Abstract
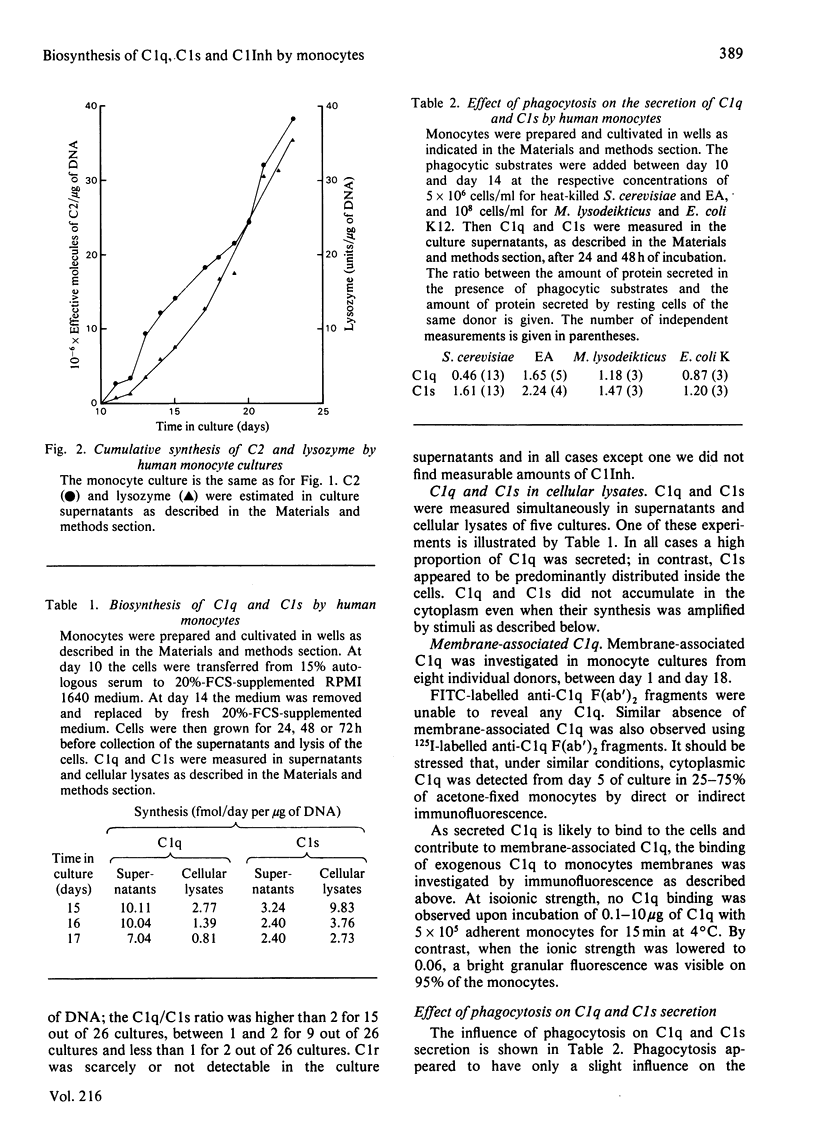
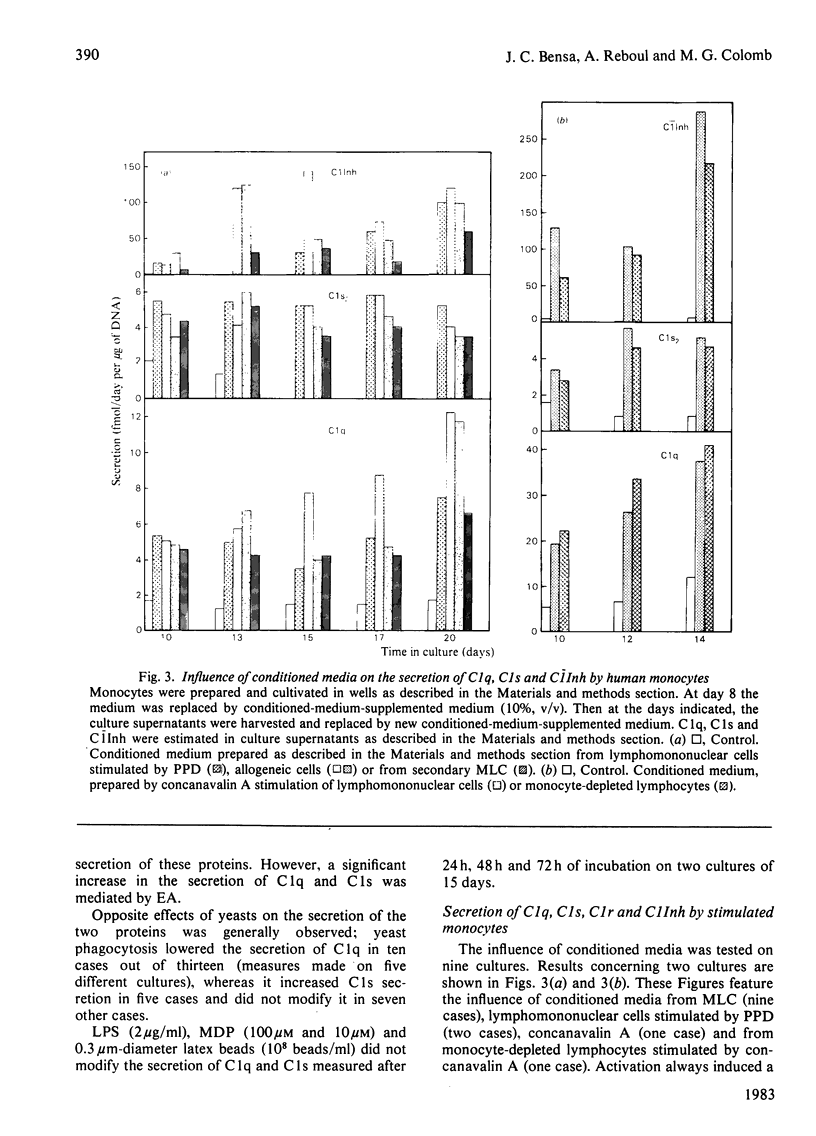
The capacity of cultured human monocytes to synthesize and to secrete the subcomponents of C1 and C1 inhibitor was examined. Non-stimulated monocytes secreted C1q and C1s from day 5 of culture. C1s reached a plateau immediately at its maximum level, whereas C1q secretion increased progressively until the end of the second week. Between day 12 and day 25, C1q secretion remained nearly constant (1-15 fmol/day per microgram of DNA, depending on the donor), whereas C1s secretion decreased and even in some cases stopped. C1r and C1 inhibitor were not secreted in detectable amounts by these resting cells. Stimulation of monocytes by yeasts, immunoglobulin G-opsonized sheep red blood cells or latex beads did not modify consistently C1q and C1s secretion. Activation by conditioned media from mitogen-, antigen- or allogeneic-stimulated lymphocyte cultures increased C1q production from 2 to 7 times and re-activated C1s secretion. Under the same conditions of activation, C1 inhibitor was secreted (up to 300 fmol/day per microgram of DNA) and C1r became detectable in culture supernatants. Isolated human monocytes are thus able to synthesize the whole C1 subcomponents; C1, if assembled, could be protected from non-immunological activation by locally produced C1 inhibitor. Activated monocytes appear to be a good tool for studying the assembly of C1 subcomponents and the role of C1 inhibitor in this process.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlaud G. J., Chesne S., Villiers C. L., Colomb M. G. A study on the structure and interactions of the C1 sub-components C1r and C1s in the fluid phase. Biochim Biophys Acta. 1980 Nov 6;616(1):105–115. doi: 10.1016/0005-2744(80)90268-5. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Reboul A., Meyer C. M., Colomb M. G. Purification of proenzymic and activated human C1s free ofC1r. Effect of calcium and ionic strength on activated C1s. Biochim Biophys Acta. 1977 Nov 23;485(1):215–225. doi: 10.1016/0005-2744(77)90208-x. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Sim R. B., Duplaa A. M., Colomb M. G. Differential elution of Clq, Clr and Cls from human Cl bound to immune aggregates. Use in the rapid purification of Cl subcomponents. Mol Immunol. 1979 Jul;16(7):445–450. doi: 10.1016/0161-5890(79)90069-5. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Villiers C. L., Chesne S., Colomb M. G. Purified proenzyme C1r. Some characteristics of its activation and subsequent proteolytic cleavage. Biochim Biophys Acta. 1980 Nov 6;616(1):116–129. doi: 10.1016/0005-2744(80)90269-7. [DOI] [PubMed] [Google Scholar]
