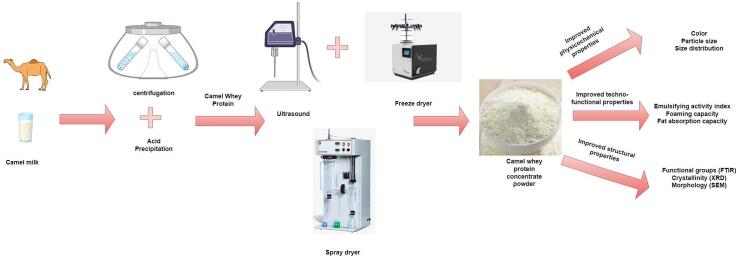
Graphical abstract
Keywords: Camel milk whey powder, Ultrasound, Spray-drying, Morphology, Technological properties, Functional properties
Abstract
Whey protein concentrates (WPCs) are gaining importance as a functional ingredient due to their high technological and functional properties and their diverse application in the food industry. In this study, Camel milk whey (CW) was separated from skimmed camel milk, then either spray-dried (SD) at 170, 185 and 200 °C, or treated by ultrasonication (US) (20 kHz) for 5, 10 and 15 min followed by freeze-drying to obtain camel milk whey powder (CWP). The structural analysis of CWP was carried out by Fourier-Transform Infrared Spectroscopy (FTIR) and X-Ray Diffraction (XRD) which showed no significant difference in the functional groups profile of US samples compared to control and SD samples. US samples showed some degree of crystallinity that was comparable to the control samples, while SD samples exhibited very low degree of crystallinity. The surface morphology, particle size, and surface charge of CWP were evaluated using scanning electron microscopy (SEM) and Zetasizer. The lowest particle size of 215.1 nm with surface charge of −21.6 mv was observed in SD-185 WPC. Moreover, SD samples revealed whiter color compared to the US-treated samples which were having lower L* values (P < 0.05). US-15 sample exhibited high protein solubility (100 %), whereas the SD-200 sample showed reduced solubility (92.7 %). Improvement in the emulsifying activity of CWP samples was observed after SD and US, with highest emulsifying activity index (EAI) values of 143.75 m2/g and 143.11 m2/g were reported for SD-185 and US-15 CWP samples, respectively. To conclude, SD and US were found to improve the physico-chemical, technological, and functional properties of CWP, and thus can be utilized as a promising strategy to preserve and enhance the technofunctional properties of CWP.
1. Introduction
Camel milk is considered a main component in the human diet in many parts of the world, especially arid countries [1]. Based on the Food and Agriculture Organization (FAO) of the United Nations (UN) statistics, in 2024 there are approximately 41 million camels in the world [2]. In the next few years, it is predicted that the population of camels and camel milk yield will increase due to increased demand and interest by consumers [3]. Camel milk contains between 2.1 and 4.9 % protein [4] comprising of casein (around 80 % of the protein content) and whey protein (WP) (around 20 % of the protein content) [5]. Camel whey protein contains serum albumin, α-lactalbumin (α-LA), immunoglobulin (Ig), lactophorin, and peptidoglycan recognition protein [6]. Camel whey protein also contains lactoperoxidase (LPO), lysozyme, and lactoferrin (LF), as well as other proteins with biological functions. Serum albumin is the major whey protein (WP) present in camel milk, with an average concentration of 10.8 g/l [6]. Camel whey protein possess high nutritional value which can be ascribed to their high content of essential amino acids [7] and being free of β-lactoglobulin prevents allergic reactions caused by this major serum protein [8]. They also have beneficial immunomodulatory roles as a natural antioxidant and reduce the risk of diabetes [9]. Moreover, camel milk whey (CW) is known to contain several minerals such as essential macro-minerals (sodium, magnesium, phosphorus, sulfur, potassium, calcium), essential micro-minerals (manganese, iron, copper, chromium, and zinc), and environmental mineral (boron, aluminum, silicon, titanium, strontium, and barium) which according to their content can influence the technological properties of camel milk whey protein [10]. Therefore, processing of camel whey protein and making them available as a shelf-stable powder is crucial for promoting camel milk proteins as functional ingredients for different food applications.
Various technologies have been applied to modify the structural and physiochemical properties of proteins. Among those, ultrasound technology as a non-thermal technique has been adapted and utilized significantly to modify proteins [11]. During ultrasound treatment, cavitation generates high pressure and shear forces, which affects the physicochemical properties of products [12]. It has been reported that sonication improves the heat stability of whey protein concentrates (WPCs) by using sonication with a frequency of 20 kHz, which leads to a reduction in the viscosity and particles size [13]. Previous study conducted by Meng et al., [14] reported that sonicated bovine whey protein (BWP) with sonication power of 600 W for 40 mins has reported significant improvement in the foam expansion from 165 % to 215 % and emulsifying activity index from 45 m2/g to 61 m2/g as well as improving the samples’ DPPH radical scavenging activity from 26 % to 42 % and ABTS radical scavenging activity from 47 % to 66.5 %.
Spray drying (SD) is the most common thermal drying and processing technique used for producing whey protein powder. Several studies have reported the effect of SD conditions on the physicochemical properties of camel milk proteins (CP) [15], [16]. The thermal stability of proteins from camel milk is superior when compared to that of bovine milk proteins (BMPs) and depends on the physicochemical properties of the proteins [17]. This has been ascribed to the absence of β-LG in camel milk, which leads to protein denaturation at a low temperature compared to BMPs [18]. A study done by Zouari et al., [19] that investigated the production of camel milk powder using spray drying has reported that the reconstituted camel milk displayed significantly higher foaming capacity and stability when compared to control skim camel milk sample. Previous studies have also shown that SD process involving high temperatures, might result in changes in protein functionality due to aggregation and denaturation of WP [20]. Overall, processing techniques like SD and US have been known to influence the physico-chemical and functional properties of whey proteins and it is important to investigate such changes occurring during processing.
There is a growing interest in the production of whey proteins powders from camel milk via technologies that can maintain or improve the CWPs’ physio-chemical, technological, and functional properties [21]. This study aimed to understand the effect of thermal (SD) and non-thermal (US) processing methods on the physicochemical technological, and functional properties of camel milk whey powder (CWP). To the best of our knowledge, there is limited literature available on that topic, which highlights the significance of this present study.
2. Material and methods
2.1. Preparation of camel milk whey
Raw milk from single breed of four different camels (Camelus dromedarius) procured from a local camel farm situated in Al Ain, United Arab Emirates was used in the present study. Camels were grown in a semi-intensive rearing system and fed with fed ad libitum on Rhodes grass (Chloris gayana) hay diet incorporated with date seed powder. After arrival in the laboratory, the raw milk samples were stored at 4 °C and processed within 2 hrs. Whey proteins were isolated from camel milk according to the previous method described by Jafar et al., [22]. Camel milk was skimmed two times through centrifugation at 4200 × g, for 15 min at 10 °C, and the resulting skimmed camel milk was subjected to acid precipitation by adjusting the pH at 4.0 using 6 M HCl , and then stored overnight at 4 °C. Milk samples were afterwards centrifuged 2 times, first at 4200 × g, for 15 min at 4 °C to separate the whey from caseins. Second at 10538 × g, for 15 min at 4 °C to completely remove the casein particles. The CW was frozen at −18 °C for further processing.
2.2. Production of camel milk whey powder (CWP) using spray drying
The CWP was produced by freeze-drying (as control) and spray-drying processes. In brief, freeze-drying was carried out on previously frozen CW at −80 °C using a Telstar Freeze-dryer (Terrassa, Spain) at 0.01 mbar. Spray-drying process was performed by using a spray dryer (Armfield, UK.), and the following operational conditions were adopted [15]: three different inlet temperatures of 170, 185 and 200 °C were explored, outlet temperature of 70 °C with a solid feed rate of 20 %, relative humidity around 8.5 %, drying air flow rate of 7.5 m3/min, and atomization pressure of 0.52 MPa that was maintained throughout the drying experiment. The CWP yield was calculated from the cyclone (cyclone recovery), and from the freely flowing powder, which was collected at the lower conical section of the drying chamber. The CWP were collected in Mylar bags (TF-4000, Impak Crop., Central City, SD; ∼1 kg per bag) and stored at −18 °C until further analysis.
2.3. Treatment of camel milk whey (CW) by ultrasonication
Ultrasonication (US) of CW was carried out using a bath-type ultrasonicator (EGS5HD, EngeSolutions, São Paulo-SP, Brazil) according to the method of Ahmadi, Razavi, Varidi, [20]. Briefly, a 10 % CW solution in deionized water was subjected to US treatment (20 kHz) for 0, 5, 10 and 15 min at 300 W. The temperature of the samples was kept below 30 °C using an ice-water bath. The obtained US samples were freeze-dried to obtain fine CWP and stored at −18 °C until further analysis.
2.4. Structural analysis of CWP
Infrared absorbance spectra of CWP samples were determined using a Fourier-Transform Infrared Spectroscopy (FTIR) Spectrometer equipped with an Attenuated Total Reflectance (ATR) Spectrum 100 (PerkinElmer Ltd, Beaconsfield, UK). in the spectral range frequencies of 4000–400 cm−1. For each spectrum, 32 scans of interferograms were averaged and the spectral resolution was 4 cm−1.
