Abstract
Left heart disease (LHD) is the most common cause of pulmonary hypertension (PH), which may be classified further as isolated post-capillary (ipcPH) or combined post- and pre-capillary PH (cpcPH). The 7th World Symposium on Pulmonary Hypertension PH-LHD task force reviewed newly reported randomised clinical trials and contemplated novel opportunities for improving outcome. Results from major randomised clinical trials reinforced prior recommendations against the use of pulmonary arterial hypertension therapy in PH-LHD outside of clinical trials, and suggested possible harm. Greater focus on phenotyping was viewed as one general strategy by which to ultimately improve clinical outcomes. This is potentially achievable by individualising ipcPH versus cpcPH diagnosis for patients with pulmonary arterial wedge pressure within a diagnostic grey zone (12–18 mmHg), and through a newly developed PH-LHD staging system. In this model, PH accompanies LHD across four stages (A=at risk, B=structural heart disease, C=symptomatic heart disease, D=advanced), with each stage characterised by progression in clinical characteristics, haemodynamics and potential therapeutic strategies. Along these lines, the task force proposed disaggregating PH-LHD to emphasise specific subtypes for which PH prevalence, pathophysiology and treatment are unique. This includes re-interpreting mitral and aortic valve stenosis through a contemporary lens, and focusing on PH within the hypertrophic cardiomyopathy and amyloid cardiomyopathy clinical spectra. Furthermore, appreciating LHD in the profile of PH patients with chronic lung disease and chronic thromboembolic pulmonary disease is essential. However, engaging LHD patients in clinical research more broadly is likely to require novel methodologies such as pragmatic trials and may benefit from next-generation analytics to interpret results.
Shareable abstract
Pulmonary hypertension (PH) associated with left heart disease is the most common cause of PH. We propose to disaggregate the classification to account for the novel approaches in cardiac disorders. We also propose a brand new staging of PH in this context. https://bit.ly/4fc5p1V
Introduction
Pulmonary hypertension (PH) is a distinct syndrome that affects many patients across the left heart disease (LHD) clinical spectrum [1, 2]. Pathogenetic changes to the morphology or function of virtually any structure between the aortic valve and large pulmonary veins can predispose to PH-LHD [3, 4]. Although PH is by-and-large associated with a change in risk profile among affected LHD patients, the therapeutic approach varies widely by underlying pathophysiology. This suggests it is timely to disaggregate PH-LHD classification, since a one-size-fit all approach to this pathophenotype is likely to miss opportunities for personalised clinical decision-making. There are three key objectives identified for the PH-LHD field by the current task force: 1) improved diagnostic accuracy; 2) appropriate treatment–patient alignment; and 3) optimising outcome. Making gains in these areas will require standardising the approach to clinical assessment [5], wider consideration to the multiorgan involvement of PH-LHD (i.e. broader phenotyping) [6] and utilising novel (i.e. decentralised) [7] clinical research methods to resolve areas of equipoise.
Definition and classification of PH-LHD
At the 6th World Symposium on Pulmonary Hypertension (WSPH), the mean pulmonary artery pressure (mPAP) threshold used to identify patients at risk for PH was decreased to >20 mmHg from  25 mmHg, [8] which is now supported by data from normative populations [9] and outcomes from large referral cohorts [10] as well as smaller studies involving well-phenotyped patients inclusive of LHD [11]. Convergent data from studies involving autopsy specimens [12] and echocardiography [13, 14] reaffirm that mildly elevated PAP is associated with substantial pulmonary arterial and venous remodelling and detrimental changes to right ventricular (RV) geometry and systolic function. Indeed, the underlying vascular pathology of PH-LHD is an important consideration to the true pathophysiological substrate [15], although imaging methods that visualise or quantitate this information remain forthcoming.
25 mmHg, [8] which is now supported by data from normative populations [9] and outcomes from large referral cohorts [10] as well as smaller studies involving well-phenotyped patients inclusive of LHD [11]. Convergent data from studies involving autopsy specimens [12] and echocardiography [13, 14] reaffirm that mildly elevated PAP is associated with substantial pulmonary arterial and venous remodelling and detrimental changes to right ventricular (RV) geometry and systolic function. Indeed, the underlying vascular pathology of PH-LHD is an important consideration to the true pathophysiological substrate [15], although imaging methods that visualise or quantitate this information remain forthcoming.
There are two haemodynamic classifications for PH-LHD: isolated post-capillary pulmonary hypertension (ipcPH) and combined post- and pre-capillary pulmonary hypertension (cpcPH). Since mPAP >20 mmHg may be observed in some patients with an immediately reversible or physiological state that increases pulmonary blood flow, the addition of pulmonary vascular resistance (PVR) to the haemodynamic criteria implicating pulmonary vascular disease in patients with PH is important. From large right heart catheterisation (RHC) referral cohorts enriched with LHD, PVR >2.0 Wood Units (WU) [16] is an independent risk factor for mortality and hospitalisation for heart failure. This observation is generally consistent with findings from other forms of pre-capillary PH [17], and, thus, PVR >2.0 WU is used to classify patients with a pre-capillary component of PH [18, 19], and has recently been confirmed in heart failure to predict outcome [20].
Using the appropriate methodology for measuring the pulmonary artery wedge pressure (PAWP) is essential to diagnose PH-LHD, and has been discussed in detail previously [21]. It has been proposed that in most situations the PAWP be measured at end-expiration and end-diastole (the latter most closely approximating left ventricular (LV) end-diastolic pressure). However, measuring at end-diastole minimises the contributions of V-waves, which are typically a result of mitral or left atrial pathology, to pulmonary pressure. Therefore, the mean PAWP that encompasses the V-wave may be the most relevant when calculating PVR. In addition, variable methodologies for assessing PAWP in PH are used clinically and in trials, particularly utilisation of computer-generated mean values. These concerns are viewed as major points of vulnerability for diagnostic (mis-)classification [22, 23]. The dependency of the PAWP on volume load complicates interpretation further, so that defining the optimal PAWP threshold to differentiate between pre- and post-capillary PH remains challenging. The current framework is supported mainly by successful pulmonary arterial hypertension (PAH) clinical trials using ≤15 mmHg as enrolment criteria [24], as well as mixed results (including concern for harm) when PAH-approved therapies are administered to patients with PAWP >15 mmHg [25].
Data from retrospective analyses in referral populations suggest that resting PAWP 12–15 mmHg may predispose to, or even represent a mild form of, post-capillary PH [26]. However, correlating PAWP >12 mmHg with evidence of pulmonary vein or arterial end-organ injury, or other concise PH end-points from prospective studies has not been shown. Furthermore, the relationship between age, sex, loading conditions, interpretation variances, physiological variance in LV structure, and other parameters with the upper limit of normal PAWP have not been reported systematically. Determining the PAWP range that defines post-capillary PH requires clinical trials that are designed to establish the upper limit of PAWP above which therapies for pre-capillary PH are ineffective, and may require novel designs that prioritise real-world data (discussed in the Clinical trial target populations, end-points and designs section). Recently, a zone of PAWP “uncertainty” has been suggested of PAWP 12–15 mmHg, possibly extended to 18 mmHg, for which PAWP is used in context with all imaging and clinical variables (including pre-test probability of PH-LHD) to phenotype and categorise PH patients [27].
Contextualising haemodynamics
In PH-LHD, dichotomising RHC data as above versus below diagnostic thresholds for mPAP, PVR, and PAWP may detract from the risk continuum associated with these haemodynamic measurements, as well as the importance of cardiac function and the clinical profile of individual patients. For example, right atrial pressure/PAWP ratio >0.5 at rest in PH-LHD could suggest biventricular heart failure and would direct attention to quantitative data on right and left ventricular geometry and systolic function. There are accumulating data suggesting that the ratio of stroke volume to pulmonary pulse pressure (a surrogate for pulmonary artery compliance (PAC)) is associated with adverse outcome in heart failure patients. An incremental decrease in PAC appears evident in patients with pathogenic elevation in mPAP despite PVR <2.0 WU, suggesting that greater attention should be placed on PAC when assessing early-stage pulmonary vascular disease [28]. Nonetheless, the precise interaction between pulmonary venous hypertension and clinical utility of PAC is not known and requires further clarification in PH-LHD. The role of pre-test probability of PH-LHD (especially in heart failure with preserved ejection fraction (HFpEF)) and provocative testing in the catheterisation laboratory is discussed later.
Recommendations, gaps in evidence and unmet needs
PAWP measurement should be interpreted in the clinical context of LHD, especially in patients with risk factors for LHD or established LHD with a value in the zone of uncertainty.
The PAWP range of uncertainty is probably 12–15 mmHg, although this may extend to 18 mmHg.
Disaggregating PH-LHD
There is a pressing need to improve alignment between PH-LHD substrate and clinical decision-making, since the clinical profile, pathophysiology, prognosis and treatment approach to PH varies across different forms of primary cardiac disease and overlapping phenotypes are common (i.e. functional mitral valvular regurgitation and heart failure). Here, we categorise the clinical relevance, therapeutic opportunities and key unanswered questions for patients with PH and different forms of LHD.
PH in mitral stenosis
Post-streptococcal rheumatic fever affects ∼30 million individuals, largely in the endemic regions of East Asia, South Asia, Oceania and central sub-Saharan Africa [29]. Inflammatory changes to mitral valve leaflets cause thickening, retraction and fusion at the commissure. This leads to mitral stenosis that causes left atrial hypertension and ipcPH or cpcPH in a large subset of patients, suggesting that rheumatic heart disease is the most common risk factor for PH-LHD globally [30]. Classical data on mitral stenosis patients with rheumatic heart disease or calcific valvular remodelling (that is due to ageing without prior infection) indicate that elevated PVR >6 WU may represent a pre-capillary resistor that protects against pulmonary oedema, which resolves following valve correction [31]. However, contemporary prospective series indicate that normalisation of PVR fails to occur longitudinally in ∼40% of symptomatic patients with severe mitral stenosis despite successful percutaneous balloon mitral valvuloplasty or mitral valve replacement [32–34], and that pre-procedural PH is a risk factor for acute and long-term adverse outcome in this subgroup [35].
Approach to management
We agree with other societies that a noninvasive estimate of pulmonary artery systolic pressure (PASP) >50 mmHg should be viewed as a potential indication for catheter-directed or surgical intervention in asymptomatic patients with severe mitral stenosis [36], recognising that adverse pulmonary vascular remodelling represents a later manifestation of disease and early intervention (prior to these changes) may improve outcome [37], In addition, we support the recommendation from the European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines that RHC should be performed prior to valvular interventions [18, 19], when PH is deemed to affect outcome and plays a role in treatment decision. PAH medications should be avoided in patients with moderate or severe mitral stenosis and PH due to concern for precipitating pulmonary oedema and lack of evidence in support of a clinical benefit in these patients.
Recommendations, gaps in evidence and unmet needs
Prospective studies using the most recent haemodynamic classification criteria for PH to risk stratify mitral valve disease patients are needed.
Data on the relevance of PVR >2.0 WU or other haemodynamic measures in addition to PAP for determining surgical or catheter-based valvular intervention candidacy are lacking.
The optimal approach to managing residual PH in patients following mitral valvular intervention is not known, but represents an overlooked subgroup in PH-LHD.
PH in aortic valve stenosis
The prevalence of PH is substantial in aortic valve stenosis, with contemporary estimates from community populations suggesting that 53% of patients have at least moderately elevated PAP [38]. The criteria used to define PH for the purpose of perioperative risk assessment vary widely across the literature (as well as at the point of care) and, in most cases, do not reference recent updates in the haemodynamic definitions (e.g. EuroSCORE II uses PASP >31 mmHg) [39]. Nonetheless, when present PH may prompt stronger consideration of transcatheter aortic valve replacement (TAVR) [40, 41], emphasising the importance of PH in prognosticating short-term and longitudinal treatment-response, heart failure readmission and other outcome measures following TAVR [42].
In one recent retrospective study of 579 consecutive patients referred for TAVR, ipcPH and cpcPH (per the 2022 ESC/ERS guideline definitions) [18, 19] were identified in 20% and 32% of patients, respectively. Elevated PVR >2.0 WU was an important risk-factor for post-procedural mortality risk, but was largely longitudinal (rather than acute), peaking >2 years post-TAVR [43]. These findings are directionally consistent with earlier retrospective data using the classical mPAP and PVR thresholds for PH of  25 mmHg and >3.0 WU, respectively, which suggested that all PH haemodynamic subgroups are associated with increased all-cause 1-year and 4-year mortality, particularly among patients with isolated pre-capillary PH [44]. Baseline left ventricular ejection fraction (LVEF) <30% and atrial fibrillation history associate with persistent PH following successful TAVR; however, models that focus on the pre-operative clinical profile to predict cardiopulmonary haemodynamics, overall, show inconsistent findings [45]. Since TAVR is often performed in elderly, frail patients with multiple cardiopulmonary comorbidities, information on nuanced PH clinical profiles at highest risk could be helpful for contemporising TAVR eligibility.
