anthracene under 18O2 and subsequent mass-spectral analysis of the metabolites indicated that hydroxylation of the methyl group and the formation of trans-dihydrodiols are catalysed by cytochrome P-450 mono-oxygenase and epoxide hydrolase enzyme systems. The results indicate that the fungal mono-oxygenase-epoxide hydrolase enzyme systems are highly stereo- and regio-selective in the metabolism of 4-methylbenz[a]anthracene.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerniglia C. E., Althaus J. R., Evans F. E., Freeman J. P., Mitchum R. K., Yang S. K. Stereochemistry and evidence for an arene oxide-NIH shift pathway in the fungal metabolism of naphthalene. Chem Biol Interact. 1983 Apr-May;44(1-2):119–132. doi: 10.1016/0009-2797(83)90134-5. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Fu P. P., Yang S. K. Metabolism of 7-methylbenz[a]anthracene and 7-hydroxymethylbenz[a]anthracene by Cunninghamella elegans. Appl Environ Microbiol. 1982 Sep;44(3):682–689. doi: 10.1128/aem.44.3.682-689.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzlik R. P., Edelman M., Michaely W. J., Scott G. Enzymatic hydration of (18O)epoxides. Role of nucleophilic mechanisms. J Am Chem Soc. 1976 Mar 31;98(7):1952–1955. doi: 10.1021/ja00423a050. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., Miwa G. T. Molecular properties and biological functions of microsomal epoxide hydrase. Annu Rev Pharmacol Toxicol. 1980;20:513–531. doi: 10.1146/annurev.pa.20.040180.002501. [DOI] [PubMed] [Google Scholar]
- Malaveille C., Tierney B., Grover P. L., Sims P., Bartsch H. High microsome-mediated mutagenicity of the 3,4-dihydrodiol of 7-methylbenz[a]anthracene in S. typhimurium TA 98. Biochem Biophys Res Commun. 1977 Mar 21;75(2):427–433. doi: 10.1016/0006-291x(77)91060-9. [DOI] [PubMed] [Google Scholar]
- Stevenson J. L., Von Haam E. Carcinogenicity of benzo(a) anthracene and benz(c)phenanthrene derivatives. Am Ind Hyg Assoc J. 1965 Sep-Oct;26(5):475–478. doi: 10.1080/00028896509342760. [DOI] [PubMed] [Google Scholar]
- Thakker D. R., Levin W., Yagi H., Turujman S., Kapadia D., Conney A. H., Jerina D. M. Absolute stereochemistry of the trans-dihydrodiols formed from benzo[a]anthracene by liver microsomes. Chem Biol Interact. 1979 Oct;27(2-3):145–161. doi: 10.1016/0009-2797(79)90122-4. [DOI] [PubMed] [Google Scholar]
- Wislocki P. G., Fiorentini K. M., Fu P. P., Yang S. K., Lu A. Y. Tumor-initiating ability of the twelve monomethylbenz[a]anthracenes. Carcinogenesis. 1982;3(2):215–217. doi: 10.1093/carcin/3.2.215. [DOI] [PubMed] [Google Scholar]
- Wislocki P. G., Juliana M. M., MacDonald J. S., Chou M. W., Yang S. K., Lu A. Y. Tumorigenicity of 7,12-dimethylbenz[a]anthracene, its hydroxymethylated derivatives and selected dihydrodiols in the newborn mouse. Carcinogenesis. 1981;2(6):511–514. doi: 10.1093/carcin/2.6.511. [DOI] [PubMed] [Google Scholar]
- Wu J., Wong L. K. Microbial transformations of 7,2-dimethylbenz[a]anthracene. Appl Environ Microbiol. 1981 Mar;41(3):843–845. doi: 10.1128/aem.41.3.843-845.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. K., Chou M. W., Fu P. P., Wislocki P. G., Lu A. H. Epoxidation reactions catalyzed by rat liver cytochromes P-450 and P-448 occur at different faces of the 8,9-double bond of 8-methylbenz[a]anthracene. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6802–6806. doi: 10.1073/pnas.79.22.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. K. The absolute stereochemistry of the major trans-dihydrodiol enantiomers formed from 11-methylbenz[a]anthracene by rat liver microsomes. Drug Metab Dispos. 1982 May-Jun;10(3):205–211. [PubMed] [Google Scholar]