The crystallographic structural analysis was carried out by using an X-Ray diffractometry Lab X–XRD–6100 (Shimadzu Corp, Japan), under the following operating conditions: X-Ray line of λ = 1.5418 Å, voltage of 40 kV and accuracy of 30 mA. The powdered samples were loaded into an aluminium plate and X-ray diffraction profiles were obtained for 2θ ranging from 10° to 50° with scanning rate of 0.02/min. The relative crystallinity (RC) was calculated using the following equation:
where Ac is crystalline area; Aa is the amorphous area on the X-Ray diffractogram.
Microstructural features of the treated and control CWP were monitored by using a JEOL JSM- 6010LA scanning electron microscope (SEM, Akishima, Tokyo, Japan) at 1,500 x magnification.
2.5. Protein hydrolysis
The protein hydrolysis following spray drying and ultrasonication was evaluated using the o-phthaldialdehyde (OPA) method as described by Kamal et al., [23]. The OPA reagent was prepared by mixing 25 mL of sodium tetraborate buffer (100 mM; pH 9.3), 2.5 mL of 20 % sodium dodecyl sulfate (SDS), 40 mg of OPA (dissolved in 1 mL methanol), 100 µL of β-mercaptoethanol, then the final volume was completed to 50 mL using ultra-pure water. 100 µL of the prepared CWP were mixed with 1 mL of the freshly prepared OPA reagent and mixed gently for 10 s, then the absorbance of the mixture was taken at 340 nm to determine the amount of free amino nitrogen (μg/mL). A standard curve was generated using tryptone to estimate the content of free amino acid (μg/mL) (FAN), and increase in FAN content in comaprison to control was presented as a mesaure of protein hydrolysis DH.
2.6. Determination of physicochemical properties of CWP
2.6.1. Color analysis
The color of the CWP samples was determined following the method previously reported by Ho et al., [3] using a Chroma meter (CR-400, Konica Minolta, USA). CWP were spread on a transparent dish and the color space of L*, a* and b* was measured which indicate the lightness/darkness, red/green, and the yellow/blue coordinates, respectively.
2.6.2. Particle size and surface charge distribution
Zeta potential and particle size distribution were evaluated following the method described by Ahmad et al., [24] using a Zeta-sizer (Nano S, Malvern Instruments, Worcestershire, U.K.). For zeta potential measurement, the CWP samples (0.01 %) were suspended in 0.1 mM KCl, and pH of the mixture was adjusted to 6.0, then samples were allowed to equilibrate overnight before measurement.
2.7. Determination of technological and-functional properties of CWP
2.7.1. Protein solubility in water
Solubility of CWP samples was determined based on the method as previously described by Al-Shamsi et al., [25]. 200 mg of CWP samples were solubilized in 20 mL of deionized water, by vortexing for 2 min followed by centrifugation at 7500 × g (25 °C) for 15 min. The protein solubility was expressed as protein content (mg of protein/g of sample) in the supernatants, which was estimated using the Biuret method.
2.7.2. Fat absorption capacity (FAC)
The FAC was evaluated as previously described by Maqsood et al., [5]. A small amount of (100 mg) of CWP samples was mixed with 1000 μL of sunflower oil using a vortex for 1 min. The protein in oil suspensions were centrifuged at 13,600 × g for 30 min at 25 °C. The supernatants were decanted, and the tubes were drained at 45° for 1–2 min. Fat absorption capacity for samples was calculated using the below mentioned formula:
2.7.3. Emulsifying activity index (EAI)
The EAI of the different CWP samples was investigated as previously described by Al-Shamsi et al., [25]. Briefly, 300 mg of control, US, and SD samples were mixed with 30 mL of deionized water (1 % protein equivalent, w/v), adjusted to pH 7.0 and 10 mL of sunflower oil were added. The mixtures were homogenized using an ultra-turrax homogenizer (Janke & Kunkel, Ultra-Turrax T25, Staufen, Germany) at 20,500 rpm for 1 min. Subsequently, an aliquot of 50 μL of the emulsion was taken from the bottom after 0 and 10 min and was mixed with 5 mL of 0.1 % sodium-dodecyl sulphate (SDS) solution. The absorbance of the diluted emulsions was measured at 500 nm using a spectrophotometer (Varian Cary® 50 UV–Vis, USA). The EAI of the different CWP samples was calculated using the following formula:
where A0 is the absorbance of the sample taken after homogenization.
2.7.4. Foaming capacity (FC)
The foaming capacity (FC) of CWP samples was estimated according to the method reported by Maqsood et al., [5]. A small amount (50 mg) of each control, US, and SD samples was mixed with 50 mL of distilled water (1 % protein equivalent, w/v), and the pH was adjusted to 2.0, 4.0, 6.0, 8.0, and 10.0 using a digital type of pH meter (Ohaus starter ST3100-B, NJ, USA). The solutions were transferred to 100 mL graduated cylinders and whipped using an ultra-turrax homogenizer at 13,500 rpm for 3 min. The total sample volume before and after whipping was measured and FC (%) was calculated as follows:
2.8. Statistical analysis
The SD and US treatments of CW were carried out in three batches and the experimental analyses were conducted in triplicate (n = 3). All data were subjected to Analysis of Variance (ANOVA) and the differences between means were evaluated by Duncan’s Multiple Range Test. SPSS statistics software (SPSS, 1.2, Version 10.0) was used for data analysis.
3. Results and discussion
3.1. Structural analysis of CWP
3.1.1. Fourier-Transform Infrared (FTIR) Spectroscopy
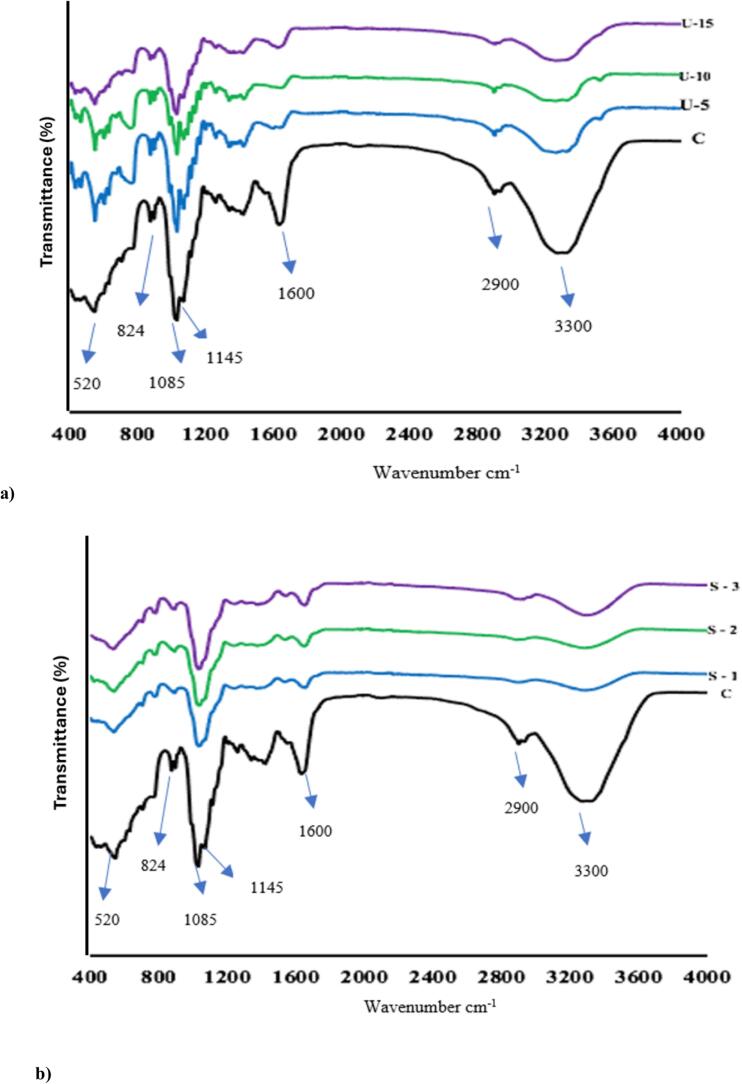
The occurrence and changes in functional group profile of CWP after sonication for 5, 10, and 15 min and SD at 170 °C, 185 °C, and 200 °C are presented in Fig. 1a and 1b, respectively. Overall, there was no significant change observed regarding the functional group profile observed for US and SD samples when compared to control sample. However, the intensity of the peak around 1600 cm−1 was higher in control sample than the peaks observed in SD and US samples. Moreover, an increase in the intensity of peaks around 1520 cm−1 was noticed after sonicating the CW for 5 and 10 min whereas it was not noticeable in US-15, SD, and control samples Fig. 1 (a and b). The peaks obtained around 520 cm−1 are linked to N-H and C-H bonds vibrations in the ion-binding peptide [26], [27]. A low intensity peak was observed in all samples around 824 cm−1 which represents the out-of-plane N-H wagging vibration in CWP [28]. The presence of peaks around 1000–1100 cm−1 either confirm the presence of lactose or due to alkoxy C-O stretching [3], [28]. Moreover, all samples obtained a peak around 1150 cm−1 which was present due to bending or stretching vibrations of C═H, C═O, and C═C [28]. The steep peak observed around 1600–1650 cm−1 was a result of C-O stretching vibration in amide I and provides information regarding the protein secondary structures (α-helix, β-sheet) [[14], [29]]. However, previous studies reported by [[29], [30]] linked the peaks around 1600 cm−1 to C═O stretching in amid I. The residue of some fat content was confirmed in all US and control samples with the presence of a low intensity peak around 2900 cm−1, whereas no fat residue was present in SD samples [[28], [29]]. The wide intensity peak, which represents symmetric or antisymmetric O-H stretching and N-H stretching that was observed at 3300 cm−1 in control sample, was totally degraded in SD samples and was presented in low intensity in all US samples [29]. The degradation or complete loss of the peak around 3300 cm−1 could be linked to loss of moisture content in US and SD samples due to the thermal treatments [3]. The FTIR results obtained from this study confirmed that the functional groups present in CW were slightly affected by US and SD treatment with some intense peaks (1600 and 3300 cm−1) being degraded in US and SD samples.