25 mmHg and >3.0 WU, respectively, which suggested that all PH haemodynamic subgroups are associated with increased all-cause 1-year and 4-year mortality, particularly among patients with isolated pre-capillary PH [44]. Baseline left ventricular ejection fraction (LVEF) <30% and atrial fibrillation history associate with persistent PH following successful TAVR; however, models that focus on the pre-operative clinical profile to predict cardiopulmonary haemodynamics, overall, show inconsistent findings [45]. Since TAVR is often performed in elderly, frail patients with multiple cardiopulmonary comorbidities, information on nuanced PH clinical profiles at highest risk could be helpful for contemporising TAVR eligibility.
Approach to management
Moderate or severe aortic valve stenosis can be a challenging confounder to clarifying the predominant underlying haemodynamic classification of PH in individual patients. At present, there is insufficient evidence to preclude TAVR in patients solely based on PH, although severe PH, particularly in the setting of RV dysfunction may ultimately associate with a unique treatment response and side-effect profile. PAH medications are not recommended to treat aortic valve stenosis prior to TAVR, nor as a challenge to discriminate PH haemodynamic subtype.
Recommendations, gaps in evidence and unmet needs
Data clarifying the relationship between PH severity/subtype and treatment responsiveness to TAVR as well as outcome post-TAVR are needed [46].
It is not known if certain forms of PH should be considered in the approach to determining TAVR inappropriateness in patients with other comorbidities.
Treatment of residual PH after TAVR remains unexplored.
PH in heart failure with reduced ejection fraction
Geometric changes to the mitral valve induced by chronic LV remodelling in the setting of ischaemic, infectious or dilated cardiomyopathy often enables pulmonary venous hypertension and is a major cause of PH in developed countries. For patients with LV systolic heart failure, chronic pulmonary venous hypertension is associated with functional PH (e.g. reversible with diuresis and/or afterload reduction); in a subset of patients, persistent elevation in PVR is evident despite normalisation of LV end-diastolic pressure. These patients are presumably characterised by initial left atrial hypertension that interacts with risk factors or specific patient characteristics that drive transition from ipcPH to cpcPH (resistant to pre-load reduction/diuresis) [47], but further natural history studies are needed to validate this. Severe PH is identified as one parameter defining high-risk mitral regurgitation [48], but systematically collected data on the relevance of PH for surgical repair or replacement or percutaneous mitral valve clip candidacy are lacking [49]. Development of PH in patients with heart failure with reduced ejection fraction (HFrEF) is associated with greater disease severity and reduced survival [50, 51], and excludes patients with advanced HFrEF and elevated PVR from cardiac transplantation as well as LV-assist device consideration in the presence of RV heart failure [52, 53],
Approach to management
The focus of PH care in patients with HFrEF should be on the underlying aetiology of heart failure, which often includes coronary artery disease, valvular disease or other nonischaemic drivers of cardiomyocyte dysfunction. Longitudinal optimisation of volume status is important to minimise pulmonary venous hypertension, which is a presumed risk factor for cpcPH. Since PH is a major determinant of outcome in patients across the heart failure spectrum, there is considerable interest in targeting modifiability of clinical risk through interventions that improve PH per se. Studies repurposing PAH therapy to HFrEF-PH patients are generally small (clinicaltrials.gov identifier NCT00309790) and associated with mixed results [54], Since the 6th WSPH, the Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction (VICTORIA) trial [55] was completed, which randomised 5050 patients with chronic heart failure and LVEF <45% to receive the soluble guanylyl cyclase (sGC) stimulator vericiguat or placebo in addition to guideline-based medical therapy. There was an absolute risk reduction for the primary end-point of death from any cause or hospitalisation for heart failure of 3.0% in the vericiguat group compared to the placebo group (hazard ratio 0.90, 95% CI 0.83–0.98; p=0.02). However, neither clinical data on the PH profile of the study patients nor the effect of therapy on key cardiopulmonary haemodynamic parameters were captured, thereby limiting insight on the mechanism of action by which sGC-modulating treatment, already in use for PAH, may have exerted benefit in the HFrEF cohort [56] (further context is given in the Future directions section).
The emergence of sodium glucose cotransporter (SGLT)-2 inhibitors in the management of patients with heart failure (across the LVEF spectrum, including HFpEF; discussed later) [57, 58] has raised interest in the potential salutary benefit of this drug class on PH-LHD given that the mechanism of action appears to mitigate key pathways involved in the pathobiology of cpcPH, including pro-inflammatory, metabolic, insulin-resistance and fluid-retention pathways (reviewed by Lopaschuck and Verma [59]). The Empagliflozin Evaluation by Measuring Impact on Hemodynamics in Patients With Heart Failure (EMBRACE-HF) [60] study was an investigator-initiated randomised, multicentre, double-blind, placebo-controlled trial that analysed the effect of SGLT-2 inhibitor therapy in patients with heart failure, including HFrEF, with a previously implanted CardioMems device for continuous PAP monitoring. After 1 week of treatment with empagliflozin, a decrease in pulmonary artery diastolic pressure was noted corresponding to an average 1.7 mmHg reduction at week 12 of the study over placebo that was not attributed to diuretic therapy. Directionally similar findings were observed in retrospective case series in which the effect of SGLT-2 inhibitor therapy including dapagliflozin on lowering PAP in patients with chronic heart failure was demonstrated [61, 62].
Recommendations, gaps in evidence and unmet needs
Data profiling the role of PH in the timing of procedural interventions that mitigate secondary mitral regurgitation, such as surgical repair or replacement or percutaneous mitral valve edge to edge repair, are needed to optimise patient candidacy.
A beneficial effect mediated by SLGT-2, glucagon-like peptide (GLP)-1, and angiotensin receptor neprilysin inhibitor therapies on PH is plausible biologically, but dedicated trials testing early clinical observations are needed, especially for patients with ipcPH versus cpcPH.
Understanding the relevance of PH to treatment response in patients that are managed through standard-of-care HFrEF therapeutics requires dedicated pulmonary vascular phenotyping in future clinical research programmes.
Specific cardiomyopathies
In obstructive hypertrophic cardiomyopathy (HCM), heart failure that limits quality of life (New York Heart Association functional class (NYHA FC) II–IV) is highly responsive to surgical myectomy in most patients [63]. Importantly, PH is common under this clinical scenario: in one study of 182 consecutive patients referred for myectomy, mPAP  25 mmHg was observed in 51%, PAWP >15 mmHg in 46%, and 32% had a haemodynamic profile consistent with pre-capillary PH with PVR >3.0 WU [64]. A subgroup of 11% of patients were identified who had PVR >3.0 WU despite PAWP ≤15 mmHg in the absence of significant comorbidities or mitral regurgitation, and with minimal use of diuretics. Data suggesting a consistent association between pre-operative PH and adverse outcome post-myectomy are lacking, and RV dysfunction that would otherwise be used to risk-stratify PH is a rare finding in HCM.
25 mmHg was observed in 51%, PAWP >15 mmHg in 46%, and 32% had a haemodynamic profile consistent with pre-capillary PH with PVR >3.0 WU [64]. A subgroup of 11% of patients were identified who had PVR >3.0 WU despite PAWP ≤15 mmHg in the absence of significant comorbidities or mitral regurgitation, and with minimal use of diuretics. Data suggesting a consistent association between pre-operative PH and adverse outcome post-myectomy are lacking, and RV dysfunction that would otherwise be used to risk-stratify PH is a rare finding in HCM.
Cardiac amyloid is characterised by myocardial deposition of light-chain (AL) or transthyretin (TTR) protein, which is insoluble and impairs cardiac lusitropy, resulting in a restrictive cardiomyopathy. In one retrospective single-centre study of 132 patients referred for RHC (presumably to assess heart failure) (52%, AL), 76% of patients had mPAP >20 mmHg and the predominant haemodynamic subgroup was cpcPH (average PVR ∼2.4 WU and PAWP ∼22 mmHg) [65]. The effect of diuresis on PH in amyloid cardiomyopathy is not well established, nor is the effect of tafamidis on PH (single disease-specific US Food and Drug Administration (FDA)-approved therapy) in TTR amyloid cardiomyopathy [66]. Patients with cardiomyopathy from Chagas and Fabry diseases may be candidates for disease-specific therapeutics, and therefore require specific consideration when contemplating the approach to clinical diagnosis and management of PH [67].
Approach to management
Individualised clinical decision-making in conjunction with an expert is recommended in patients with obstructive HCM-PH [68], since the use of diuretics may exacerbate LV outflow tract gradient to worsen symptoms, and the use of pre-operative PAH pharmacotherapy is not evidence-based and may be harmful. The approach to patients with cardiac amyloidosis should follow clinical trial data irrespective of PH status, and the routine use of PAH pharmacotherapy is not indicated.
Recommendations, gaps in evidence and unmet needs
Further data are needed to clarify the clinical relevance of PH in post-myectomy patients that may experience residual heart failure symptoms (NYHA FC I or II post-myectomy) [69], although this is not observed commonly in clinical practice.
The role of PH in stratifying patient candidacy for myosin inhibitor therapy in HCM as well as PH response to therapy is not known.
Prospective data informing the clinical relevance of PH on outcome in TTR amyloid-treated patients may be helpful for understanding optimal timing of treatment in TTR amyloid cardiomyopathy patients.
Overlapping phenotypes
Encountering a patient with pure PH-LHD in “real world” practice is rare. Instead, there is substantial overlap between LHD and other diseases that increase the risk of developing PH. From the Pulmonary Vascular Disease Phenomics (PVDOMICS) study [70], for example, 57% of patients classified as WSPH group 2 also could be classified as group 2–3 overlap. A retrospective analysis of the Atherosclerosis Risk in Communities (ARIC) study [71] showed that ∼75% of patients with cardiovascular disease that increases PH-LHD risk also had at least mild COPD or restrictive lung disease. This was supported further by a recent meta-analysis of 31 677 patients, which showed that obstructive and restrictive lung pathophysiology was associated with increased risk of incident heart failure by 17% and 43%, respectively, particularly HFpEF [72]. Notably, the effect of this association was akin to systemic hypertension and diabetes.
Importantly, PH-LHD overlapping syndromes are reported for diseases that otherwise have been historically viewed as isolated subphenotypes, such as chronic thromboembolic pulmonary hypertension (CTEPH). Gerges et al. [73] showed that 11% and 37% of CTEPH patients had elevated LV filling pressure when using a threshold of >15 mmHg and >11 mmHg, respectively. This profile corresponded to greater age, prevalence of systemic hypertension, diabetes and atrial fibrillation, and higher left atrial volume. In patients diagnosed with PAH, the presence of multiple LHD risk factors itself is associated with diminished efficacy and tolerability of PAH medications, including higher rates of treatment failure compared to similar patients without LHD risk factors.
Despite these collective observations, there is little guidance on methodologies to address the dilemma of overlapping phenotypes in clinical practice, even though incomplete clinical assessment in at-risk patients is common [74]. Comprehensive phenotyping that spans appropriate lung diagnostics (i.e. pulmonary function testing, computed tomographic chest imaging, diffusing capacity of the lung for carbon monoxide, others) as well as cardiopulmonary exercise testing (to establish a pulmonary mechanical limit to exercise) in heart failure patients can be helpful for unmasking key comorbidities that require disease-specific treatments. Indeed, it is important to distinguish between LHD risk factors from bona fide LHD comorbidities (table 1). Furthermore, the extent to which an LHD comorbidity arbitrates the probability of PH-LHD versus PAH is probably graded, suggesting that building a staging system focusing on comorbidities per se is important (table 2).
TABLE 1.
Selected common left heart disease (LHD) comorbidities and risk factors
| Common LHD comorbidities | Common LHD risk factors |
|---|---|
| Obesity | Hypercholesterolaemia |
| Systemic hypertension | Tobacco use and second-hand smoke exposure |
| Coronary artery disease | Sedentary lifestyle |
| Diabetes | Illicit drug use |
| Valvular heart disease | Chronic alcohol use |
| Arrhythmia | Infectious exposures in endemic regions |
| Mild reduction in left ventricular systolic function | |
| Peripheral artery disease |
TABLE 2.