- Colten H. R., Borsos T., Rapp H. J. In vitro synthesis of the first component of complement by guinea pig small intestine. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1158–1163. doi: 10.1073/pnas.56.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colten H. R., Gordon J. M., Rapp H. J., Borsos T. Synthesis of the first component of guinea pig complement by columnar epithelial cells of the small intestine. J Immunol. 1968 Apr;100(4):788–792. [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusser C., Boesman M., Nordin J. H., Isliker H. Effect of chemical and enzymatic radioiodination on in vitro human Clq activities. J Immunol. 1973 Mar;110(3):820–828. [PubMed] [Google Scholar]
- Higgins T. J., O'Neill H. C., Parish C. R. A sensitive and quantitative fluorescence assay for cell surface antigens. J Immunol Methods. 1981;47(3):275–287. doi: 10.1016/0022-1759(81)90283-0. [DOI] [PubMed] [Google Scholar]
- Kilchherr E., Fuchs H., Tschopp J., Engel J. Dissociation of C1 and concentration dependence of its activation kinetics. Mol Immunol. 1982 May;19(5):683–691. doi: 10.1016/0161-5890(82)90370-4. [DOI] [PubMed] [Google Scholar]
- Loos M., Storz R., Müller W., Lemmel E. M. Immunofluorescence studies on the subcomponents of the first component of complement (C1): detection of C1q and C1s in different cells of biopsy material and on human as well as on guinea pig peritoneal macrophages. Immunobiology. 1981;158(3):213–224. doi: 10.1016/S0171-2985(81)80071-X. [DOI] [PubMed] [Google Scholar]
- Loos M. The functions of endogenous C1q, a subcomponent of the first component of complement, as a receptor on the membrane of macrophages. Mol Immunol. 1982 Oct;19(10):1229–1238. doi: 10.1016/0161-5890(82)90288-7. [DOI] [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- Morris K. M., Colten H. R., Bing D. H. The first component of complement. A quantitative comparison of its biosynthesis in culture by human epithelial and mesenchymal cells. J Exp Med. 1978 Oct 1;148(4):1007–1019. doi: 10.1084/jem.148.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Hanauske-Abel H., Loos M. Biosynthesis of the first component of complement by human and guinea pig peritoneal macrophages: evidence for an independent production of the C1 subunits. J Immunol. 1978 Oct;121(4):1578–1584. [PubMed] [Google Scholar]
- PORTER R. R. The fractionation of rabbit gamma-globulin by partition chromatography. Biochem J. 1955 Mar;59(3):405–410. doi: 10.1042/bj0590405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahl J. W., Porter R. R. Allotype-related sequence variation of the heavy chain of rabbit immunoglobulin G. Biochem J. 1968 May;107(6):753–763. doi: 10.1042/bj1070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul A., Arlaud G. J., Sim R. B., Colomb M. G. A simplified procedure for the purification of C1-inactivator from human plasma. Interaction with complement subcomponents C1r and C1s. FEBS Lett. 1977 Jul 1;79(1):45–50. doi: 10.1016/0014-5793(77)80347-5. [DOI] [PubMed] [Google Scholar]