Fig. 1.
FTIR spectra of ultrasonicated (US) (a) and spray-dried (SD) (b) CWP samples in the wavenumber range 400 to 4000 cm−1. Keynotes: Control: freeze-dried CWP; US-5: sonicated for 5 min; US-10: sonicated for 10 min; and US-15: sonicated for 15 min; S-1: SD at 170 °C; S-2: SD at 185 °C; and S-3: SD at 200 °C,
3.1.2. X-ray diffraction (XRD) analysis
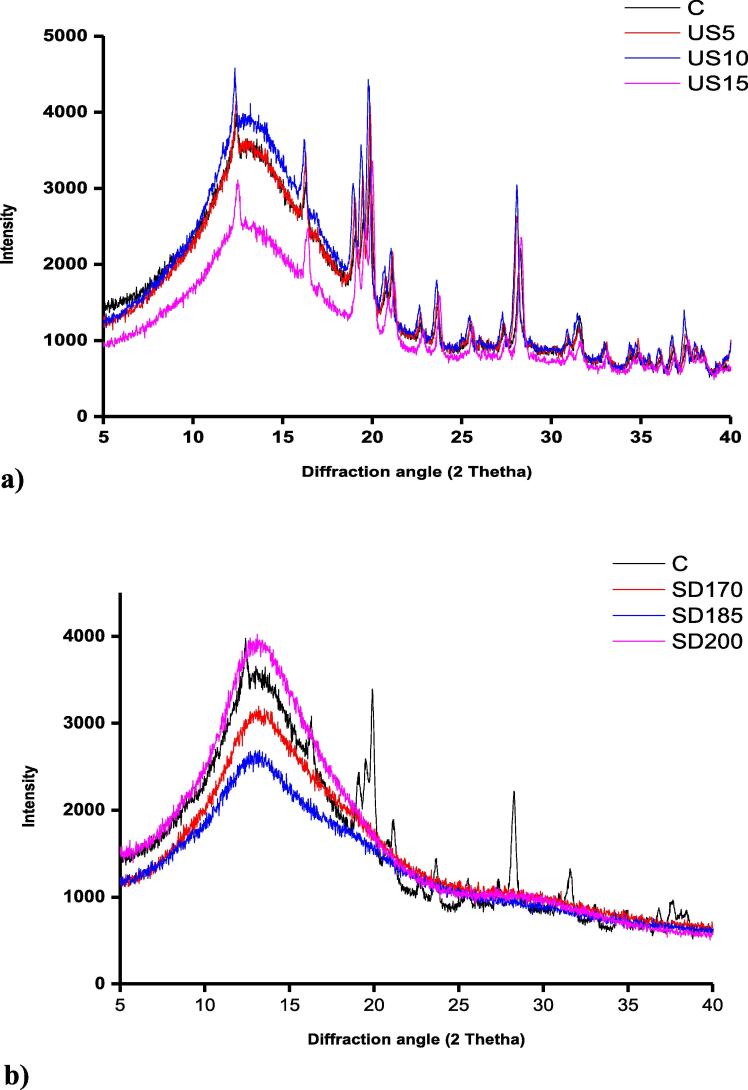
The analysis of CWP with X-ray diffraction (XRD) showed some degree of crystallinity was present in all US and control samples since few sharp peaks were detected around 13°, 17°, 20°, 24°, and 28° (Fig. 2a). A previous study investigated the effect of US treatment on the crystal structure of sunflower protein has reported that from the XRD diffractogram of control (untreated), US-probe, and US-bath samples, there were no significant (P < 0.05) difference observed among the mentioned samples and confirmed that all samples had two intense peaks around 10° and 20° [31]. Peaks obtained around 10° and 20° mainly suggest the presence of crystalline region I and II, respectively and as well as the presence of noncovalent bonds [32]. This repeating trend indicates that US treatment did not influence the crystallinity of substances. However, the obtained X-ray diffractogram of all SD CWP samples confirmed that they had very low or neglected degree of crystallinity (Fig. 2b). Overall, no significant difference in crystallization was observed among SD samples treated with different inlet temperatures, 170 °C, 185 °C, and 200 °C respectively. These finding are in line with results reported by Ho et al., [3], which confirmed using X-ray diffractogram that control samples showed several sharp peaks which verify the high degree of crystallinity, whereas SD camel milk powder exhibited more amorphous structure with few small peaks indicating low degree of crystallinity. Moreover, spray-drying of bovine serum albumin at 120 °C produced samples that exhibited low degree of crystallinity with few minor peaks observed [33]. In this study, XRD in US and control (freeze-dried) samples showed high degree of crystallinity, with few sharp peaks present around 13°, 17°, 20°, 24°, and 28°. US treatment had no effect on the crystallinity as there was no difference noticed in US samples when compared to control samples. However, in SD samples, low degree of crystallinity was observed, and samples had more amorphous structure.
Fig. 2.
X-Ray diffractograms (XRD) for control CWP and ultrasonicated (US) samples (a) treated for 5, 10, and 15 mins and spray-dried (SD) CWP samples (b) processed at 170 °C, 185 °C, and 200 °C. For keynotes, please see figure legend of Fig. 1.
3.1.3. Scanning electron microscopy (SEM)
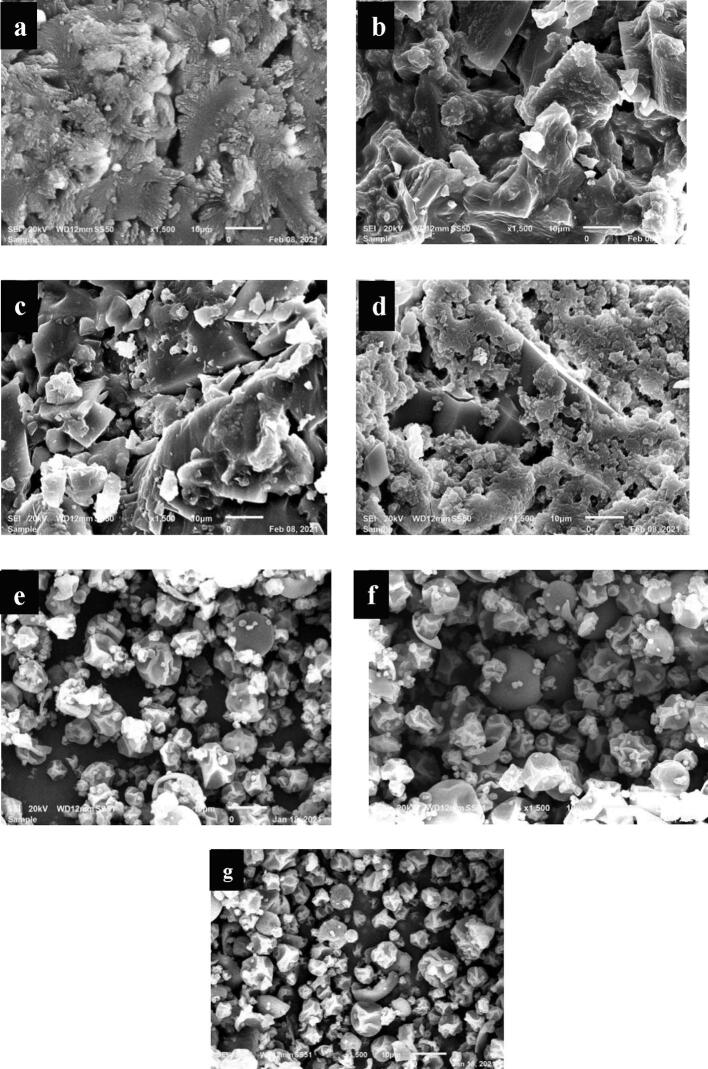
The morphology of control, US, and SD CWP samples was determined using SEM and the obtained SEM images for all samples are presented in Fig. 3. SEM images of control and US samples, which were freeze-dried to obtain the powder showed that the samples did not exhibit a uniform shape or size as well as confirmed the formation of small aggregates. In US samples, partial deagglomeration of CWP caused by acoustic cavitation may be the reason for the formation of aggregates [12]. Freeze-drying of CW resulted in production of powder with a spherical shape with clear signs of aggregation [24]. In contrast, SD samples dried at different temperatures showed a uniform spherical shape in different sizes with no sign of aggregation (Fig. 3). Previous studies reported that SD of camel milk powder at different temperatures produced particles with spherical shape and did not show any signs of aggregation, which is in agreement with results obtained in this study [3], [16]. Moreover, it was clear from the results that there was a noticeable difference between the particle size and uniformity of shape of SD samples when compared to control and US samples.