Left heart disease (LHD) comorbidity staging
| Mild | Moderate | Severe | |
|---|---|---|---|
| Obesity | 30–35 kg·m−2 | 35–40 kg·m−2 | >40 kg·m−2 |
| Systemic hypertension | Treated ≤2 drugs | Treated >3 drugs | Uncontrolled |
| Diabetes | Insulin resistance/pre-diabetes | Type 2 diabetes | Type 2 diabetes with vascular complications |
| Coronary artery disease | Single vessel disease | NSTEMI Multiple vessels disease Multiple percutaneous interventions Single episode of SCA |
CABG (any time) Repeated SCA STEMI Symptomatic Persistent ischaemia Diffuse disease |
| Arrhythmia | Single episode of atrial arrhythmia Absence of AF at diagnosis |
Repeated episodes of Afl/AF ≥1 treatment for arrhythmia |
Permanent Afl/AF Ventricular arrhythmias Repeated ablation Implantation of pacemaker/ ICD CRT |
| PAD | Asymptomatic large vessels atheromatosis | Nonsignificant stenosis (carotid, femoral) Previous single percutaneous intervention |
Previous surgery for large vessels disease Stage 2b PAD |
| Low | Intermediate | High | |
|---|---|---|---|
| Combined LHD comorbidities | ≥1 mild-stage LHD comorbidity | >1 moderate-stage LHD comorbidity or ≥3 mild-stage LHD comorbidities |
>1 severe-stage LHD comorbidity |
PAD: peripheral arterial disease; NSTEMI: non-ST segment elevation myocardial infarction; SCA: sudden cardiac arrest; CABG: coronary artery bypass graft; AF: atrial fibrillation; Afl: atrial flutter; ICD: implantable cardioverter defibrillator; CRT: cardiac resynchronisation therapy.
Approach to patients with group 1–2 overlap
Diagnosis
Differentiating group 1 from group 2 PH remains challenging, but should align with the prior ESC/ERS guideline recommendations [18, 19] and the WSPH clinical classification system. As outlined in the 6th WSPH LHD statement, establishing the pre-test probability of LHD is the first step. The H2FpEF [75] score and Heart Failure Association Pre-test Assessment, Echocardiography and Natriuretic Peptide, Functional Testing, Final Aetiology (HFA-PEFF) [76] are widely utilised to predict the diagnosis of HFpEF in patients with dyspnoea. These scores may be also used in concert with the previously published WSPH algorithm to determine the pre-test probability of LHD [77]. However, it is important to remember neither have been validated to discriminate PAH from PH-LHD in isolation, and invasive haemodynamic evaluation is still required in most cases.
In patients with suspected PH and an intermediate to high pre-test probability of LHD, several factors should be considered. Diuresis is commonplace prior to haemodynamic evaluation; however, consideration of earlier RHC for diagnostic purposes may be helpful to avoid misdiagnosing isolated pre-capillary PH in PH-LHD due to the salutary effect of volume optimisation on left atrial hypertension [5]. While contributions of pericardial restraint to measured PAWP is possible in the setting of RV dysfunction and volume overload, significant pericardial restraint is rare in the setting of chronic PH and should also be readily recognisable if present (e.g. right atrial pressure greater than or equal to PAWP). If volume status optimisation has already occurred and haemodynamics suggest pre-capillary PH despite risk factors, provocative testing may be considered. Previously this was suggested only in patients with borderline PAWP (13–15 mmHg), although newer data suggest that lower PAWP may also be seen with patients with occult LHD, particularly with diuretic use [78–81].
Exercise provocation can add significant diagnostic and prognostic value when coupled to invasive haemodynamics [82, 83]. However, data supporting the utility of exercise to specifically differentiate group 1 versus group 2 PH remains limited. In addition to uncertainty surrounding what constitutes a normal PAWP response in the setting of PH [84]. exercise protocols and measurement techniques differ widely, even at expert centres [85–87]. As an example, a retrospective analysis of the REDUCE LAP-HF II study suggested that an exercise PVR >1.7 WU, coined “latent pulmonary vascular disease (PVD)”, was common and discriminated responders to intra-atrial septal device therapy from nonresponders [88]. Interestingly, resting PVR did not predict response, nor did it predict the presence of latent PVD. In a subsequent study by Caravita et al. [89] in which exercise cardiac output was assessed with the gold-standard direct Fick (as opposed to indirect Fick or thermodilution), the authors found latent PVD to be exceedingly rare.
Given the complexities outlined here, provocation with a fluid challenge (500 mL of normal saline or 7 mL·kg−1 over 5–10 min) was recommended at the 6th WSPH, with a post-fluid PAWP >18 mmHg indicating an abnormal or positive response. Since that time, a handful of studies have further investigated this manoeuvre (table 3). In a study by Agrawal et al. [80], which included both retrospective (n=126) and prospective (n=52) PH cohorts, 21% and 10% of patients, respectively, were found to have a positive fluid challenge (PFC) suggestive of occult left heart disease. Early mitral inflow velocity (E) and the ratio of E to mitral tissue Doppler (e′) were higher in those with PFC. In another study of PH patients with normal resting PAWP, Moghaddam et al. [81] found that 18% of patients with a PFC were more likely to have clinical characteristics, haemodynamics and echocardiographic features suggestive of LHD. Two studies have suggested lack of diagnostic classification agreement between fluid challenge and exercise [94, 95]. Exercise diagnostic criteria were different in each study and likely partially contribute to lack of agreement. Finally, a PFC was shown to alter therapeutic decision making by PH providers [96]. More robust data on treatment decisions and longitudinal outcomes of individuals with PFC are lacking.
TABLE 3.
Contemporary studies of provocative manoeuvres to improve phenotyping patients with pulmonary hypertension in heart failure with preserved ejection fraction (HFpEF)
| First author, year [reference] | Subjects n | Modality of provocation | Response to provocation | Future directions |
|---|---|---|---|---|
| Vasodilator testing | ||||
| Schwartzenberg, 2012 [90] | 257 | i.v. sodium nitroprusside 0.25–0.5 μg·kg−1·min−1 until PAWP <15 mmHg and SBP <90 mmHg or symptoms | HFpEF had a greater reduction in stroke volume with vasodilation when compared to HFrEF | Assess afterload reduction to better delineate severity of fixed pulmonary vascular resistance in HFpEF |
| Ghio, 2021 [91] | 140 | i.v. sodium nitroprusside 0.25–0.5 μg·kg−1·min−1 until PAWP <15 mmHg, SBP <90 mmHg or symptoms | DBP of <70 mmHg, PVR >5 WU, and PAC <1.2 mL·mmHg−1 were associated with persistent elevation of PVR despite reduction in PAWP | Identify haemodynamic patterns that indicate clinical benefit or risk of pulmonary vasodilator therapy |
| Krishtopaytis, 2023 [92] | 104 | Administered iNO (40 ppm) for 5 min carried by  4 L·min−1 oxygen 4 L·min−1 oxygen |
mPAP >20 mmHg, PAWP >15 mmHg, and PVR >2 WU: iNO increased PAWP and decreased PVR, neither of which correlated with tolerance of pulmonary vasodilator therapy | |
| Volume loading | ||||
| Agrawal, 2019 [80] | 178 | In selected patients with mPAP >25 mmHg and PAWP  15 mmHg 15 mmHgInfusion of 500 mL or 10–15 mL·kg−1 of 0.9% sodium chloride over 5 min via central venous access |
Early diastolic mitral-inflow velocity was higher and ratio of E velocity to average mitral annular tissue Doppler velocity was higher in patients with occult diastolic dysfunction unmasked by saline challenge or resting diastolic dysfunction | Identify noninvasive measures that correlate with degree of pulmonary vascular disease measured by haemodynamics Clarify “abnormal” response following fluid challenge, and if ULN is age-dependent |
| Exercise provocation | ||||
| Gorter, 2018 [93] | 161 | Supine recumbent bicycle: initial measures when supine, feet on pedals and unloaded then serial measurements with an increase workload by 10–20 W every 3 min until exhaustion | Greater increase in PAWP/CO, reduced PAC, higher PVR with exercise | Define what defines an abnormal RV response to exercise Distinguish exercise haemodynamic parameters that indicate a favourable response to different therapies such as PDEi, SGLT2i, GLP-1 |
| Müller, 2023 [85] | 121 | Semi-supine exercise on cycle ergometer with stepwise incremental protocol (increase by 10–20 W every 3 min) | PAH and CTEPH patients  50 years had significantly higher PAWP/CO slope than patients <50 years without exceeding PAWP 25 mmHg during exercise 50 years had significantly higher PAWP/CO slope than patients <50 years without exceeding PAWP 25 mmHg during exercise |
Clarify the population in which iCPET testing is useful clinically Characterise the association between underlying lung disease (or spirometry abnormalities) on pulmonary vascular and RV responses to exercise |
| Caravita, 2023 [89] | 86 | Passive leg raise (feet on the pedals) and invasive cycle cardiopulmonary exercise testing | Few HFpEF patients have latent PVD | |
| Comparison of volume, exercise and passive leg raise | ||||
| Ewert, 2020 [94] | 49 | Exercise: partially upright cycle ergometer cardiopulmonary exercise testing, unloaded cycling at 45 rpm for 5 min and increase in workload by 25 W every 5 min Volume: after normalisation of haemodynamics and vitals, 500 mL of 0.9% sodium chloride administered by i.v. over 5–10 min |
Lack of correlation between volume versus exercise provocation for detection of occult HFpEF | Determine the most reliable protocol |
| Montané, 2022 [95] | 85 | Passive leg raise, load-targeted supine bicycle exercise at 60 rpm starting at 20 W and increasing by 20 W every 2 min to a maximum of 60 W or symptoms required termination, and rapid crystalloid fluid infusion by i.v. of 500 mL of 0.9% sodium chloride over 5 min | Moderate correlation between exercise versus volume in RAP, mPAP, PAWP and cardiac index Passive leg raise correlated moderately with volume and exercise |
i.v.: intravenous; PAWP: pulmonary artery wedge pressure; SBP: systolic blood pressure; HFrEF: heart failure with reduced ejection fraction; DBP: diastolic blood pressure; PVR: pulmonary vascular resistance; WU: Wood Units; PAC: pulmonary arterial compliance; iNO: inhaled nitric oxide; mPAP: mean pulmonary artery pressure; E: mitral inflow velocity; ULN: upper limit of normal; CO: cardiac output; PVD: pulmonary vascular disease; RV: right ventricular; PDEi: phosphodiesterase inhibitor; SGLT: sodium-glucose transport protein; GLP: glucagon-like peptide; CTEPH: chronic thromboembolic pulmonary hypertension; iCPET: invasive cardiopulmonary exercise testing; rpm: revolutions per minute; RAP: right atrial pressure.
A leg-raise manoeuvre increases venous return to the heart, partially mimicking a fluid bolus, and may increase PAWP in patients with occult LHD [79, 97]. Using exercise haemodynamics as the ultimate adjudicator for LHD, PAWP ≤11 mmHg during leg raise ruled out LHD, whereas PAWP >18 mmHg ruled in LHD [79]. However, 75% of the cohort had an intermediate result (PAWP 12–18 mmHg) during leg raise, perhaps limiting the clinical utility of this manoeuvre alone. Several additional reports of potential discriminators of group 1 versus group 2 PH have been made since the 6th WSPH. These include differential response of right atrial pressure [98] and systemic oxygen levels [99] during exercise testing. D’Alto et al. [100] found that combining use of lung ultrasound and echocardiography before and after fluid challenge may improve discrimination.
Overlap syndromes
The above discussion relies on the premise that the pathophysiology of group 1 and 2 PH are not only distinct, but exist in isolation; in reality, there is probably coexistence of both conditions [101]. For example, patients with systemic sclerosis (Ssc), which itself is an independent risk factor for PAH, may present with LV dysfunction. When present, LV dysfunction occurring in the presence or absence of comorbid PH is associated with adverse outcome [102, 103]. Occult diastolic dysfunction, elicited by exercise or fluid challenge, is also common [104–106]. Management of SSc patients with pre-capillary PH and either concomitant overt or occult left heart disease remains challenging and largely unexplored. In a retrospective study of the Ambrisentan and Tadalafil in Patients with Pulmonary Arterial Hypertension (AMBITION) trial, patients with characteristics suggestive of LHD (or restrictive lung disease) were less likely to have benefit from dual therapy versus monotherapy compared with patients with typical PAH [107]. Concomitant LHD may also be present in other group 1 PH patients, particularly with ageing, as well as group 3, 4 and 5 PH [73]. More data are needed to understand and define predominant phenotypes and develop therapeutic strategies in overlap syndromes.
Recommendations, gaps in evidence and unmet needs
Exercise protocols for invasive haemodynamics require standardisation.
Pulmonary vascular structural changes should be better understood through imaging methods to differentiate complex or mixed forms of PH in the setting of LHD.
For patients with LHD risk factors and PFC, data on outcomes with and without PH therapy are needed.
