Abstract
Pulmonary hypertension (PH) associated with chronic lung disease (CLD) is both common and underrecognised. The presence of PH in the setting of lung disease has been consistently shown to be associated with worse outcomes. Recent epidemiological studies have advanced understanding of the heterogeneity of this patient population and shown that defining both the specific type of CLD as well as the severity of PH (i.e. deeper phenotyping) is necessary to inform natural history and prognosis. A systematic diagnostic approach to screening and confirmation of suspected PH in CLD is recommended. Numerous uncontrolled studies and one phase 3 randomised, controlled trial have suggested a benefit in treating PH in some patients with CLD, specifically those with fibrotic interstitial lung disease (ILD). However, other studies in diseases such as COPD-PH showed adverse outcomes with some therapies. Given the expanding list of approved pharmacological treatments for pulmonary arterial hypertension, developing a treatment algorithm for specific phenotypes of CLD-PH is required. This article will summarise existing data in COPD, ILD and other chronic lung diseases, and provide recommendations for classification of CLD-PH and approach to the diagnosis and management of these challenging patients.
Shareable abstract
This manuscript includes a summary of the newly published data since the 6th WSPH, existing gaps in evidence and recommendations for future research directions for pulmonary hypertension associated with chronic lung diseases. https://bit.ly/3xPsnLe
Introduction
It has long been known that chronic lung disease (CLD) is frequently complicated by pulmonary hypertension (PH) and right ventricular (RV) dysfunction. Despite this clear association, the prevalence, clinical significance and treatment options for PH in CLD are still under investigation. The heterogeneity of PH in CLD increases the difficulty in characterising this group. In addition, the definitions of PH in general have evolved. Since the 6th World Symposium on Pulmonary Hypertension (WSPH), the definition of PH was further revised by the 2022 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines to include patients with a mean pulmonary arterial pressure (mPAP) >20 mmHg and a pulmonary vascular resistance (PVR) >2 Wood Units (WU) [1, 2]. How this change affects estimates of PH prevalence in CLD is not entirely known. However, it is inevitable that lowering the PVR threshold will increase the number of patients with CLD diagnosed with PH [3–7]. For example, in a study of 8991 patients with idiopathic pulmonary fibrosis (IPF) listed for lung transplantation through the United Network for Organ Sharing, PH was more prevalent using the 6th WSPH definition (73.6%) compared to the former version (47.6%), with the pre-capillary phenotype being present in 36.8% of IPF patients [8]. Similarly, the prevalence of PH associated with COPD numerically increased from 52.4% to 82.4%, of whom 28.1% had pre-capillary PH [8]. The prevalence data for interstitial lung disease (ILD)-PH or COPD-PH defined by PVR >2 WU have yet to be published.
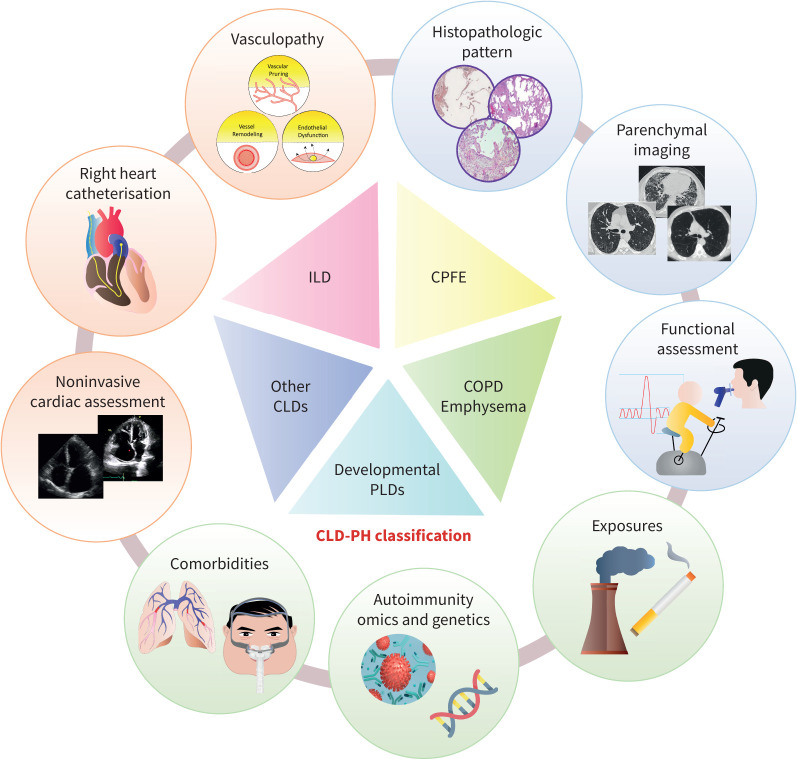
In addition to better understanding the epidemiology of PH in CLD, defining distinct clinical phenotypes is critical in both understanding unique pathogenetic mechanisms (supplementary material) as well as in designing and testing therapies. Until recently, various phenotypes of CLD-PH have been grouped together in both registries and clinical trials. Although there are some commonalities among various phenotypes of CLD-PH, recent literature demonstrates that there are important pathogenic differences contributing to PH complicating different CLDs (figure 1). Based on the emerging data on different behavioural disease patterns according to the underlying parenchymal disease, group 3 classification was updated by the 7th WSPH (figure 1) [9].