Fig. 3.
Scanning electron microscopy (SEM) images for control, ultrasonicated (US) and spray-dried (SD) CWP samples. Keynotes: a) Control (freeze-dried CWP); b) sonicated for 5 min; c) sonicated for 10 min; d) sonicated for 15 min; e) SD at 170 °C; f): SD at 185 °C; g) SD at 200 °C.
3.1.4. Degree of hydrolysis (DH)
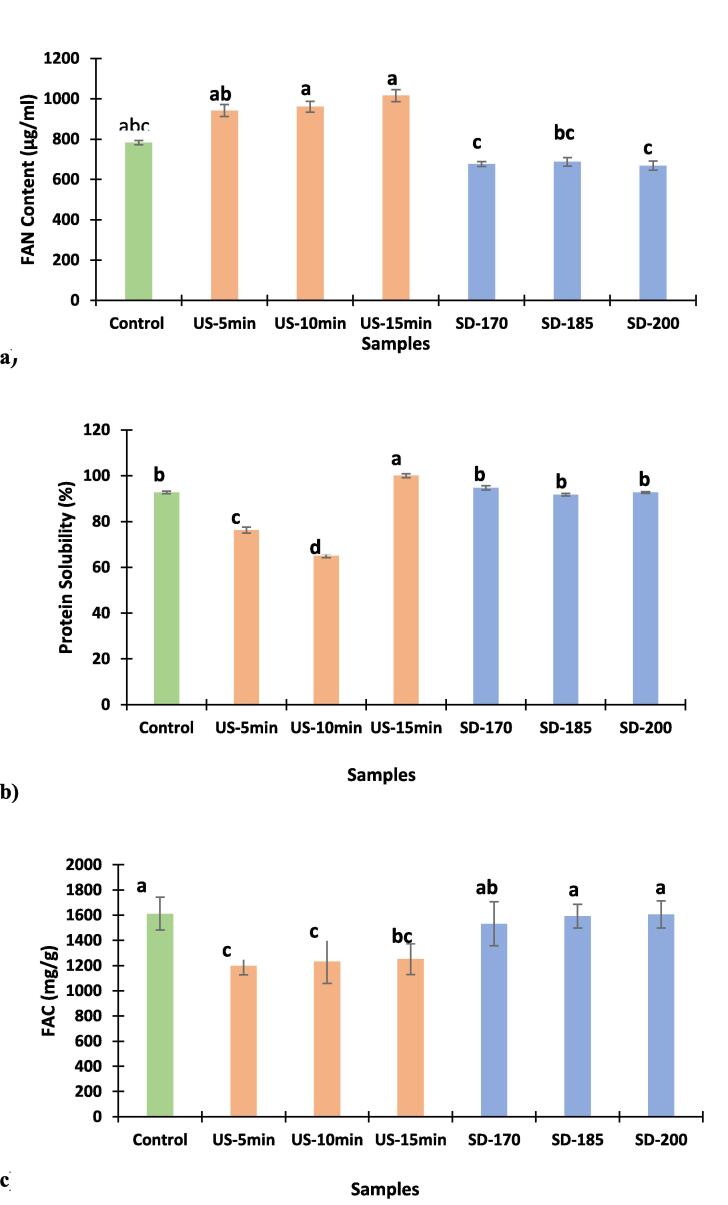
The influence of US and SD on the degree of hydrolysis (DH) and the release of free amino nitrogen (FAN) was determined and the results are presented in Fig. 4. Maximum DH was observed in US-15 samples with FAN value of 1015.9 µg/ml which is significantly higher when compared to the DH of control (freeze-dried) and SD samples with FAN values of 782.75, 676.04, 687.20, and 667.95 µg/ml, respectively. There was no significant (P > 0.05) difference in DH observed between US-5, US-10, and US-15 samples having strong hydrolytic efficiency (Fig. 4). Treatment of CW using US resulted in the breakdown of the whey proteins into free amino acids (FAA) and smaller peptides as a result of high temperature and pressure. According to previous studies, small protein fragments and FAA in general exhibited improved bioactive properties and nutritional values when compared to intact proteins [22], [23]. Isolated whey protein (WPI) from rice was treated by ultrasound (400 W) and microwave (75 °C) for 15 min, respectively. Both treatments significantly increase the release of free amino group from WPI and had the ability to modify overall protein structure [34]. A study investigated the effect of US treatment WPC for 5 and 15 min on the DH and reported that US-15 samples of whey protein concentrate (WPC) showed significant increase in DH of around 8.7 % when compared to the control sample, while US-5 samples showed no change [35]. In this study, SD samples exhibited a decline in DH and release of FAN when compared to control samples, which were in agreements with the results reported by Yang et al., [36], the authors spray-dried a mixture of β-LG and α-lactose at 180 °C and observed a significant decrease in the DH and release of FAN when compared to control samples. In conclusion, the US treatment has a significant positive effect on increasing the release of FAN from CW, while SD reduced the rate of FAN released when compared to control.
Fig. 4.
Degree of Hydrolysis (DH) based on free amino nitrogen content (a), protein solubility (b) and fat absorption capacity (c) of CWP of the control: freeze-dried, US: Sonicated and spray-dried CWP samples. Values presented are mean ± standard deviation (n = 3). Different letters on the different bars indicates that there is a significant difference (P < 0.05). For keynotes, please see footnote of Table 1.
3.2. Physicochemical properties of CWP
3.2.1. Color analysis
Color measurements (L*, a*, and b* values) of control, US, and SD samples are presented in Table 1. It can be concluded from the results that there was a significant difference (P < 0.05) observed in L*, a*, and b* values among SD, US, and control samples, while there was no significant difference observed between the US sample and control sample. In general, L* values indicate lightness/darkness coordinate which means if the value is 100, the color is white, and if the value is 0, the color is black [37] while a* values indicate redness (+) or greenness (−) and b* values indicate yellowness (+) or blueness (−) [3]. Previous studies have reported that the whitish color of camel milk is linked to the presence of small-sized fat globules and lower carotene content [[3], [29]]. L* values of SD samples (95.2–95.92) were higher when compared to the control (91.57) and US samples (87.32–89.89). Whereas the a* values (0.89–0.97) and b*values (2.89–2.92) of SD samples were lower than US samples with a* values between 0.43–0.67 and b* values between 9.7–10.67, confirming that a greener color was observed in the SD samples, and a more yellowish color was observed especially in the US samples (Table 1). The observed difference in the color of samples can be linked to the type of treatment that has been used for each sample. A previous study reported that the L*, a*, and b* values of SD camel milk at 200 °C were 97.73, −1.08, and 7.9, respectively [37]. Raising the temperature above 170 °C during SD of samples slightly decreased the L* and b* values of powders, which can be observed in the values presented in Table 1. A previous study reported that during SD of camel milk powder at 110 °C, 120 °C, and 130 °C, the inlet drying temperature had a significant effect on decreasing the L* value of dried samples [16]. Increasing the US treatment time from 5 to15 min did not have a significant effect on L*, a*, and b* when compared to the control sample. A previous study has reported that sonication of skim milk concentrates had affected color values in terms of L*, a*, and b* when subjected to sonication treatment at a gradual increase of time from 5 to 60 min. The results showed an increase in L* and b* values from 5-60 min samples (78.21––81.2) and (10.14––13.92) respectively, while it decreased for a* value from 5-60 min samples (−6.08- −7.91) [37]. In this study, the obtained values of L*, a*, and b* generally indicated that control and US samples were yellower than SD samples whereas the SD samples were whiter than control and US samples.
Table 1.
Color values of the ultrasonicated (US) and spray-dried (SD) CWP powders.
| Samples | L* | a* | b* |
|---|---|---|---|
| Control | 91.57 ± 0.01b | −0.17 ± 0.004a | 8.64 ± 0.049b |
| US-5 | 89.6 ± 1.13bc | −0.67 ± 0.243bc | 9.7 ± 0.761ab |
| US-10 | 87.32 ± 3.58c | −0.64 ± 0.297bc | 10.19 ± 0.704ab |
| US-15 | 89.89 ± 0.44bc | −0.43 ± 0.081ab | 10.67 ± 1.429a |
| SD-170 | 95.92 ± 0.26a | −0.93 ± 0.022c | 2.92 ± 0.24c |
| SD-185 | 95.52 ± 0.38a | −0.97 ± 0.041c | 2.91 ± 0.1c |
| SD-200 | 95.2 ± 0.49a | −0.89 ± 0.034c | 2.89 ± 0.064c |
Control: freeze-dried CWP; SD: spray-dried samples at 170 °C (SD-170), 185 °C(SD-185), and 200 °C (SD-200); US: Sonicated samples for 5 mins (US-15), 10 mins (US-10), and 15 mins (US-15). Values are expressed as mean ± SD.