Treatment strategies for overlapping group 1 and 2 PH phenotypes are lacking, particularly SSc.
The role of next-generation machine learning and artificial intelligence to improve phenotyping of patients with group 1 and other PH groups should be defined [108].
Staging PH-LHD
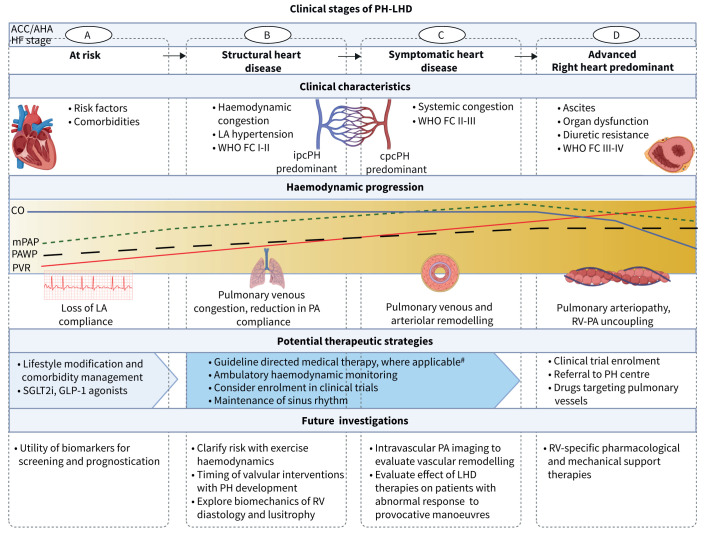
We propose a system that stages PH in LHD in alignment with risk factors alone (stage A) and the identification or evolution of other clinical characteristics including echocardiography patterns, haemodynamics and exercise capacity that suggest progressive disease (stages B–D) (table 4 and figure 1). The goal of establishing this framework is to aim to 1) recognise that PH is a distinct and potentially modifiable entity within the wider heart failure syndrome; 2) approximate PH to LHD severity; and 3) contextualise data on PH for the purpose of LHD and PH treatment selection and escalation. This conceptual framework emphasises disease severity, enabling referral to specialty heart failure or PH centres prior to late-stage disease and could be important for supporting clinical trials that explore novel approaches to the treatment of at-risk patients. This topic is discussed in this section for patients with HFpEF (without valvular disease), while the role of PH in other forms of LHD is addressed in a different section.
TABLE 4.
Proposed clinical staging of in pulmonary hypertension (PH) in heart failure with preserved ejection fraction (HFpEF)
| A At risk |
B Structural heart disease |
C Symptomatic heart disease |
D Right heart predominant |
|
|---|---|---|---|---|
| Clinical profile | Risk factors for HFpEF-PH BMI >30 kg·m−2 Systemic hypertension Glucose intolerance/diabetes Atrial fibrillation Sleep apnoea? |
±Risk factors for HFpEF-PH | ±Risk factors for HFpEF-PH | ±Risk factors for HFpEF-PH |
| Symptom burden | None | WHO FC I–II | WHO FC II–III | WHO FC IV |
| Exercise capacity | Normal |
V′O2peak >80% predicted V′E/V′CO2 slope <30 |
V′O2peak 50–80% predicted with RER >1.05 V′E/V′CO2 slope 30–37 |
V′O2peak <50% predicted with RER >1.05 V′E/V′CO2 slope >37 |
| Typical echo findings | No abnormalities | Mildly elevated pTRV Mildly enlarged LAVI Grade 1–2 diastolic dysfunction ±Valvular disease Normal RV function |
Mild–moderately elevated pTRV Mild–moderately enlarged LAVI Grade 2 diastolic function Mild–moderate valvular disease Mild–moderate RV dysfunction |
Severely elevated pTRV Severe enlarged LAVI Grade >2 diastolic dysfunction Severe valvular disease Severe RV dysfunction |
| Typical haemodynamics | mPAP 19–24 mmHg PAC <3.0 mL·mmHg−1 |
mPAP 25–35 mmHg PVR >2.0–3.0 WU with PAWP >15 mmHg ±PVR <2.0 with after diuresis ±RAP elevation |
mPAP >35 mmHg PVR >5.0 WU with PAWP >15 mmHg PVR >3.0 WU after diuresis Cardiac index <2.2 L·min−1·m−2 Significantly elevated RAP Low PAPi |
|
| NT-proBNP | Normal | Normal or mildly elevated | Pre-diuresis: >>ULN Post-diuresis: <ULN or >ULN |
>>>ULN even after diuresis |
| PH management approach | Treat comorbidities Risk factor modification Dietary modification |
Treat comorbidities Risk factor modification Dietary modification Optimise GDMT Annual echo Clinical trial enrolment (including studying PAH medications) |
Risk factor modification Dietary modification Optimise GDMT Biannual follow-up with functional test and echo Treat comorbidities Clinical trial enrolment (including studying PAH medications) Consider PAP monitoring device Consider referral to PH centre |
Risk factor modification Dietary modification Optimise GDMT Treat comorbidities Bi-annual follow-up with functional test and echo Clinical trial enrolment Consider PAP monitoring device Clinical trial enrolment (including studying PAH medications) Referral to PH centre for individualised therapy Referral to PH/HF centre for individualised management |
echo: two-dimensional surface echocardiogram; NT-proBNP: N-terminal pro-brain natriuretic peptide; BMI: body mass index; WHO FC: World Health Organization functional class; V′O2peak: peak oxygen consumption; V′E: minute ventilation; V′CO2: carbon dioxide production; RER: respiratory exchange ratio; pTRV: peak tricuspid regurgitant velocity; LAVI: left atrial volume index; RV: right ventricular; mPAP: mean pulmonary artery pressure; PAC: pulmonary arterial compliance; PVR: pulmonary vascular resistance; WU: Wood Units; PAWP: pulmonary artery wedge pressure; RAP: right atrial pressure; PAPi: pulmonary artery pressure index; ULN: upper limit of normal; GDMT: goal-directed medical therapy; PAH: pulmonary arterial hypertension; HF: heart failure.
FIGURE 1.
Stages of pulmonary hypertension (PH) in left heart disease (LHD). ACC: American College of Cardiology; AHA: American Heart Association; HF: heart failure; LA: left atrial; WHO FC: World Health Organization functional class; ipcPH: isolated post-capillary pulmonary hypertension; cpcPH: combined post- and pre-capillary pulmonary hypertension; CO: cardiac output; mPAP: mean pulmonary artery pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; PA: pulmonary artery; RV: right ventricle; SGLTi: sodium glucose transport protein 2 inhibitor; GLP: glucagon-like peptide. #: disease-specific guidelines must be applied, i.e. HF (HF with reduced ejection fraction, HF with mid-range ejection fraction, HF with preserved ejection fraction), specific cardiomyopathies, valvular heart diseases. Created with BioRender.com.
In patients with HFpEF, a mildly elevated PASP estimated by echocardiography is independently associated with increased RV volume, decreased RV systolic function quantitated by tricuspid plane of systolic excursion (TAPSE), abnormal RV–pulmonary artery coupling estimated by TAPSE/systolic PAP, which may occur early and be load-independent, among other parameters, and a substantial increase in risk for hospitalisation and mortality [13, 14, 109]. The possibility remains (and in some patient subsets is likely) that outcome is driven by comorbidities or effects on cardiovascular function alternative to PH mediated by the underlying left heart substrate. However, the preponderance of data suggests that mild PH may be an overlooked biomarker in the prognosis of LHD. Further information on the precise alignment between PH severity and LHD symptom burden is needed, but it seems unlikely that moderate or severe heart failure symptom burden is attributed to mildly elevated PASP alone, reinforcing the potential opportunity for utilising mild PH as a cornerstone finding defining early (stage B) disease and an entryway into mitigating or preventing disease progression.
In this scenario, the presence of LHD risk factors in patients with subtle changes in functional capacity may correspond to echocardiographic findings suggesting PH-LHD, including left atrial enlargement, impaired left arial strain and abnormal mitral valve inflow patterns. This group is at risk for adverse clinical events linked to PH and, thus, is designated as stage B despite lacking data to profile PH per se or substantial symptom burden. In patients for whom PH-LHD is associated with objective impairment in exercise capacity leading to referral for RHC confirming the diagnosis of PH-LHD, consideration to additional clinical data is advised to stratify risk further. For example, mortality risk increases approximately two-fold for mPAP >30 mmHg and 1.5-fold for PVR >3.0 WU, while peak oxygen consumption V′O2peak <60% predicted on standard cardiopulmonary exercise testing is an independent risk factor for adverse outcome in HFpEF patients [110, 111]. Delineating stage B and stage C PH-LHD patients aligns with the aggregate risk estimates across different parameters, but probably differs across individuals with consideration to age, sex, frailty and other comorbidities.
Treatment considerations by HFpEF-PH stage
Risk factor modification, adherence to dietary guidelines, and optimisation of guideline-directed pharmacotherapy for heart failure [112] define the strategic aims of PH-LHD stage B and C patients. In particular, SGLT2 inhibitor therapies may exert a salutary benefit in heart failure by reducing plasma volume and, thus, were shown to affect PAP or possibly other PH haemodynamics. Individualised strategies to palliate symptoms that could include PAH therapy may be reasonable to consider in stage D. However, in these patients, clear discontinuation metrics should be established to avoid unnecessary and prolonged therapy for drugs that are otherwise unproven and/or may be harmful in clinical trials (table 5 presents a summary of recent trials involving PAH medications and PH-LHD). Further details on the general approach to treating HFpEF-PH patients are provided later.
TABLE 5.
Selected phase II/III clinical trials since the 6th World Symposium on Pulmonary Hypertension
| Study [reference] | Study drug | Dose | Subjects n | Duration | Population | Primary outcome | Result |
|---|---|---|---|---|---|---|---|
| HELP (phase 2) [113] | Levosimendan | 0.075–0.1 μg·kg−1·min−1 for 24 h × 6 weeks | 44 (37 after run-in) | 6 weeks | LVEF ≥40%, mPAP ≥35 mmHg, PAWP ≥20 mmHg | Exercise PAWP | No change in exercise PAWP Reduction in PAWP across all stages Increase in 6MWD |
| DYNAMIC (phase 2B) [114] | Riociguat | 1.5 mg three times daily | 114 | 26 weeks | LVEF ≥50%, mPAP ≥25 mmHg and PAWP ≥15 mmHg WHO FC II–IV |
Change in CO | Increase in CO (LS mean difference 0.54 (0.112–0.971) L·min−1 No change in NT-proBNP, WHO FC, exercise capacity or QoL Higher dropout rates in riociguat group |
| SPHERE HF (phase 2) [115] | Mirabegron | 200 mg daily | 80 | 16 weeks | Any LVEF; mPAP ≥25 mmHg and PVR ≥3 WU and/or DPG ≥7 mmHg or TPG ≥12 mmHg; NYHA FC II–III | Change in PVR | No significant change in PVR Increase in MRI RVEF No change in FC or QoL |
| SERENADE (phase 2B) [116] | Macitentan | 10 mg daily | 300 (142 enrolled) | 52 weeks (shortened to 24 weeks) | HFpEF (LVEF ≥40%) with structural echo abnormalities, diuretic use, NYHA FC II–III Elevated NT-proBNP or BNP PVD or RVD |
% change from baseline in NT-proBNP at week 24 | Terminated early No difference in primary end-point High run-in failure rate due to fluid retention Not published |
| PASSION (phase 3) [117] | Tadalafil | 40 mg daily | 372 (125 patients enrolled) | 24 weeks and individual end of study | LVEF ≥50%, elevated BNP or NT-proBNP, and one additional HFpEF criteria mPAP ≥25 mmHg, PAWP >15 mmHg, PVR >3 WU |
Event-free survival (adjudicated HF-related hospitalisation or any cause death) | Terminated early (disruption in study medication supply) No change in primary end-point Increase in all-cause mortality No difference in other secondary end-points |
LVEF: left ventricular ejection fraction; mPAP: mean pulmonary artery pressure; PAWP: pulmonary artery wedge pressure; 6MWD: 6-min walk distance; WHO: World Health Organization; FC: functional class; CO: cardiac output; LS: least squares; NT-proBNP: N-terminal pro-brain natriuretic peptide; QoL: quality of life; PVR: pulmonary vascular resistance; WU: Wood Units; DPG: diastolic pulmonary gradient; TPG: transpulmonary gradient; NYHA: New York Heart Association; MRI: magnetic resonance imaging; RVEF: right ventricular ejection fraction; HFpEF: heart failure with preserved ejection fraction; PVD: pulmonary vascular disease; RVD: right ventricular dysfunction; HF: heart failure.
Recommendations, gaps in evidence and unmet needs
Comprehensive phenotyping data are needed to affirm or refine the parameters that define each PH-LHD stage.