FIGURE 1.
Major components of the updated classification of group 3 pulmonary hypertension (PH). Chronic lung diseases (CLDs) encompass a wide range of parenchymal processes which often have unique pathogenetic mechanisms and clinical presentations. Recent literature demonstrates that there are important pathogenic differences contributing to PH complicating different CLDs, which is likely to impact how these entities respond to therapy. These considerations led to a change in classification of group 3 PH that placed COPD, interstitial lung disease (ILD), combined pulmonary fibrosis emphysema (CPFE) and other parenchymal lung diseases in different categories, in addition to developmental lung diseases, nonparenchymal restriction and hypoxia without lung disease (which are not represented on the figure). Making a diagnosis of CLD-PH requires a multimodal approach, with particular emphasis on a high-quality radiographic assessment.
This article focuses primarily on COPD-PH and ILD-PH, and describes subphenotypes of both conditions based on parenchymal disease and haemodynamic impairment, outlines a diagnostic algorithm, summarises current data on safety and efficacy of treatments, and offers a pragmatic therapeutic approach to the management of ILD-PH. It provides an update on PH associated with several other chronic lung diseases, including several non-group 3 diseases that often involve parenchyma, such as sarcoidosis and Langerhans cell histiocytosis. Finally, current gaps in evidence are identified and directions for future research are provided.
PH in the setting of COPD and emphysema
Prevalence, mortality and predictors of outcomes
COPD is a common condition in the general population, with a 7–9% prevalence in developed countries, and hence might be a comorbidity in any type of PH [10]. Furthermore, COPD is frequently associated with other diseases that also cause PH, in particular left heart disease [11]. Most studies on PH in COPD have been conducted in patients who were candidates for lung volume reduction surgery or lung transplantation or patients with severe PH [3, 6, 8]. When considering PH phenotypes in COPD, it is necessary to place them in the context of the airway/parenchymal disease and concomitant comorbidities. COPD severity is commonly categorised using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria based on post-bronchodilator forced expiratory volume in 1 s (FEV1) percentage (stage 1: FEV1 ≥80%; stage 2: FEV1 50–79%; stage 3: FEV1 30–49%; and stage 4: FEV1 <30%) [12]. COPD comprises a range of patterns of emphysema, chronic bronchitis and nonemphysematous obstruction due to small-airways disease that vary among individuals [13]. Importantly, individuals with similar levels of physiological impairment may have very different computed tomography (CT) morphological appearances. Additionally, cigarette smokers who do not have obstructive physiology can exhibit emphysema on CT scan [14]. Given that a subset of patients with COPD have emphysema without evidence of obstruction, we suggest incorporating the Fleischner radiographic staging (trace/mild, moderate, confluent) while phenotyping pulmonary vascular disease in patients with COPD, as CT has been validated extensively as a tool for the assessment of the presence, pattern and severity of emphysema [15].