3.2.2. Particle size and charge distribution of CWP
Particle sizes of control, US, and SD samples are presented in Table 2. Control sample displayed a bimodal size distribution with two intensity peaks centered around 82 nm and 468 nm, respectively, and with z-average diameter and polydispersity index (PDI) of 878.3 nm and 0.849, respectively. US samples showed a significant decrease in particle size compared to the control sample (878.3 nm). When the samples were treated at 5, 10, and 15 min with constant US power of 200 W at 20 kHz, a bimodal size distribution with the intensity peaks around (78 nm and 441 nm), (53 nm and 290 nm), and (44 nm and 255 nm), respectively, and with z-average diameter and PDI of 778.3 nm and 0.892, 466.1 nm and 0.469, and 360.8 nm and 0.692, respectively was reported. A previous study reported that treatment of camel milk casein and whey particles with US probe for 45 min at 400 W resulted in a 50 % reduction in the average particle diameter [12]. Another study reported that treating WP at a 20 kHz US probe for 15–30 min decreased the particle size [11]. The obvious decrease of particle size of WP after sonication can be linked to the cavitation phenomenon in which hotspot temperature and pressure is released where the bursted cavitation bubbles were located and this high temperature and pressure are capable of breaking-down polymers and particles. For the surface charge, there was no significant difference between US samples compared to the control sample (−18.7 mV). All the samples had particle charge in the range of −18.3 mV to −21.5 mV, except US sample at 15 min which showed charge values of −21.6 mV. A previous study reported that treating WPI from bovine with US for 20 min at 20 % amplitude achieved a significant increase in the zeta potential from −14.9 mV to −27.94 mV [38].
Table 2.
Particle size (z-average), polydispersity index (PDI) and zeta potential for the ultrasonicated (US) and spray-dried (SD) CWP powders.
| Samples | Z-average (nm) | Poly dispersity index (PDI) | Zeta potential (mv) |
|---|---|---|---|
| Control | 878.3 ± 10.9a | 0.692 ± 0.021c | −18.7 ± 0.9ab |
| SD-170 | 400.0 ± 15.5d | 0.447 ± 0.032d | −18.6 ± 1.3ab |
| SD-185 | 215.1 ± 6.4f | 0.643 ± 0.048c | −21.6 ± 0.7b |
| SD-200 | 352.7 ± 10.7e | 0.426 ± 0.053d | −17.2 ± 1.8a |
| US-5 | 778.3 ± 21.3b | 1.000 ± 0.000a | −18.3 ± 0.6ab |
| US-10 | 466.1 ± 14.1c | 0.849 ± 0.011b | −18.9 ± 1.4ab |
| US-15 | 360.8 ± 4.2e | 0.469 ± 0.035d | −21.5 ± 2.3b |
Control: freeze-dried CWP; SD: spray-dried samples at 170 °C (SD-170), 185 °C(SD-185), and 200 °C (SD-200); US: Sonicated samples for 5 mins (US-15), 10 mins (US-10), and 15 mins (US-15). Values are expressed as mean ± SD.
SD samples at 170, 185, and 200 °C exhibited a noticeable reduction in particle size compared to the control sample. SD samples displayed a trimodal size distribution with three intensity peaks around (47 nm, 348.2, and 5341 nm), (24.26 nm, 319.0, and 5017), and (38 nm, 373.1 nm, and 4799 nm), respectively. The average particle size and PDI of SD samples processed at 170, 185, and 200 °C outlet temperature was (400.0 nm and 0.447), (215.1 nm and 0.643), and (352.7 nm and 0.426), respectively. A previous study has reported that during SD of skimmed camel milk at 160 °C, 180 °C, and 200 °C a decrease in the particle size was noticed with increase in the SD temperature with values of 12.6 µm, 9.2 µm, and 8.8 µm, respectively, which confirm that temperature have a significant effect in decreasing the particle size during SD treatment [18]. Previous study done by Park et al., [39] reported that SD BWP concentrated at 180 °C achieved a lower particle size of 36.8 µm when compared to 200 °C and 220 °C with particle sizes of 41.0 µm and 41.7 µm which are in lined with results obtained in this study as SD at 180 °C achieved lower particle size than SD at 200 °C. The surface charge of spray-dried CWP at 170 °C (−18.6 mV), 185 °C (−21.6 mV), and 200 °C (−17.2 mV) showed no significant difference when compared to the surface charge of the control sample (−18.7 mV), whereas, there was no significant difference observed between US samples compared to control sample which all having particle charge in the range of −18.3 mV to −21.5 mV, with −21.6 mV belong to US sample for 15 min. A previous study reported that SD concentrated (28 %) camel milk at 190 °C yielded powder with more surface hydrophobicity than powders produced by SD at 200 °C and 210 °C [40]. Overall, both US and SD samples showed a significant reduction in particle size compared to the control sample. US samples displayed a bimodal size for particle distribution, whereas SD samples displayed a trimodal size particle distribution. SD samples exhibited the highest reduction in particle size and better size distribution within all tested samples.
3.3. Technological and functional properties of CWP
3.3.1. Protein solubility in water
Protein solubility is an important functional attribute that influences several functional properties such as gelation, emulsification, and foaming. As shown in Fig. 4b, all samples show significantly high protein solubility (P < 0.05). US samples treated for 15 min showed the highest protein solubility (100 %), which is significantly higher than US samples for 5 min and 10 min, respectively (Fig. 4b). SD samples at 170 °C had a solubility of 94.7 %, further increasing the SD temperature to 185 °C and 200 °C, slightly reduced the solubility to 91.74 and 92.67, respectively. Moreover, US-15 and the SD-170 samples had higher protein solubilities when compared to the control sample, that had a protein solubility of 92.71 %. A previous study reported that reducing the molecular weight of the protein has increased the protein hydrolysates solubility [25]. Deshwal et al., [29] reported that SD whole and skim camel milk powder at 170 °C had solubilities of 65.47 % and 72.96 %, respectively and claimed that SD camel milk powders had significantly lower solubility than the freeze-dried sample as freeze-dried whole camel milk powder had 88.77 % protein solubility. This claim is both against and in agreement with the results obtained in this study, as samples treated with US for 5–10 min and SD at 185 °C and 200 °C had produced samples with protein solubility lower than the control sample while increasing the US treatment to 15 min and adjusting the inlet SD temperature at 170 °C have significantly increased the solubility of the CWP to surpass the control sample. Another previous study reported that SD of 2.4 % camel milk at 160 °C produced camel milk powder with 98.62 % protein solubility [3]. Zouari et al., [41] reported that spray-dried camel milk at 180 °C had a protein solubility of 96.2 % which is close to the protein solubility achieved in this study by SD at 170 °C (94.7 %). Perusko et al., [39] also reported that increasing the SD temperature from 170 °C to 250 °C maximized the loss of solubility of camel milk protein, which supported the results obtained in this study that SD-170 samples had higher protein solubilities when compared to SD-185 and SD-200, respectively (Fig. 4b). A previous study reported that US treatment of bovine WPC for 1 min at 31 W slightly retained the solubility of the samples around 90 % after 60 days of storage at 25 °C, while non-sonicated bovine WPC stored under the same conditions exhibited solubilities around 50 % [42]. A study done by Yanjun et al., [43] on bovine milk protein concentrates that have initial solubility of 35.78 % reported that US treatment of the samples for 5 min then SD of the sonicated samples at 130 °C have significantly increased the sample’s solubility to 88.30 %. This significant increase in solubility after US treatment is attributed to the ability of US to break down the static interaction between COO– and NH-2 located on the surface of the protein and cause more protein dispersion, which subsequently improves the solubility. Results of this study showed that US-15 samples exhibited the highest protein solubility (100 %) since US treatment generates more peptides that can have exposed hydrophilic groups and thus display an increase in the solubility of the powder. Further increasing the SD temperature from 185 °C to 200 °C showed slightly reduced the solubility (P > 0.05), as the CWP particle size produced after SD at 200 °C was higher than the particle size of powder produced at 185 °C.