Further data are needed to affirm or refine the alignment of parameters and relative importance of each parameter toward defining each PH-LHD stage.
Prospective data are needed to clarify appropriate risk strata, progression of risk, modifiability of risk, and best approach to mitigate risk for patients in each PH-LHD stage.
The generalisability of data defining each PH-LHD stage to patients across demographic, geographic and socioeconomic profiles is not known, and should be the target of further empiric studies.
Determination of the role of current therapies, specifically SGLT2 inhibitors and GLP-1 agonists, in prevention of progression to PH-LHD in patients with high risk or early imaging manifestations of left atrial hypertension is needed.
Recent studies, presented since the 6th WSPH, have reinforced our position that PAH-specific medications should not be used in PH-LHD.
Forward-thinking concepts
Switching focus to preventative medicine in PH-LHD
There is opportunity to leverage mildly elevated estimated PASP (and possibly other echocardiographic parameters) for early detection of patients at risk of PH-LHD. Doing so would mark a shift in emphasis toward preventative medicine in this disease by 1) enabling prospective longitudinal follow-up for patients with mild PH for whom evidence-based guidance on nuanced risk stratification is much needed; and 2) initiation of research protocols that focus on multidisciplinary strategies, particularly nonpharmacological interventions such as prescribed exercise and individualised dietary strategies with monitoring using contemporary methodologies (e.g. digital or wearable health technologies), for inhibiting progression to moderate- or severe-stage disease. Owing to the large and heterogeneous universe of PH-LHD patients, establishing dedicated clinical referral centres that are managed by mid-level providers in conjunction with clinical experts could represent a new model for shared clinical decision-making in PH-LHD primary prevention.
Decentralising research to address areas of equipoise and diversify PH-LHD research data
There is growing emphasis on post-market deployment of cardiovascular, metabolic and diabetes therapeutics to subpopulations that qualify for use, but for whom access to care is limited or unavailable. This trend is consistent with policy initiatives from international health policy stakeholders (e.g. FDA, others) that increasingly prioritise the assembly of clinical research data that reflect communities at hand. For example, <5% of patients enrolled in the VICTORIA trial were Black, even though the prevalence of HFrEF is highest in Black patients, who, in turn, also bear the majority disease burden [118]. Similar trends are observed for other subgroups in which the root-cause basis of health inequity is emphasised; i.e. socioeconomic, geographical and in-groups with limited trust between patients and medical institutions. Overall, centralised research in quaternary referral centres is viewed as an impediment to health equity, is largely impractical for addressing areas of clinical equipoise and provides little information on drug efficacy outside of the rigorous confines of a phase III clinical trial.
These collective dilemmas set the stage in favour of pragmatic and community-based clinical trials that emphasise decentralised clinical research with minimal disruption to workflow. Next-generation computational analytics (e.g. network medicine, artificial intelligence) [119, 120] and other contemporary methods should be considered to address numerous topics that are important but are unlikely to garner the funding support needed to implement at large-scale using an inclusive research design. Global feasibility of pragmatic trials hinge somewhat on capabilities of healthcare and hospital systems as well as electronic medical records. The current task force identifies the following PH-LHD topics for consideration in pragmatic trials.
Should pragmatic clinical trials emphasise patients with a diagnosis of HFpEF with NYHA FC II–III, PVR >5 WU and PAWP >15 mmHg at rest?
Should pragmatic clinical trials emphasise patients with a diagnosis of HFpEF with NYHA FC II–III and PAWP >12 mmHg at rest, but >18 mmHg after fluid loading on exercise, N-terminal pro-brain natriuretic peptide >1200 pg·mL−1 (or >1500 pg·mL−1 if patient is in atrial fibrillation) and signs of RV dysfunction such as impaired TAPSE/systolic PAP?
Future directions
Cardiovascular medicine continues to fractionate with proliferation and, in many cases, isolation among subspecialities, which can be appreciated by reviewing the programming tracks at international meetings and monitoring the emergence of sub-sub-specialty journals. In this regard, the task force identifies opportunity to stimulate closer collaboration between the LHD and PH fields, which at present track largely parallel and is evident, in part, by virtue of limited information on PH clinical data in many large-scale and highly influential LHD/heart failure clinical trials. This is particularly the case for studies focusing on percutaneous tricuspid valve and mitral valve therapies, HFpEF and HFrEF. Greater effort should be made to bridge this gap through collaborative alignment, which is expected to benefit patients. Diversifying fundamental knowledge on PH-LHD pathobiology is also an important future direction, particularly as this may relate to broader understanding of pulmonary vein biology in HFpEF, pathogenetic mechanisms that regulate the transition from ipcPH to cpcPH, and molecular pathways that are important to lung disease and LHD that may converge to induce extreme phenotypes in affected patients.
Diagnosis
Our field should strive to move away from pure haemodynamic diagnoses, especially with strict and sometimes arbitrary thresholds. While incorporating haemodynamics with or without provocative challenges remains an integral part of the evaluation process, development of other platforms, biomarkers, imaging modalities and strategies to determine predominant pathology in overlap syndromes are needed.
Clinical trial target populations, end-points and designs
The primary guiding principle underpinning the design of a clinical trial is the trade-off between validity and efficiency. The goal is to formulate a trial design that will lead to a valid answer to the research question but at minimal allowable cost, sample size and time frame. Table 6 addresses the differences between a randomised clinical trial, an observational study and a pragmatic trial. The latter is attractive, since this approach can assess the benefit of a strategy more than a specific intervention [124, 125]. Table 7 provides insights into gaps and challenges of studying PH associated with LHD. Assuming that the mechanisms of PH are different across the spectrum of LHD, each clinical scenario is associated with specific challenges. One is the selective use of RHC in the assessment of PH-LHD. Current recommendations in HFrEF, valvular heart disease and cardiomyopathies restrict the indications of RHC as guidance for surgical decisions. Therefore, trials in group 2 PH associated with PH-HFpEF should be restricted to patients with stage C or D (see table 4 for PH-HFpEF staging) (table 8). PH and RV function should not only be assessed in trials of LHD, but also should be included as end-points. Increased collaboration between PH and heart failure specialists when designing clinical trials is needed.
TABLE 6.
Study designs
| Randomised controlled clinical trials |
Observational studies | Pragmatic clinical trials | |
|---|---|---|---|
| Purpose | Understand whether an intervention: Is beneficial (efficacious) in a highly selected population Elucidates potential adverse effects (safety and tolerability) |
Primary focus: hypothesis generating through “real-life” evaluation: Insights into efficacy and safety Assessing how RCT learnings are implemented |
Understand whether an intervention: Is beneficial in a broader population Informs clinical practice or policy decisions by providing evidence for adoption of intervention in real-world clinical practice |
| Subtypes | Prospective 1) Traditional nonadaptive superiority RCT 2) Noninferiority or equivalence RCT: Appropriate for specific situations where proposed Therapy has clear benefit over established therapy; i.e. less costly, more convenient, better tolerated 3) Adaptive clinical trial design: Newer design; allows planned assessment and action on data as it accrues; adaptation of design in real time so more efficient and less costly |
Prospective and retrospective: 1) Ecological 2) Cross-sectional 3) Cohort 4) Case–control |
Prospective: Not well delineated, but generally clinical trial designs which de-emphasise strict trial procedures in favour of rapid recruitment of a wide-ranging population allowing for flexible follow-up protocols that resemble clinical practice |
| Pros (advantages) | Robust statistical inference; unbiased estimates of treatment effect; accepted by regulators | Serve to emulate large randomised trials Less costly than RCTs Efficient (especially case–control studies) Convenient (reflects clinical practice) |
Emphasis on informing practice rather than efficacy Patient population relevant for intervention Usually large sample sizes =/− Multidisciplinary, +/− multicentric Ability to leverage electronic medical records or existing registries (lower cost; learning healthcare systems approach with data collected by default) Provide long-term safety data for unselected populations Implementation: at time of a complex intervention or the post-marketing phase of drug evaluation to understand the effect of introducing the new strategies on overall public health |
| Cons (disadvantages/challenges) | 1) Superiority: expensive; inefficient; requires a priori knowledge 2) Noninferiority: often requires sample size >1 3) Adaptive: caveat for validity |
Concerns for confounding, bias, and validity Regulatory agency disadvantage |
Not clearly defined May overestimate benefits, underestimate harm in smaller sample sizes Recruitment and participation challenges Large sample sizes required Financial disincentives may limit enrolment Informed consent barrier to unselected participant recruitment Interpretation of data (self-reporting, incompleteness, coding variability) in registries; introduction of bias Funding challenge (industry–government) |
TABLE 7.
Suitability of study design# for clinical question and knowledge gap in World Symposium on Pulmonary Hypertension group 2 pulmonary hypertension (PH). Different clinical scenarios are considered, depending on the underlying left heart disease (LHD)
| Questions, gaps, unmet needs | Randomised controlled trials | Observational studies | Pragmatic trials |
|---|---|---|---|
| PH associated with HFpEF | |||
| Routine use of RHC for phenotyping¶ | +++ | +++ | +++ |
| Population 1: HFpEF with NYHA II–III, PVR >5 WU, PAWP >15 mmHg | +++ | ++ | +++ |
| Population 2: HFpEF, NYHA II–III, PAWP >12 mmHg at rest, >18 mmHg with fluid or exercise, NT-proBNP >1200 pg·mL−1 or >1500 pg·mL−1 with AF, signs of RV dysfunction | +++ | + | +++ |
| Population 3: PAH trial in patients with HFpEF, but PAWP <15 mmHg | ++ | + | ++ |
| PH associated with HFrEF | |||
| Routine use of RHC for phenotyping | + | ++ | |
| Efficacy of SGLT2i, GLP-1, ARNi on PH (with proper haemodynamic assessment) | +++ | ++ | ++ |
| Valvular disease | |||
| Routine use of RHC for phenotyping | + | ++ | |
| Relevance of PVR >2 WU for surgical valvulotomy candidacy | +++ | ++ | +++ |
| Haemodynamic classification of PH to predict risk and outcome | ++ | +++ | +++ |
| Optimal approach to manage residual PH post-valvular intervention | +++ | ++ | +++ |
| Delineate association between PH subtype and TAVR timing | +++ | ++ | +++ |
| Cardiomyopathies | |||
| Role of PH in stratifying candidacy for septal reduction surgery or myosin inhibitors in HCM | +++ | ++ | +++ |
| Clinical relevance of PH on outcome in treatment of ATTR | ++ | +++ | +++ |
| Overlapping phenotypes | |||
| Routine use of RHC for phenotyping | +++ | +++ | +++ |
| Standardisation of exercise protocols for invasive haemodynamics | +++ | ++ | ++ |
| LHD and comorbidities outcome data with and without PH | ++ | +++ | +++ |
| Treatment strategies for group 1 and 2 PH phenotypes, especially scleroderma spectrum | + | + | ++ |
| Machine learning/AI analysis of capabilities | + | ++ | +++ |
| Phenotyping data to affirm or refine range of parameters that define each PH-LHD stage | + | ++ | +++ |
| Generalisability of data to define each PH-LHD stage across broad patient profiles | + | ++ | +++ |
HFpEF: heart failure with preserved ejection fraction; RHC: right heart catheterisation; NYHA: New York Heart Association; PVR: pulmonary vascular resistance; WU: Wood Units; PAWP: pulmonary artery wedge pressure; NT-proBNP: N-terminal pro-brain natriuretic peptide; AF: atrial fibrillation; RV: right ventricular; PAH: pulmonary arterial hypertension; HFrEF: heart failure with reduced ejection fraction; SGLTi: sodium-glucose transport protein-2 inhibitors; GLP: glucagon-like peptide; ARNi: angiotensin receptor neprilysin inhibitor; TAVR: transaortic valve replacement; HCM: hypertrophic cardiomyopathy; ATTR: transthyretin amyloid; AI: artificial intelligence. #: strength of trial design: +: low, ++: moderate, +++: high; ¶: RHC evaluation is more common in HFpEF, as it may be part of the differential diagnosis with idiopathic PAH.
TABLE 8.