- Reid K. B. C1q. Methods Enzymol. 1982;82(Pt A):319–324. doi: 10.1016/0076-6879(82)82069-7. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Solomon E. Biosynthesis of the first component of complement by human fibroblasts. Biochem J. 1977 Dec 1;167(3):647–660. doi: 10.1042/bj1670647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. C., Schumaker V. N. Measurement of the association constants of the complexes formed between intact C1q or pepsin-treated C1q stalks and the unactivated or activated C1r2C1s2 tetramers. Mol Immunol. 1983 Jan;20(1):53–66. doi: 10.1016/0161-5890(83)90105-0. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Analysis of receptor-mediated C1q binding to human peripheral blood mononuclear cells. J Immunol. 1980 Oct;125(4):1658–1664. [PubMed] [Google Scholar]
- Thielens N. M., Villiers M. B., Reboul A., Villiers C. L., Colomb M. G. Human complement subcomponent C2: purification and proteolytic cleavage in fluid phase by C1s, C1r2-C1s2 and C1. FEBS Lett. 1982 May 3;141(1):19–24. doi: 10.1016/0014-5793(82)80006-9. [DOI] [PubMed] [Google Scholar]
- Valet G., Cooper N. R. Isolation and characterization of the proenzyme form of the C1r subunit of the first complement component. J Immunol. 1974 May;112(5):1667–1673. [PubMed] [Google Scholar]
- Valet G., Cooper N. R. Isolation and characterization of the proenzyme form of the C1s subunit of the first complement component. J Immunol. 1974 Jan;112(1):339–350. [PubMed] [Google Scholar]
- Villiers C. L., Chesne S., Lacroix M. B., Arlaud G. J., Colomb M. G. Structural features of the first component of human complement, C1, as revealed by surface iodination. Biochem J. 1982 Apr 1;203(1):185–191. doi: 10.1042/bj2030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiers C. L., Duplaa A. M., Arlaud G. J., Colomb M. G. Fluid phase activation of proenzymic C1r purified by affinity chromatography. Biochim Biophys Acta. 1982 Jan 4;700(1):118–126. doi: 10.1016/0167-4838(82)90299-0. [DOI] [PubMed] [Google Scholar]
- Ziccardi R. J. A new role for C-1-inhibitor in homeostasis: control of activation of the first component of human complement. J Immunol. 1982 Jun;128(6):2505–2508. [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Modulation of the antigenicity of C1r and C1s by C1 inactivator. J Immunol. 1978 Dec;121(6):2148–2152. [PubMed] [Google Scholar]