3.3.2. Fat absorption capacity (FAC)
The protein’s ability to absorb fat and form strong interactions with oils in emulsions is considered a valuable attribute that can be utilized in food formulations. There are many factors that affect the protein’s FAC such as the quantity of protein used, protein-lipid-carbohydrate interactions, and the number of nonpolar sites [25]. Previous study reported that protein powders with low density and small particles have improved FAC since the increased protein surface area will entrap more volumes of oil than protein powder with big particles and high density [44]. In this study, treating CW with US for 5, 10, and 15 min had a negative effect on the FAC of the sonicated samples (Fig. 4c). The obtained FAC values were 1197.5, 1230.8, and 1251.3 mg/g, respectively which is lower than the FAC of the control sample that had a FAC of 1612.0 mg/g. However, a previous study that treated bovine WPI using US has reported that sonication has exponentially improved the oil holding capacity (OHC) of treated samples [45]. Al-Dowaila et al., [5] also reported that the FAC of freeze-dried skimmed camel whey protein samples collected from four different camel breeds ranged from 1617.70-1811.26 mg/g. SD at 170 °C, 185 °C, and 200°C have produced CWP samples that have very comparable but lower FAC values (1531.8, 1591.7, and 1605.8 mg/g, respectively) when compared to the FAC of the control samples (Fig. 4c). Freeze-dried whole camel milk samples had FAC of 1850 mg/g, while spray-dried skimmed and whole camel milk at 170 °C had FAC values of 7400 mg/g and 5460 mg/g [29]. A study reported that heating goat whey proteins (WP) at 65 °C for 30 min, 85 °C for 15 sec, 125 °C for 4 sec, and 135 °C for 4 sec have significantly improved the OHC of goat WP with the values of 5300, 5900, 5100, and 5000 mg/g, respectively compared to the OHC of the control sample (3600 mg/g) [46]. Moreover, enzymatic hydrolyses of skimmed camel whey protein using alcalase, bromelain, and papain have significantly reduced the FAC of the hydrolyzed samples (650.26, 644.53, and 641.06 mg/g, respectively) when compared to unhydrolyzed skim camel milk (714.43 mg/g) [24]. The findings of this study showed that the control CWP had an FAC value of 1612.0 mg/g which was higher than US and SD samples. However, further increasing the US treatment time and SD temperature resulted in increased FAC of samples.
3.3.3. Emulsifying activity index (EAI)
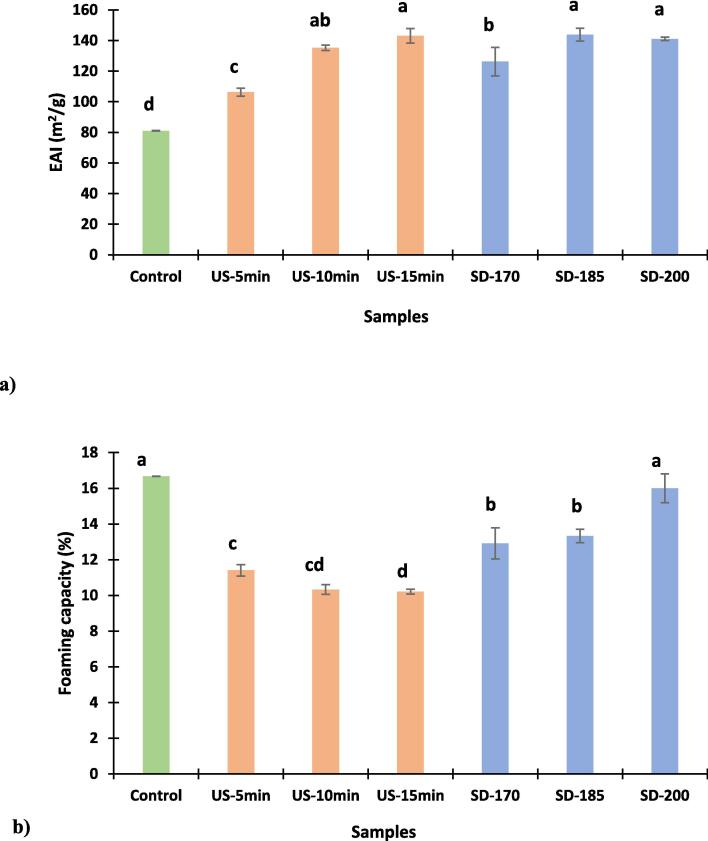
Milk proteins are known for their good emulsifying properties owing to their amphiphilic nature, high surface activity, and rapid unfolding and adsorption at the oil/water interface [25]. As shown in Fig. 5(a) sonication has significantly improved the samples’ EAI. US-15 samples showed the highest EAI (143.11 m2/g) among US samples, which is significantly higher than US-5 (106.25 m2/g) and the control sample (81.04 m2/g) as depicted in Fig. 5(a). The noticeable increase in EAI after sonication can be linked to the ability of ultrasound via cavitation to produce hot spot temperature that is capable of partially denaturing/unfolding the CW and exposing the hydrophobic side of the protein which subsequently improves the emulsifying properties. A previous study conducted by Shen et al., [38] reported that treating WPI with US for 20 min at 20 % amplitude before thermal aggregation or after thermal aggregation has considerably improved the EAI of the WPI. The presence of minerals enhances the ionic strength which lower the electrostatic repulsion and promote phase separation and bind to oppositely charged groups on the surface of emulsion droplets, decreasing their zeta potential and thus reducing electrostatic repulsion [10]. Untreated sample before and after thermal aggregation had EAI value of 3.6 m2/g, whereas post-treated WPI with US had EAI value of 5.25 m2/g and EAI of post treated WPI with US was 6.1 m2/g, which supports the claim that US treatment of protein to a certain extend had a positive effect on improving the EAI. Enzymatic hydrolysis of skimmed camel whey protein samples using three different enzymes alcalase, bromelain, and papain at different pH levels have significantly increased their EAI when compared to unhydrolyzed skimmed camel whey protein. The positive impact of enzymatic hydrolysis on EAI of skimmed camel whey protein was caused by the exposure of more hydrophobic functional groups when the skimmed camel whey protein was broken-down. Among the hydrolysed samples, Papain hydrolyzed samples had the highest EAI (86.135 m2/g), followed by bromelain (70.132 m2/g) and alcalase (68.901 m2/g), while unhydrolyzed camel milk proteins displayed the lowest EAI value (55.361 m2/g) [25]. EAI values obtained in this current study where significantly higher than EAI values obtained after enzymatic hydrolysis using above mentioned enzymes, which confirm that the level of denaturation/unfolding achieved by US treatment up to 15 min and SD at 185 °C was more suitable to enhance the EAI. The FTIR results obtained in this study have shown peaks around 1000–1100 cm−1 with can be attributed to the presence of lactose (Fig. 1). A study done by Zayas, [47], reported that the presence of lactose in high amounts in whey protein concentrate hinders protein propagation on the interface surface consequently might reduce the sample’s EAI. All SD samples in this study exhibited higher EAI when compared to the control samples due to the heat-induced unfolding of the protein. SD-170 samples had EAI values of 126.19 m2/g, further increase of SD temperature to 185 °C has significantly increased the EAI to 143.75 m2/g (Fig. 5a). However, when SD was performed at 200 °C, a slight decrease in the EAI of CWP was noticed. The EAI of proteins can be reduced when excessive denaturation/unfolding of protein takes place and negatively affects the protein’s surface hydrophobicity [48]. A previous study reported that SD of bovine WPC powder (76.8 %) at 145 °C while adjusting the pH of the solution to 3 and 10 have significantly improved the EAI, with EAI of 94 m2/g for WPC-SD-pH3 and 95 m2/g for WPC-SD-pH10 when compared to control sample (87 m2/g) [3]. The pH of protein solution is considered one of the main factors influencing the EA of proteins, where the EA of protein is minimum at the protein’s isoelectric point and maximum at acidic and alkaline pH [5]. Overall, the results presented in Fig. 5a showed that EAI has been significantly improved with increasing the US treatment time. For SD samples, samples dried at 185 °C exhibited the best EAI among all SD samples.
Fig. 5.
Emulsifying activity index (EAI) (a) and Foaming capacity (FC) (b) of freeze-dried (control), US, and SD of CWP samples. Values presented are mean ± standard deviation (n = 3). Different letters on the different bars indicates that there is a significant difference (P < 0.05). For keynotes, please see footnote of Table 1.
3.3.4. Foaming capacity (FC)
The mechanical agitation of a liquid mixture containing WP, will lead to the absorption and formation of gas bubbles, which subsequently produce foam. The mechanical and structural properties of air/water interfaces need to be understood to control the behavior of the foam system [30]. The good surface activity of WPs allows them to be used as stabilizers in food systems as a functional ingredient [18]. US treatment had a negative effect on FC of sonicated samples as the FC kept reducing with increasing the US treatment time, with highest FC (11.41 %) was observed for sample US treated for 5 min (Fig. 5b). Freeze-dried samples that served as control possessed the highest FC (16.67 %). Sonication of WPC powder for 2.5, 5, and 7.5 min was not effective in improving the FC of treated WPC with average FC of all samples was around 40 % and only slight improvement in FC was noticed after samples were treated for 7.5 min with FC of 45 % [21]. A previous study that investigated treating of WPI using high-pressure homogenization (120 MPa) coupled with US (600 W, 30 min) has reported a 26.10 % increase in the FC of samples [49]. Regarding the potential presence of lactose in the CWP prepared in this study. according to the study done by Ho et al., [50], lactose has high water holding capacity and its presence can increase the viscosity of the sample and hinder the molecular diffusion and protein adsorption on the interfacial region and subsequently reduce the foaming ability. It was noticed in this study that increasing the inlet temperature during SD has enhanced the FC of SD samples (Fig. 5b). However, when comparing the FC of SD samples to the control samples it can be concluded that both SD and US treatment did not improve the FC of CWP samples. A previous study stated that fresh camel milk was dried using freeze-dryer and spray dryer, then measured the effect of both drying methods on FC has reported that SD of fresh camel milk produced powders with better FC (40.88 %) when compared with freeze-dried powder (25.41 %) [29]. These results contradict the findings of this current study that found the control CWP had higher FC than SD samples. The low FC of CWP samples tested in this study can be linked to the fact that camel milk does not have β-LG and has a high α-LA, thereby affecting its complexation when heated, and it can have anti-foaming property [29]. Previous studies reported that partial denaturation of proteins can improve the FC. However, excessive degradation can have a negative effect on the FC [[3], [46], [47]]. Various factors, such as protein concentration, protein conformation, pH, temperature, speed of whipping, method of foaming, and mixing time can also affect the FC of proteins [25]. It is reported that subjecting CWP samples to high temperatures will produce CWP with low electrostatic repulsion that will allow better protein absorption at the air/water interface and hence the formation of stable foams [29]. The current study reported that ultrasound treatment and spray-drying had a negative effect on the FC, while the highest FC percentage belonged to the control samples.