Design and end-points in clinical trials for World Symposium on Pulmonary Hypertension group 2 pulmonary hypertension (PH)#
| Randomised controlled trials | Observational studies | Pragmatic trials | |
|---|---|---|---|
| Haemodynamic | |||
| PVR | +++ Preferred primary end-point in phase 2 |
++ | +++ |
| PA compliance | ? | ? | ? |
| Other haemodynamic end-points | Not utilised | + | ++ |
| Exercise capacity | |||
| 6MWD | +++ Preferred secondary end-point in phase 2 May be considered as primary? |
+ Variability concerns |
++ |
| CPET derived variable (V′O2peak,V′E/V′CO2 slope) | + Feasibility concerns Centralised reading required (core lab) |
− Not suitable |
+ Feasibility concerns Centralised reading required (core lab) |
| iCPET and exercise haemodynamics | + Standardised procedure, limited by the number of centres Centralised reading required (core lab) |
− Not suitable |
−/+ May be feasible in selected centres Centralised reading required (core lab) |
| Other tools (i.e. accelerometry) | + | − | − |
| Biomarkers | |||
| NT-proBNP | ++ Prone to variability in HF, in association with congestion |
−/+ Quality control and variability concerns |
+ Quality control concerns |
| Other biomarkers | ? Exploratory |
− | − |
| Imaging | |||
| Echo-derived variables | ++ Ideal setting, feasible Core lab recommended Centralised reading required (core lab) |
− | + May be considered, but requires core lab reading |
| cMRI-derived variables | + Core lab Centralised reading required (core lab) |
− Standardisation concerns |
− Feasibility and standardisation concerns Centralised reading required (core lab) |
| Clinical events | |||
| Combined all-cause mortality + hospitalisation | +++ Highest evidence Adjudication required Feasibility unlikely |
++ Events easily collected No adjudication |
+++ Events easily collected Potential for adjudication |
| Combined cardiovascular mortality + hospitalisation for HF | +++ Preferred design Robust Adjudication mandated Common in HF trials |
+ Events easily collected No adjudication Depends on centre's practice |
+ Lack of adjudication limits feasibility |
A focus on PH associated with heart failure with preserved ejection fraction (HFpEF) and selected cases of PH associated with left heart disease (LHD) where valvular heart disease is mild and/or was corrected irrespective of the technique is assumed. It is important to stress that the latter population may be heterogeneous across the globe, with a preference for transcatheter aortic valve replacement interventions in older and more comorbid patients. Additionally, it is assumed that the underlying disease must be stabilised and treated before considering patients for a clinical trial. PVR: pulmonary vascular resistance; PA: pulmonary artery: 6MWD: 6-min walk distance; (i)CPET: (invasive) cardiopulmonary exercise testing; V′O2peak: peak volume of oxygen consumption; V′E: minute ventilation; V′CO2: carbon dioxide production; NT-proBNP: N-terminal pro-brain natriuretic peptide; cMRI: cardiac magnetic resonance imaging; HF: heart failure. #: strength of trial entity: − unnecessary, + low, ++ moderate, +++ high.
Recommendations, gaps in evidence and unmet needs
Should PAH trials be open to patients with HFpEF traits, predominant PVD, but normal PAWP at rest?
Should PH HFpEF trials be open to patients with an established HFpEF diagnosis, but PAWP below the threshold of 15 mmHg?
Should patients with an abnormal response to a fluid loading test be included in PH-HFpEF trials?
Drug development targets
There is a paucity of data on the pathobiology or pathogenesis of adverse pulmonary venous remodelling due to left atrial hypertension. It is imperative that fundamental observations on unique -omics profiles of pulmonary venous cell types inferred from human atlas studies [126] are validated with histologically confirmed specimens. Wider studies characterising the effect of vascular congestion, neurohumoral overactivation, increased vascular oxidant stress and other endophenotypes implicated in PH-LHD [127] on sclerotic, fibrotic and hypertrophic remodelling patterns are needed. Comparative studies involving pulmonary arterial cell types are likely to yield novel therapeutic target specific to the pulmonary venous circulation that can then be tested in patients with cpcPH. Additionally, further work exploring the molecular mechanism and biophysics that regulate LV and RV cardiomyocyte lusitropy, lymphatic reserve [128] and left atrial compliance [129] are well positioned to yield new insights on HFpEF pathophysiology and treatment opportunities. Finally, the task force identifies a need for betting understanding early signs of RV stress, particularly impaired lusitropy, in clinical circumstances.
Shareable PDF
Footnotes
Conflict of interest: B.A. Maron reports grants from NIH/NHLBI and Deerfield Company, consultancy fees from Actelion and Tenax Therapeutics, and the following unlicensed patents: PCT/US2019/059890, #9,605,047 and PCT/US2020/066886. G. Bortman reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Bago, Tuteur, Raffo, Aerovate, Novartis, United, Biosidus, Sandoz, Glaxo, Gador and MKD, support for attending meetings from Tuteur, Bago, Biosidus, Raffo, Sandoz, Boehringer, Gador, Glaxo and MKD, participation on a data safety monitoring board or advisory board with Aerovate and MKD, and is a member of the Argentina Heart Society, Argentina Transplant Society and LHL. T. De Marco reports grants from CareDx and Acceleron Pharm, consultancy fees from Aerovate, Boston Scientific, Atara, Kamada, Kerios, Merck, Natera, Pulnovo, Tectonic and United Therapeutics, payment or honoraria for lectures, presentations, manuscript writing or educational events from Simply Speaking PAH CME lecture, support for attending meetings from CareDx, Atara, Kamada and United Therapeutics, and participation on a data safety monitoring board or advisory board with Merck, Keros Therapeutics, Tectonic and BIAL. J.H. Huston has no potential conflicts of interest to disclose. I.M. Lang reports grants from AOP-Health, consultancy fees from Pulnovo, Janssen, MSD, Novo Norrdisk, Daiichi and Amarin, payment or honoraria for lectures, presentations, manuscript writing or educational events from MSD, Sanofi, Janssen and Daiichi, and support for attending meetings from Medtronic. S.H. Rosenkranz reports grants from Actelion, AstraZeneca, Bayer, Janssen, Lempo and MSD, consultancy fees from Abbott, Acceleron, Actelion, Aerovate, Altavant, AOP, AstraZeneca, Bayer, Ferrer, Gossamer, Janssen, Liquidia, MSD and UT, support for attending meetings from Bayer, participation on a data safety monitoring board or advisory board with Acceleron, Actelion, Aerovate, Altavant, AOP, Gossamer, Janssen, Liquidia, MSD and UT, and was ESC task force chair for the 2022 ESC/ERS guidelines on pulmonary hypertension. J-L. Vachiery reports consultancy fees from Merck, payment or honoraria for lectures, presentations, manuscript writing or educational events from Janssen, Merck, Boehringer Ingelheim and Novartis, support for attending meetings from Merck, participation on a data safety monitoring board or advisory board with Moderna, and has leadership roles with ISHLT and ESC. R.J. Tedford reports consultancy fees from Abbott, Acorai, Aria CV Inc., Acceleron/Merck, Alleviant, Boston Scientific, Cytokinetics, Edwards LifeSciences, Endotronix, Gradient, Medtronic, Morphic Therapeutics, Restore Medical and United Therapeutics, support for attending meetings from Abiomed, participation on a data safety monitoring board or advisory board with Restore Medical, stock (or stock options) with Aria CV, and is deputy editor for JHLT, and co-chair of the PH-LHD task force for WSPH.
This article has an editorial commentary: https://doi.org/10.1183/13993003.01222-2024
References
- 1.Rosenkranz S, Gibbs JSR, Wachter R,et al. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016; 37: 942–954. doi: 10.1093/eurheartj/ehv512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brittain EL, Thenappan T, Huston JH, et al. Elucidating the clinical implications and pathophysiology of pulmonary hypertension in heart failure with preserved ejection fraction: a call to action: A science advisory from the American Heart Association. Circulation 2022; 146: e73–e88. doi: 10.1161/CIR.0000000000001079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alushi B, Beckhoff F, Leistner D, et al. Pulmonary hypertension in patients with severe aortic stenosis: prognostic impact after transaortic valve replacement: pulmonary hypertension in patients undergoing TAVR. JACC Cardiovasc Imaging 2019; 12: 591–601. doi: 10.1016/j.jcmg.2018.02.015 [DOI] [PubMed] [Google Scholar]
- 4.Latson LA, Prieto LR. Congenital and acquired pulmonary vein stenosis. Circulation 2007; 115: 103–108. doi: 10.1161/CIRCULATIONAHA.106.646166 [DOI] [PubMed] [Google Scholar]
- 5.Maron BA, Kovacs G, Vaidya A, et al. Cardiopulmonary hemodynamics in pulmonary hypertension and heart failure: JACC review topic of the week. J Am Coll Cardiol 2020; 76: 2671–2681. doi: 10.1016/j.jacc.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenkranz S, Howard LS, Gomberg-Maitland M, et al. Systemic consequences of pulmonary hypertension and right-sided heart failure. Circulation 2000; 141: 678–993. doi: 10.1161/CIRCULATIONAHA.116.022362 [DOI] [PubMed] [Google Scholar]
- 7.Usman MS, Van Spall HGC, Greene SJ, et al. The need for increased pragmatism in cardiovascular clinical trials. Nat Rev Cardiol 2022; 19: 737–750. doi: 10.1038/s41569-022-00705-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovacs G, Berhold A, Scheidl S, et al. Pulmonary arterial systolic pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 2009; 34: 888–894. doi: 10.1183/09031936.00145608 [DOI] [PubMed] [Google Scholar]
- 10.Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking program. Circulation 2016; 133: 1240–1248. doi: 10.1161/CIRCULATIONAHA.115.020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douschan P, Kovacs G, Avian A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med 2018; 197: 509–516. doi: 10.1164/rccm.201706-1215OC [DOI] [PubMed] [Google Scholar]
- 12.Maron BA, Kleiner DE, Arons E, et al. Evidence of advanced pulmonary vascular remodeling in obstructive hypertrophic cardiomyopathy with pulmonary hypertension. Chest 2023; 163: 678–686. doi: 10.1016/j.chest.2022.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huston JH, Maron BA, French J, et al. Association of mild echocardiographic pulmonary hypertension with mortality and right ventricular function. JAMA Cardiol 2019; 4: 1112–1121. doi: 10.1001/jamacardio.2019.3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strange G, Stewart S, Celermajer DS, et al. Threshold of pulmonary hypertension associated with increased mortality. J Am Coll Cardiol 2019; 73: 2660–2672. doi: 10.1016/j.jacc.2019.03.482 [DOI] [PubMed] [Google Scholar]
- 15.Fayyaz AU, Edwards WD, Maleszewski JJ, et al. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure with preserved or reduced ejection fraction. Circulation 2018; 137: 1796–1810. doi: 10.1161/CIRCULATIONAHA.117.031608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maron BA, Brittain EL, Hess E, et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: a retrospective cohort study. Lancet Respir Med 2020; 8: 873–884. doi: 10.1016/S2213-2600(20)30317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xanthouli P, Jordan S, Milde N, et al. Haemodynamic phenotypes and survival in patients with systemic sclerosis: the impact of the new definition of pulmonary arterial hypertension. Ann Rheum Dis 2020; 79: 370–378. doi: 10.1136/annrheumdis-2019-216476 [DOI] [PubMed] [Google Scholar]
- 18.Humbert M, Kovacs G, Hoeper M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023; 61: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 19.Humbert M, Kovacs G, Hoeper M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 20.Fauvel C, Damy T, Berthelot E, et al. Post-capillary pulmonary hypertension in heart failure: impact of current definition in the PH-HF multicentre study. Eur Heart J 2024; 43: 3618. 10.1093/eurheartj/ehae467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacs G, Avian A, Pienn M, et al. Reading pulmonary vascular pressure tracings. How to handle the problems with zero leveling and respiratory swings. Am J Respir Crit Care Med 2014; 190: 252–257. doi: 10.1164/rccm.201402-0269PP [DOI] [PubMed] [Google Scholar]
- 22.Ryan JJ, Rich JD, Thiruvoipati T, et al. Current practice for determining pulmonary capillary wedge pressure predisposes to serious errors in classification of patients with pulmonary hypertension. Am Heart J 2012; 163: 589–594. doi: 10.1016/j.ahj.2012.01.024 [DOI] [PubMed] [Google Scholar]
- 23.Sahay S, Lane J, Sharpe MG, et al. Impact of pulmonary hypertension hemodynamic classification based on the methodology used to measure pulmonary artery wedge pressure and cardiac output. Ann Am Thorac Soc 2023; 20: 1752–1759. doi: 10.1513/AnnalsATS.202303-216OC [DOI] [PubMed] [Google Scholar]
- 24.Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. doi: 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- 25.Vachiéry JL, Delcroix M, Al-Hiti H, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J 2018; 51: 1701886. doi: 10.1183/13993003.01886-2017 [DOI] [PubMed] [Google Scholar]
- 26.Oldham WM, Hess E, Waldo SW, et al. Integrating haemodynamics identifies an extreme pulmonary hypertension phenotype. Eur Respir J 2021; 58: 2004625. doi: 10.1183/13993003.04625-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rayner SG, Tedford RJ, Leary PJ, et al. “This patient needs a doctor – not a guideline!” The zone of uncertainty in pulmonary artery wedge pressure measurement. Am J Respir Crit Care Med 2024; 210: 712–714. Doi: 10.1164/rccm.202402-0359VP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tampakakis E, Shah SJ, Borlaug BA, et al. Pulmonary effective arterial elastance as a measure of right ventricular afterload and its prognostic value in pulmonary hypertension due to left heart disease. Circ Heart Fail 2018; 11: e004436. doi: 10.1161/CIRCHEARTFAILURE.117.004436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med 2017; 377: 713–722. doi: 10.1056/NEJMoa1603693 [DOI] [PubMed] [Google Scholar]
- 30.Bigna JJ, Noubiap JJ, Nansseu JR, et al. Prevalence and etiologies of pulmonary hypertension in Africa: a systematic review and meta-analysis. BMC Pulm Med 2017; 17: 183. doi: 10.1186/s12890-017-0549-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood P, Besterman EM, Towers MK, et al. The effect of acetylcholine on pulmonary vascular resistance and left atrial pressure in mitral stenosis. Br Heart J 1957; 19: 279–286. doi: 10.1136/hrt.19.2.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamra H, Zhang HP, Allen JW, et al. Factors determining normalization of pulmonary vascular resistance following successful balloon mitral valvotomy. Am J Cardiol 1999; 83: 392–395. doi: 10.1016/S0002-9149(98)00875-3 [DOI] [PubMed] [Google Scholar]
- 33.Nair KK, Pillai HS, Titus T, et al. Persistent pulmonary artery hypertension in patients undergoing balloon mitral valvotomy. Pulm Circ 2013; 3: 426–431. doi: 10.4103/2045-8932.114779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins N, Sugito S, Davies A, et al. Prevalence and survival associated with pulmonary hypertension after mitral valve replacement: national echocardiography database of Australia study. Pulm Circ 2022; 12: e12140. doi: 10.1002/pul2.12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincens JJ, Temizer D, Post JR, et al. Long-term outcome of cardiac surgery in patients with mitral stenosis and severe pulmonary hypertension. Circulation 1995; 92: II137–II142. doi: 10.1161/01.CIR.92.9.137 [DOI] [PubMed] [Google Scholar]
- 36.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol 2021; 77: e25–e197. doi: 10.1016/j.jacc.2020.11.018 [DOI] [PubMed] [Google Scholar]
- 37.Yang B, DeBenedictus C, Watt T, et al. The impact of csoncomitant pulmonary hypertension on early and late outcomes following surgery for mitral stenosis. J Thorac Cardiovasc Surg 2016; 152: 394–400. doi: 10.1016/j.jtcvs.2016.02.038 [DOI] [PubMed] [Google Scholar]
- 38.Ratwate S, Stewart S, Strange G, et al. Prevalence of pulmonary hypertension in aortic stenosis and its influence on outcomes. Heart 2023; 109: 1319–1326. doi: 10.1136/heartjnl-2022-322184 [DOI] [PubMed] [Google Scholar]
- 39.Nashef SAM, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012; 41: 734–744. doi: 10.1093/ejcts/ezs043 [DOI] [PubMed] [Google Scholar]
- 40.Werner N, Zahn R, Beckmann A, et al. Patients at intermediate surgical risk undergoing isolated interventional or surgical aortic valve implantation for severe symptomatic aortic valve stenosis. Circulation 2018; 138: 2611–2623. doi: 10.1161/CIRCULATIONAHA.117.033048 [DOI] [PubMed] [Google Scholar]
- 41.Gössl M, Kane GC, Mauermann W, et al. Pulmonary hypertension in patients undergoing transcatheter aortic valve replacement: discussion of the current evidence. Circ Cardiovasc Interv 2015; 8: e002253. doi: 10.1161/CIRCINTERVENTIONS.114.002253 [DOI] [PubMed] [Google Scholar]
- 42.Auffret V, Bakhti A, Leurent G, et al. Determinants and impact of heart failure readmission following transaortic valve replacement. Circ Cardiovasc Interv 2020; 13: e008959. doi: 10.1161/CIRCINTERVENTIONS.120.008959 [DOI] [PubMed] [Google Scholar]
- 43.Cardaioli F, Fovino LN, Fabris T, et al. Updated definition of pulmonary hypertension and outcome after transcatheter aortic valve implantation. Heart 2023; 110: 27–34. doi: 10.1136/heartjnl-2023-322881 [DOI] [PubMed] [Google Scholar]
- 44.Schewel J, Schmidt T, Kuck K-H, et al. Impact of pulmonary hypertension hemodynamic status on long-term outcome after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2019; 12: 2155–2168. doi: 10.1016/j.jcin.2019.08.031 [DOI] [PubMed] [Google Scholar]
- 45.Gilard M, Eltchaninoff H, Lung B, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med 2012; 366: 1705.–. doi: 10.1056/NEJMoa1114705 [DOI] [PubMed] [Google Scholar]
- 46.Généreux P. Pulmonary hypertension and aortic stenosis: a piece of the puzzle. J Am Coll Cardiol 2022; 80: 1614–1616. doi: 10.1016/j.jacc.2022.09.005 [DOI] [PubMed] [Google Scholar]
- 47.Babu G, Annis JS, Garry JD, et al. Clinical features do not identify risk of progression from isolated postcapillary pulmonary hypertension to combined pre- and postcapillary pulmonary hypertension. Pulm Circ 2023; 13: e12249. doi: 10.1002/pul2.12249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011; 364: 1395–1406. doi: 10.1056/NEJMoa1009355 [DOI] [PubMed] [Google Scholar]
- 49.Ubben T, Frerker C, Fujita B, et al. Association of pulmonary hypertension with the outcome in patients undergoing edge-to-edge mitral valve repair. Heart 2024; 110: 800–807. doi: 10.1136/heartjnl-2023-323473 [DOI] [PubMed] [Google Scholar]
- 50.Kjaergaard J, Akkan D, Karmark Iversen K, et al. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol 2007; 99: 1146–1150. doi: 10.1016/j.amjcard.2006.11.052 [DOI] [PubMed] [Google Scholar]
- 51.Adir Y, Guazzi M, Offer A, et al. Pulmonary hemodynamics in heart failure patients with reduced or preserved ejection fraction and pulmonary hypertension: similarities and disparities. Am Heart J 2017; 192: 120–127. doi: 10.1016/j.ahj.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 52.Goland S, Czer LSC, Kass RM, et al. Pre-existing pulmonary hypertension in patients with end-stage heart failure: impact on clinical outcome and hemodynamic follow-up after orthotopic heart transplantation. J Heart Lung Transplant 2007; 26: 312–318. doi: 10.1016/j.healun.2006.12.012 [DOI] [PubMed] [Google Scholar]
- 53.Rame JE, Pagani FD, Kieran MS, et al. Evolution of late right heart failure with left ventricular assist devices and association with outcomes. J Am Coll Cardiol 2021; 78: 2294–2308. doi: 10.1016/j.jacc.2021.09.1362 [DOI] [PubMed] [Google Scholar]
- 54.Bonderman D, Ghio S, Felix SB, et al. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation 2013; 128: 502–511. doi: 10.1161/CIRCULATIONAHA.113.001458 [DOI] [PubMed] [Google Scholar]
- 55.Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020; 382: 1883–1893. doi: 10.1056/NEJMoa1915928 [DOI] [PubMed] [Google Scholar]
- 56.Ryan JJ, Maron BA. Vericiguat in heart failure with reduced ejection fraction. N Engl J Med 2020; 383: 1497. doi: 10.1056/NEJMc2027731 [DOI] [PubMed] [Google Scholar]
- 57.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 58.Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022; 387: 1089–1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 59.Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the art review. JACC Basic Transl Sci 2020; 5: 632–644. doi: 10.1016/j.jacbts.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nassif ME, Qintar M, Windsor SL, et al. Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE-HF trial. Circulation 2021; 143: 1673–1686. doi: 10.1161/CIRCULATIONAHA.120.052503 [DOI] [PubMed] [Google Scholar]
- 61.Kirschbaum K, Vasa-Nicotera M, Zeiher AM, et al. SGLT2 inhibitor therapy and pulmonary artery pressure in patients with chronic heart failure-further evidence for improved hemodynamics by continuous pressure monitoring. Clin Res Cardiol 2022; 111: 469–472. doi: 10.1007/s00392-021-01954-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mullens W, Martens P, Forouzan O, et al. Effects of dapagliflozin on congestion assessed by remote pulmonary artery pressure monitoring. ESC Heart Fail 2020; 7: 2071–2073. doi: 10.1002/ehf2.12850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui H, Schaff HV, Wang S, et al. Survival following alcohol septal ablation or septal myectomy for patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2022; 79: 1647–1655. doi: 10.1016/j.jacc.2022.02.032 [DOI] [PubMed] [Google Scholar]
- 64.Covella M, Rowin EJ, Hill NS, et al. Mechanism of progressive heart failure and significance of pulmonary hypertension in obstructive hypertrophic cardiomyopathy. Circ Heart Fail 2017; 10: e0036989. doi: 10.1161/CIRCHEARTFAILURE.116.003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Longinow J, Buggey J, Jacob M, et al. Significance of pulmonary hypertension in cardiac amyloidosis. Am J Cardiol 2023; 192: 147–154. doi: 10.1016/j.amjcard.2023.01.014 [DOI] [PubMed] [Google Scholar]
- 66.Maurer M, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379: 1007–1016. doi: 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- 67.Ribeiro AL, Nunes MP, Teixeira MM, et al. Diagnosis and management of Chagas disease and cardiomyopathy. Nat Rev Cardiol 2012; 9: 576–589. doi: 10.1038/nrcardio.2012.109 [DOI] [PubMed] [Google Scholar]
- 68.Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2020; 142: e558–e631. doi: 10.1161/CIR.0000000000000938 [DOI] [PubMed] [Google Scholar]
- 69.Maron MS, Rastegar H, Dolan N, et al. Outcomes over follow-up ≥10 years after surgical myectomy for symptomatic obstructive hypertrophic cardiomyopathy. Am J Cardiol 2022; 163: 91–97. doi: 10.1016/j.amjcard.2021.09.040 [DOI] [PubMed] [Google Scholar]
- 70.Hemnes AR, Leopold JA, Radeva MK, et al. Clinical characteristics and transplant-free survival across the spectrum of pulmonary vascular disease. J Am Coll Cardiol 2022; 80: 697–718. doi: 10.1016/j.jacc.2022.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension, and cardiovascular disease in COPD. Eur Respir J 2008; 32: 962–969. doi: 10.1183/09031936.00012408 [DOI] [PubMed] [Google Scholar]
- 72.Eckhardt CM, Balte PP, Barr RG, et al. Lung function impairment and risk of incident heart failure: the NHLBI Pooled Cohorts Study. Eur Heart J 2022; 43: 2196–2208. doi: 10.1093/eurheartj/ehac205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerges C, Pistritto A-M, Gerges M, et al. Left ventricular filling pressure in chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2023; 81: 653–664. doi: 10.1016/j.jacc.2022.11.049 [DOI] [PubMed] [Google Scholar]
- 74.Maron BA, Choudhary G, Khan UA, et al. Clinical profile and underdiagnosis of pulmonary hypertension in US veteran patients. Circ Heart Fail 2013; 6: 906–912. doi: 10.1161/CIRCHEARTFAILURE.112.000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reddy YNV, Carter RE, Obokata M, et al. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018; 138: 861–870. doi: 10.1161/CIRCULATIONAHA.118.034646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barandiarán Aizpurua AB, Sanders-van Wijk S, Brunner-La Rocca H-P, et al. Validation of the HFA-PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail 2020; 22: 413–421. doi: 10.1002/ejhf.1614 [DOI] [PubMed] [Google Scholar]
- 77.Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J 2019; 53: 1801897. doi: 10.1183/13993003.01897-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.D'Alto M, Badesch D, Bossone E, et al. A fluid challenge test for the diagnosis of occult heart failure. Chest 2021; 159: 791–797. doi: 10.1016/j.chest.2020.08.019 [DOI] [PubMed] [Google Scholar]