3.4. Conclusion
This study reports the production of SD and US CWP samples and effect on physicochemical technological, and functional properties was investigated. SD samples exhibited more whiter color (L* values), lower average particle size and better size distribution compared to US and control samples. US samples exhibited a decrease in the FAC compared to control samples, while EAI was improved, and FC was reduced after US and SD treatment. US treatment showed higher degradation of the protein bands with US-15 samples showing the highest DH. Spray drying was able to produce uniform spherical shaped particles with no sign of aggregation. As per our knowledge, this study is the first to evaluate an efficient drying method to produce CWP from camel milk with desired physicochemical, technological, and functional properties. CWP can be utilized as a functional ingredient in the production of several food products.
4. Data availability statement
All data generated or analyzed during this study are included in this published article.
5. Author's contribution
A. Al-Thaibani conducted the experiments and wrote the first draft. H. Mostafa: experimentation, writing-original draft, data analysis, M. Alalawi: methodology, investigation. A. Sboui: Writing: Revision and editing; suggestions. Mudgil and F, Hamed: experimentation and revised the manuscript. S. Maqsood: conceptualization, validation, supervision, funding, writing: reviewing and editing.
6. Declarations
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: Not applicable.
Funding: No funding was obtained for this study
CRediT authorship contribution statement
Alanoud Al-Thaibani: Writing – original draft, Investigation, Formal analysis. Hussein Mostafa: Writing – original draft, Investigation, Formal analysis. Mariam Al Alawi: Methodology, Investigation. Amel Sboui: Writing – review & editing. Fathalla Hamed: Writing – review & editing, Formal analysis. Priti Mudgil: Writing – review & editing, Methodology, Formal analysis, Conceptualization. Sajid Maqsood: Writing – review & editing, Validation, Supervision, Resources, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors are thankful to United Arab Emirates University for funding this grant through a Summer Undergraduate Research Experience-Plus (SURE-Plus) project with grant code G00003973 awarded to PI-Sajid Maqsood.
References
- 1.S. Saibhavana, S.M. Vasukhi, R. Shreya, R. Rajakumari, A.S. Abhijith, K.S. Adithya, C. Deepak, Prospective nutritional, therapeutic, and dietary benefits of camel milk making it a viable option for human consumption: current state of scientific knowledge, (2023).
- 2.Food and Agriculture Organization of the United Nations (FAO). (2024) Camels. [Online] https://www.fao.org/dairy-production-products/dairy-animal/camels/en#:∼:text=The%20global%20camel%20population%20is,percent%20of%20total%20milk%20production.
- 3.Ho T.M., Chan S., Yago A.J.E., Shravya R., Bhandari B.R., Bansal N. Changes in physicochemical properties of spray-dried camel milk powder over accelerated storage. Food Chem. 2019;295:224–233. doi: 10.1016/j.foodchem.2019.05.122. [DOI] [PubMed] [Google Scholar]
- 4.A.A. Redha, H. Valizadenia, S.A. Siddiqui, S. Maqsood, A state-of-art review on camel milk proteins as an emerging source of bioactive peptides with diverse nutraceutical properties. Food Chemistry, (2022) 373, 131444. [DOI] [PubMed]
- 5.Maqsood S., Al-Dowaila P., Mudgil H., Kamal B., Jobe H.M. Hassan, Comparative characterization of protein and lipid fractions from camel and cow milk, their functionality, antioxidant and antihypertensive properties upon simulated gastro-intestinal digestion. Food Chem. 2019;279:328–338. doi: 10.1016/j.foodchem.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Badr G., Ramadan N.K., Sayed L.H., Badr B.M., Omar H.M., Selamoglu Z. Why whey? Camel whey protein as a new dietary approach to the management of free radicals and for the treatment of different health disorders. Iran. J. Basic Med. Sci. 2017;20(4):338. doi: 10.22038/IJBMS.2017.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lajnaf R., Attia H., Ayadi M.A. Technological properties and biological activities of camel α-lactalbumin – a review. Int. Dairy J. 2023;139 doi: 10.1016/j.idairyj.2022.105563. [DOI] [Google Scholar]
- 8.Kamal H., Jafar S., Mudgil P., Hamdi M., Ayoub M.A., Maqsood S. Camel whey protein with enhanced antioxidative and antimicrobial properties upon simulated gastro-intestinal digestion. Nutr. Health. 2022 doi: 10.1177/02601060221122213. [DOI] [PubMed] [Google Scholar]
- 9.Ayoub M.A., Palakkott A.R., Ashraf A., Iratni R. The molecular basis of the anti-diabetic properties of camel milk. Diabetes Res. Clin. Pract. 2018;146:305–312. doi: 10.1016/j.diabres.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Amalfitano N., Patel N., Haddi M.L., Benabid H., Pazzola M., Vacca G.M., Bittante G. Detailed mineral profile of milk, whey, and cheese from cows, buffaloes, goats, ewes and dromedary camels, and efficiency of recovery of minerals in their cheese. J. Dairy Sci. 2024 doi: 10.3168/jds.2023-24624. [DOI] [PubMed] [Google Scholar]
- 11.Jambrak A.R., Mason T.J., Lelas V., Paniwnyk L., Herceg Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 2014;121:15–23. doi: 10.1016/j.jfoodeng.2013.08.012. [DOI] [Google Scholar]
- 12.Gammoh S., Alu’datt M.H., Tranchant C.C., Al-U’datt D.G., Alhamad M.N., Rababah T., Kubow S., Haddadin M.S.Y., Ammari Z., Maghaydah S., Banat H. Modification of the functional and bioactive properties of camel milk casein and whey proteins by ultrasonication and fermentation with Lactobacillus delbrueckii subsp. lactis. LWT. 2020;129 doi: 10.1016/j.lwt.2020.109501. [DOI] [Google Scholar]
- 13.Khatkar A.B., Kaur A., Khatkar S.K., Mehta N. Characterization of heat-stable whey protein: Impact of ultrasound on rheological, thermal, structural and morphological properties. Ultrason. Sonochem. 2018;49:333–342. doi: 10.1016/j.ultsonch.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Meng Y., Liang Z., Zhang C., Hao S., Han H., Du P., Li A., Shao H., Li C., Liu L. Ultrasonic modification of whey protein isolate: Implications for the structural and functional properties. LWT. 2021;152 doi: 10.1016/j.lwt.2021.112272. [DOI] [Google Scholar]
- 15.Habtegebriel H., Edward D., Wawire M., Sila D., Seifu E. Effect of operating parameters on the surface and physico-chemical properties of spray-dried camel milk powders. Food Bioprod. Process. 2018;112:137–149. doi: 10.1016/j.fbp.2018.09.010. [DOI] [Google Scholar]
- 16.Ogolla J.A., Kulig B., Bădulescu L., Okoth M.W., Esper G., Breitenbach J., Hensel O., Sturm B. Influence of inlet drying air temperature and milk flow rate on the physical optical and thermal properties of spray-dried camel milk powders. Food Bioproc Tech. 2019;12:751–768. doi: 10.1007/s11947-019-2243-5. [DOI] [Google Scholar]
- 17.Hailu Y., Hansen E.B., Seifu E., Eshetu M., Ipsen R., Kappeler S. Functional and technological properties of camel milk proteins: a review. J. Dairy Res. 2016;83:422–429. doi: 10.1017/S0022029916000686. [DOI] [PubMed] [Google Scholar]
- 18.Seifu E. Camel milk products: innovations, limitations and opportunities. Food Production, Processing and Nutrition. 2023;5(1):15. [Google Scholar]
- 19.Zouari A., Lajnaf R., Lopez C., Schuck P., Attia H., Ayadi M.A. Physicochemical, techno-functional, and fat melting properties of spray-dried camel and bovine milk powders. J. Food Sci. 2021;86:103–111. doi: 10.1111/1750-3841.15550. [DOI] [PubMed] [Google Scholar]
- 20.Carter B., Patel H., Barbano D.M., Drake M. The effect of spray drying on the difference in flavor and functional properties of liquid and dried whey proteins, milk proteins, and micellar casein concentrates. J. Dairy Sci. 2018;101:3900–3909. doi: 10.3168/jds.2017-13780. [DOI] [PubMed] [Google Scholar]