- 79.van de Bovenkamp AA, Wijkstra N, Oosterveer FPT, et al. The value of passive leg raise during right heart catheterization in diagnosing heart failure with preserved ejection fraction. Circ Heart Fail 2022; 15: e008935. doi: 10.1161/CIRCHEARTFAILURE.121.008935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agrawal V, D'Alto M, Naeije R, et al. Echocardiographic detection of occult diastolic dysfunction in pulmonary hypertension after fluid challenge. J Am Heart Assoc 2019; 8: e012504. doi: 10.1161/JAHA.119.012504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moghaddam N, Swiston JR, Levy RD, et al. Clinical and hemodynamic factors in predicting response to fluid challenge during right heart catheterization. Pulm Circ 2019; 9: 2045894018819803. doi: 10.1177/2045894018819803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baratto C, Caravita S, Soranna D, et al. Current limitations of invasive exercise hemodynamics for the diagnosis of heart failure with preserved ejection fraction. Circ Heart Fail 2021; 14: e007555. doi: 10.1161/CIRCHEARTFAILURE.120.007555 [DOI] [PubMed] [Google Scholar]
- 83.Ho JE, Zern EK, Lau ES, et al. Exercise pulmonary hypertension predicts clinical outcomes in patients with dyspnea on effort. J Am Coll Cardiol 2020; 75: 17–26. doi: 10.1016/j.jacc.2019.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Omote K, Verbrugge FH, Sorimachi H, et al. Central haemodynamic abnormalities and outcome in patients with unexplained dyspnoea. Eur J Heart Fail 2023; 25: 185–196. doi: 10.1002/ejhf.2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Müller J, Mayer L, Schneider SR, et al. Pulmonary arterial wedge pressure increase during exercise in patients diagnosed with pulmonary arterial or chronic thromboembolic pulmonary hypertension. ERJ Open Res 2023; 9: 00379-2023. doi: 10.1183/23120541.00379-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berlier C, Saxer S, Lichtblau M, et al. Influence of upright versus supine position on resting and exercise hemodynamics in patients assessed for pulmonary hypertension. J Am Heart Assoc 2022; 11: e023839. doi: 10.1161/JAHA.121.023839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grinstein J, Sinha SS, Goswami RM, et al. Variation in hemodynamic assessment and interpretation: a call to standardize the right heart catheterization. J Card Fail 2023; 29: 1507–1518. doi: 10.1016/j.cardfail.2023.06.009 [DOI] [PubMed] [Google Scholar]
- 88.Borlaug BA, Blair J, Bergmann MW, et al. Latent pulmonary vascular disease may alter the response to therapeutic atrial shunt device in heart failure. Circulation 2022; 145: 1592–1604. doi: 10.1161/CIRCULATIONAHA.122.059486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caravita S, Baratto C, Filippo A, et al. Shedding light on latent pulmonary vascular disease in heart failure with preserved ejection fraction. JACC Heart Fail 2023; 11: 1427–1438. doi: 10.1016/j.jchf.2023.03.003 [DOI] [PubMed] [Google Scholar]
- 90.Schwartzenberg S, Redfield MM, From AM, et al. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol 2012; 59: 442–451. doi: 10.1016/j.jacc.2011.09.062 [DOI] [PubMed] [Google Scholar]
- 91.Ghio S, Crimi G, Houston B, et al. Nonresponse to acute vasodilator challenge and prognosis in heart failure with pulmonary hypertension. J Card Fail 2021; 8: 869–876. doi: 10.1016/j.cardfail.2021.01.021 [DOI] [PubMed] [Google Scholar]
- 92.Krishtopaytis E, Al Ampriti S, Obeidat M, et al. Can inhaled nitric oxide response predict tolerance to therapies and survival in patients with combined precapillary and postcapillary pulmonary hypertension? Am J Cardiol 2023; 207: 363–369. doi: 10.1016/j.amjcard.2023.09.032 [DOI] [PubMed] [Google Scholar]
- 93.Gorter TM, Obokata M, Reddy YNV, et al. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 2018; 39: 2825–2835. doi: 10.1093/eurheartj/ehy331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ewert R, Heine A, Müller-Heinrich A, et al. Exercise and fluid challenge during right heart catheterisation for evaluation of dyspnoea. Pulm Circ 2020; 10: 10.1177_2045894020917887. doi: 10.1177/2045894020917887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Montané B, Tonelli AR, Arunachalam A, et al. Hemodynamic responses to provocative maneuvers during right heart catheterization. Ann Am Thorac Soc 2022; 19: 1977–1985. doi: 10.1513/AnnalsATS.202201-077OC [DOI] [PubMed] [Google Scholar]
- 96.Moghaddam N, Swiston JR, Weatherald J, et al. Impact of saline loading at cardiac catheterization on the classification and management of patients evaluated for pulmonary hypertension. Int J Cardiol 2020; 306: 181–186. doi: 10.1016/j.ijcard.2019.11.104 [DOI] [PubMed] [Google Scholar]
- 97.Tossavainen E, Wikström G, Henein MY, et al. Passive leg-lifting in heart failure patients predicts exercise-induced rise in left ventricular filling pressures. Clin Res Cardiol 2020; 109: 498–507. doi: 10.1007/s00392-019-01531-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baratto C, Caravita S, Dewachter C, et al. Right heart adaptation to exercise in pulmonary hypertension: an invasive hemodynamic study. J Card Fail 2023; 29: 1261–1272. doi: 10.1016/j.cardfail.2023.04.009 [DOI] [PubMed] [Google Scholar]
- 99.Hardin KM, Giverts I, Campain J, et al. Systemic arterial oxygen levels differentiate pre- and post-capillary predominant hemodynamic abnormalities during exercise in undifferentiated dyspnea on exertion. J Card Fail 2024; 30: 39–47. doi: 10.1016/j.cardfail.2023.05.023 [DOI] [PubMed] [Google Scholar]
- 100.D'Alto M, Liccardo B, Di Maio M, et al. Lung ultrasound, echocardiography, and fluid challenge for the differential diagnosis of pulmonary hypertension. J Am Soc Echocardiogr 2023; 36: 1181–1189. doi: 10.1016/j.echo.2023.07.010 [DOI] [PubMed] [Google Scholar]
- 101.Opiz CF, Hoeper MM, Gibbs JSR, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol 2016; 68: 368–378. doi: 10.1016/j.jacc.2016.05.047 [DOI] [PubMed] [Google Scholar]
- 102.Tennøe AH, Murbræch K, Andreassen JC, et al. Left ventricular diastolic dysfunction predicts mortality in patients with systemic sclerosis. J Am Coll Cardiol 2018; 72: 1804–1813. doi: 10.1016/j.jacc.2018.07.068 [DOI] [PubMed] [Google Scholar]
- 103.Bourji KI, Kelemen BW, Mathai SC, et al. Poor survival in patients with scleroderma and pulmonary hypertension due to heart failure with preserved ejection fraction. Pulm Circ 2017; 7: 409–420. doi: 10.1177/2045893217700438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fox BD, Shimony A, Langleben D, et al. High prevalence of occult left heart disease in scleroderma-pulmonary hypertension. Eur Respir J 2013; 42: 1083–1091. doi: 10.1183/09031936.00091212 [DOI] [PubMed] [Google Scholar]
- 105.Mohri T, Goda A, Takeuchi K, et al. High prevalence of occult left ventricular diastolic dysfunction detected by exercise stress test in systemic sclerosis. Sci Rep 2022; 12: 2423. doi: 10.1038/s41598-022-06400-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.D'Alto M, Romeo E, Argiento P, et al. Hemodynamic changes after acute fluid loading in patients with systemic sclerosis without pulmonary hypertension. Pulm Circ 2019; 9: 2045894018816089. doi: 10.1177/2045894018816089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kuwana M, Blair C, Takahashi T, et al. Initial combination therapy of ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH) in the modified intention-to-treat population of the AMBITION study: post hoc analysis. Ann Rheum Dis 2020; 79: 626–634. doi: 10.1136/annrheumdis-2019-216274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Swinnen K, Verstraete K, Baratto C, et al. Machine learning to differentiate pulmonary hypertension due to left heart disease from pulmonary arterial hypertension. ERJ Open Res 2023; 9: 00229-2023. doi: 10.1183/23120541.00229-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Z-W, Chung Y-W, Cheng J-F, et al. Right ventricular-vascular uncoupling predicts pulmonary hypertension in clinically diagnosed heart failure with preserved ejection fraction. J Am Heart Assoc 2024; 13: e030025. doi: 10.1161/JAHA.123.030025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.La Gerche A, Howeden EJ, Haykowsky MJ, et al. Heart failure with preserved ejection fraction as an exercise deficiency syndrome: JACC Focus Seminar 2/4. J Am Coll Cardiol 2022; 80: 1177–1191. doi: 10.1016/j.jacc.2022.07.011 [DOI] [PubMed] [Google Scholar]
- 111.Ho J, Zern EK, Wooster L, et al. Differential clinical profiles, exercise responses, and outcomes associated with existing HFpEF definitions. Circulation 2019; 140: 353–365. doi: 10.1161/CIRCULATIONAHA.118.039136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3526. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 113.Burkoff D, Borlaug BA, Shah SJ, et al. Levosimendan improves hemodynamics and exercise tolerance in PH-HFpEF: results of the randomized placebo-controlled HELP trial. JACC Heart Fail 2021; 9: 360–370. doi: 10.1016/j.jchf.2021.01.015 [DOI] [PubMed] [Google Scholar]
- 114.Dachs TM, Duca F, Rettl R, et al. Riociguat in pulmonary hypertension and heart failure with preserved ejection fraction: the haemoDYNAMIC trial. Eur Heart J 2022; 43: 3402–3413. doi: 10.1093/eurheartj/ehac389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.García-Álvarez A, Blanco I, García-Lunar I, et al. β3 adrenergic agonist treatment in chronic pulmonary hypertension associated with heart failure (SPHERE-HF): a double blind, placebo-controlled, randomized clinical trial. Eur J Heart Fail 2023; 25: 373–385. doi: 10.1002/ejhf.2745 [DOI] [PubMed] [Google Scholar]
- 116.Voors A. SERENADE: Macitentan in Heart Failure with preSERved ejEction fractioN and pulmonAry Vascular DiseasE. Presented at the European Society of Cardiology on 21 May 2022; Barcelona, Spain. https://esc365.escardio.org/presentation/250300.
- 117.Hoeper MM, Oerke B, Wissmüller M, et al. Tadalafil for treatment of combined postcapillary and precapillary pulmonary hypertension in patients with heart failure and preserved ejection fraction: a randomized controlled phase 3 study. Circulation 2024; 150: 600–610. doi: 10.1161/CIRCULATIONAHA.124.069340 [DOI] [PubMed] [Google Scholar]
- 118.Nayak A, Hicks AJ, Morris AA. Understanding the complexity of heart failure risk and treatment in black patients. Circ Heart Fail 2020; 13: e007264. doi: 10.1161/CIRCHEARTFAILURE.120.007264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang RS, Huang S, Waldo SW, et al. Elevated pulmonary arterial compliance is associated with survival in pulmonary hypertension: results from a novel network medicine analysis. Am J Respir Crit Care Med 2023; 208: 312–321. doi: 10.1164/rccm.202211-2097OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eliott J, Menakuru N, Martin JK, et al. iCPET calculator: a web-based application to standardize the calculation of alpha distensibility in patients with pulmonary arterial hypertension. J Am Heart Assoc 2023; 12: e0299667. doi: 10.1161/JAHA.123.029667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mauri L, D'Agostino RB. Challenges in the design and interpretation of noninferiority trials. N Engl J Med 2017; 377: 1357–1367. doi: 10.1056/NEJMra1510063 [DOI] [PubMed] [Google Scholar]
- 122.Pallmann P, Bedding AW, Choodari-Oskooei B, et al. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med 2018; 16: 29. doi: 10.1186/s12916-018-1017-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ford I, Norrie J. Pragmatic trials. N Engl J Med 2016; 375: 454–463. doi: 10.1056/NEJMra1510059 [DOI] [PubMed] [Google Scholar]
- 124.Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med 2023; 389: 1069–1084. doi: 10.1056/NEJMoa2306963 [DOI] [PubMed] [Google Scholar]
- 125.Mebazza A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet 2022; 400: 1938–1952. doi: 10.1016/S0140-6736(22)02076-1 [DOI] [PubMed] [Google Scholar]
- 126.Schupp JC, Adams TS, Cosme C, et al. Integrated single-cell atlas of endothelial cells of the human lung. Circulation 2021; 144: 286–302. doi: 10.1161/CIRCULATIONAHA.120.052318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: a review. JAMA 2023; 329: 827–838. doi: 10.1001/jama.2023.2020 [DOI] [PubMed] [Google Scholar]
- 128.Rossitto G, Mary S, McAllister C, et al. Reduced lymphastic reserve in heart failure with preserved ejection fraction. J Am Coll Cardiol 2020; 76: 2817–2829. doi: 10.1016/j.jacc.2020.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cramariuc D, Alfraidi H, Nagata Y, et al. Atrial dysfunction in significant atrial functional mitral regurgitation: phenotypes and prognostic implications. Circ Cardiovasc Imaging 2023; 16: e015089. doi: 10.1161/CIRCIMAGING.122.015089 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01344-2024.Shareable (282.5KB, pdf)