- 21.Ahmadi Z., Razavi S.M.A., Varidi M. Sequential ultrasound and transglutaminase treatments improve functional, rheological, and textural properties of whey protein concentrate. Innov. Food Sci. Emerg. Technol. 2017;43:207–215. doi: 10.1016/j.ifset.2017.08.013. [DOI] [Google Scholar]
- 22.Jafar S., Kamal H., Mudgil P., Hassan H.M., Maqsood S. Camel whey protein hydrolysates displayed enhanced cholesteryl esterase and lipase inhibitory, anti-hypertensive and anti-haemolytic properties. LWT. 2018;98:212–218. doi: 10.1016/j.lwt.2018.08.024. [DOI] [Google Scholar]
- 23.Kamal H., Jafar S., Mudgil P., Murali C., Amin A., Maqsood S. Inhibitory properties of camel whey protein hydrolysates toward liver cancer cells, dipeptidyl peptidase-IV, and inflammation. J. Dairy Sci. 2018;101:8711–8720. doi: 10.3168/jds.2018-14586. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad M., Mudgil P., Gani A., Hamed F., Masoodi F.A., Maqsood S. Nano-encapsulation of catechin in starch nanoparticles: characterization, release behavior and bioactivity retention during simulated in-vitro digestion. Food Chem. 2019;270:95–104. doi: 10.1016/j.foodchem.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Al-Shamsi K.A., Mudgil P., Hassan H.M., Maqsood S. Camel milk protein hydrolysates with improved technofunctional properties and enhanced antioxidant potential in in vitro and in food model systems. J. Dairy Sci. 2018;101:47–60. doi: 10.3168/jds.2017-13194. [DOI] [PubMed] [Google Scholar]
- 26.Chen D., Liu Z., Huang W., Zhao Y., Dong S., Zeng M. Purification and characterisation of a zinc-binding peptide from oyster protein hydrolysate. J. Funct. Foods. 2013;5:689–697. doi: 10.1016/j.jff.2013.01.012. [DOI] [Google Scholar]
- 27.Zhao L., Huang S., Cai X., Hong J., Wang S. A specific peptide with calcium chelating capacity isolated from whey protein hydrolysate. J. Funct. Foods. 2014;10:46–53. doi: 10.1016/j.jff.2014.05.013. [DOI] [Google Scholar]
- 28.Ye Q., Woo M.W., Selomulya C. Modification of molecular conformation of spray-dried whey protein microparticles improving digestibility and release characteristics. Food Chem. 2019;280:255–261. doi: 10.1016/j.foodchem.2018.12.074. [DOI] [PubMed] [Google Scholar]
- 29.Deshwal G.K., Singh A.K., Kumar D., Sharma H. Effect of spray and freeze drying on physico-chemical, functional, moisture sorption and morphological characteristics of camel milk powder. LWT. 2020;134 doi: 10.1016/j.lwt.2020.110117. [DOI] [Google Scholar]
- 30.Meng Y., Li C. Conformational changes and functional properties of whey protein isolate-polyphenol complexes formed by non-covalent interaction. Food Chem. 2021;364 doi: 10.1016/j.foodchem.2021.129622. [DOI] [PubMed] [Google Scholar]
- 31.Malik M.A., Saini C.S. Rheological and structural properties of protein isolates extracted from dephenolized sunflower meal: effect of high intensity ultrasound. Food Hydrocoll. 2018;81:229–241. doi: 10.1016/j.foodhyd.2018.02.052. [DOI] [Google Scholar]
- 32.Li K., Pan B., Ma L., Miao S., Ji J. Effect of dextrose equivalent on maltodextrin/whey protein spray-dried powder microcapsules and dynamic release of loaded flavor during storage and powder rehydration. Foods. 2020;9:1878. doi: 10.3390/foods9121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y., Ling J., Li M., Su Y., Arte K.S., Mutukuri T.T., Taylor L.S., Munson E.J., Topp E.M., Zhou Q.T. Understanding the impact of protein-excipient interactions on physical stability of spray-dried protein solids. Mol. Pharm. 2021;18:2657–2668. doi: 10.1021/acs.molpharmaceut.1c00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W., Zhao P., Li J., Wang X., Hou J., Jiang Z. Effects of ultrasound synergized with microwave on structure and functional properties of transglutaminase-crosslinked whey protein isolate. Ultrason. Sonochem. 2022;83 doi: 10.1016/j.ultsonch.2022.105935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alizadeh O., Aliakbarlu J. Effects of ultrasound and ohmic heating pretreatments on hydrolysis, antioxidant and antibacterial activities of whey protein concentrate and its fractions. LWT. 2020;131 doi: 10.1016/j.lwt.2020.109913. [DOI] [Google Scholar]
- 36.Yang S., Tu Z., Wang H., Huang T. The reduction in the immunoglobulin G and immunoglobulin E binding capacity of β-lactoglobulin via spray-drying technology. J. Dairy Sci. 2020;103:2993–3001. doi: 10.3168/jds.2019-17322. [DOI] [PubMed] [Google Scholar]
- 37.Sulieman Abdel Moneim E., Elamin O.M., Elkhalifa E.A., Loouis L. Comparison of physicochemical properties of spray-dried camel’s milk and cow’s milk powder, international journal of food science and nutrition. Engineering. 2014;4:15–19. [Google Scholar]
- 38.Shen X., Fang T., Gao F., Guo M. Effects of ultrasound treatment on physicochemical and emulsifying properties of whey proteins pre- and post-thermal aggregation. Food Hydrocoll. 2017;63:668–676. doi: 10.1016/j.foodhyd.2016.10.003. [DOI] [Google Scholar]
- 39.Park C.W., Bastian E., Farkas B., Drake M. The effect of feed solids concentration and inlet temperature on the flavor of spray dried whey protein concentrate. J. Food Sci. 2014;79 doi: 10.1111/1750-3841.12279. [DOI] [PubMed] [Google Scholar]
- 40.Perusko M., Ghnimi S., Simovic A., Stevanovic N., Radomirovic M., Gharsallaoui A., Smiljanic K., Van Haute S., Stanic-Vucinic D., Cirkovic Velickovic T. Maillard reaction products formation and antioxidative power of spray dried camel milk powders increases with the inlet temperature of drying. LWT. 2021;143 doi: 10.1016/j.lwt.2021.111091. [DOI] [Google Scholar]
- 41.Zouari A., Schuck P., Gaucheron F., Triki M., Delaplace G., Gauzelin-Gaiani C., Lopez C., Attia H., Ayadi M.A. Microstructure and chemical composition of camel and cow milk powders’ surface. LWT. 2020;117 doi: 10.1016/j.lwt.2019.108693. [DOI] [Google Scholar]
- 42.Chandrapala J., Zisu B., Palmer M., Kentish S.E., Ashokkumar M. Sonication of milk protein solutions prior to spray drying and the subsequent effects on powders during storage. J. Food Eng. 2014;141:122–127. doi: 10.1016/j.jfoodeng.2014.05.017. [DOI] [Google Scholar]
- 43.Yanjun S., Jianhang C., Shuwen Z., Hongjuan L., Jing L., Lu L., Uluko H., Yanling S., Wenming C., Wupeng G., Jiaping L. Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate. J. Food Eng. 2014;124:11–18. doi: 10.1016/j.jfoodeng.2013.09.013. [DOI] [Google Scholar]
- 44.Kinsella J.E., Melachouris N. Functional properties of proteins in foods: a survey. CRC Critical Reviews in Food Science and Nutrition. 1976;7:219–280. doi: 10.1080/10408397609527208. [DOI] [Google Scholar]
- 45.Liu Z.D., Guo B.H., Su M.Y., Wang Y.Y. Effect of ultrasonic treatment on the functional properties of whey protein isolates. Adv. Mat. Res. 2012;443–444:660–665. doi: 10.4028/www.scientific.net/AMR.443-444.660. [DOI] [Google Scholar]
- 46.Zhao X., Cheng M., Zhang X., Li X., Chen D., Qin Y., Wang J., Wang C. The effect of heat treatment on the microstructure and functional properties of whey protein from goat milk. J. Dairy Sci. 2020;103:1289–1302. doi: 10.3168/jds.2019-17221. [DOI] [PubMed] [Google Scholar]
- 47.Zayas J.F. Springer Science & Business Media; 2012. Functionality of proteins in food. [Google Scholar]
- 48.Jain A., Prakash M., Radha C. Extraction and evaluation of functional properties of groundnut protein concentrate. J. Food Sci. Technol. 2015;52:6655–6662. doi: 10.1007/s13197-015-1758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi R., Liu Y., Hu J., Gao H., Qayum A., Bilawal A., Munkh-Amgalan G., Jiang Z., Hou J. Combination of high-pressure homogenization and ultrasound improves physiochemical, interfacial and gelation properties of whey protein isolate. Innov. Food Sci. Emerg. Technol. 2020;65 doi: 10.1016/j.ifset.2020.102450. [DOI] [Google Scholar]
- 50.Ho T.M., Xiong X., Bhandari B.R., Bansal N. Foaming properties and foam structure of milk determined by its protein content and protein to fat ratio. Food Bioproc. Tech. 2024;1–14 doi: 10.1007/s11947-024-03407-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.