A meta-analysis of 38 studies of patients across a wide spectrum of COPD severity showed a prevalence for PH of 39.2% [16]. In patients with severe COPD by GOLD criteria, up to 90% of patients have a mPAP >20 mmHg, with the majority ranging between 20 and 35 mmHg. The prevalence of PH in GOLD 1 and 2 COPD is much lower (∼5%) [17, 18]. Only a small subset of these patients (1–5%) have severely increased mPAP (≥35 mmHg) [19, 20].
The presence of PH is associated with worsened functional class, reduced exercise tolerance, increased rate of COPD exacerbations and hospitalisations as well as higher healthcare utilisation cost [11, 21–24]. The presence of PH is strongly associated with mortality. Several recent analyses have demonstrated that the 6-min walk distance (6MWD) and PVR were significantly associated with survival, with the latter being the strongest independent haemodynamic predictor of mortality [1, 5, 25–27].
COPD-associated PH phenotypes
Haemodynamic phenotypes
Severe COPD-PH
PH in COPD is usually of mild-to-moderate severity and progresses slowly. A subset of COPD patients develops severe PH, which is associated with higher mortality. Different cut-offs have been proposed previously to define severe PH in COPD. While analysing different cut-off values, Zeder et al. [26] found that a PVR value >5 WU had the strongest predictive value for mortality in a cohort of patients with moderate airflow obstruction. Based on these data, the 2022 ESC/ERS guidelines recommended PVR >5 WU as the haemodynamic definition of severe PH in COPD [1]. Thereafter, another study confirmed the significance of PVR >5 WU in a COPD cohort with a wider range of airway obstruction and pulmonary vasculopathy [6]. Most patients with this degree of PVR elevation usually have mPAP ≥35 mmHg, one of the prior definitions of severe PH in CLD [28].
COPD with post-capillary PH
Patients with COPD have a high prevalence of associated cardiac diseases, particularly coronary artery disease, heart failure (especially with preserved ejection fraction (HFpEF)) and atrial fibrillation, likely due to common risk factors (older age, smoking, sedentary lifestyle, obesity) and pathobiological mechanisms [29, 30]. Cardiovascular diseases are a major cause of death in COPD, particularly in patients with mild-to-moderate airflow obstruction [31]. Isolated post-capillary and combined pre-/post-capillary PH have been reported in 23% of patients with advanced COPD listed for lung transplantation [8].
Phenotypes related to severity of COPD/emphysema
PH with mild COPD (GOLD stage 1–2) and/or trace or mild centrilobular emphysema
Several publications have described a group of patients with significant PH in the presence of mild COPD including GOLD stage 1 and 2 airflow obstruction [20, 32]. Registries of patients with COPD-PH have also reported that most patients with severe PH have mild-to-moderate airflow obstruction [27, 33]. Based on previously published data, the term “pulmonary vascular phenotype” of COPD-PH has been proposed to describe patients with severe PH, moderate airflow limitation, no hypercapnia, severely reduced diffusion capacity of the lung for carbon dioxide (DLCO) and a circulatory exercise limitation or progressive RV failure [34]. The Registry to Evaluate Early and Long-Term PAH Disease (REVEAL) database reported that 20% of patients with pulmonary arterial hypertension (PAH) had COPD as comorbidity. A cluster analysis of the Comparative Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) registry identified a subgroup (52%) of patients diagnosed with idiopathic PAH (IPAH), who were characterised by male predominance, older age, history of smoking and DLCO <45% predicted, and coined it “IPAH with lung phenotype”. These patients had worse survival and poorer response to treatment than the classical IPAH phenotype [35]. An analysis of the COMPERA and Assessing the Severity of Pulmonary Hypertension in a Pulmonary Hypertension Referral Centre (ASPIRE) registries showed that patients who carried a diagnosis of IPAH with “lung phenotype” had similar characteristics to those diagnosed with the “pulmonary vascular phenotype” in terms of age, sex distribution, DLCO, arterial partial pressure of oxygen, response to PH therapies and survival [4]. This analysis was limited by the lack of radiographic data, which was not collected in COMPERA and available only in a minority of patients from the ASPIRE registry. In the cohort with available CT data, approximately half of the patients had either no or mild emphysema, and the rest had moderate-to-severe parenchymal involvement, thus raising the question of whether the latter group should have been classified as having group 3 PH for the purposes of analysis. Furthermore, a study of histological patterns in IPAH patients (excluding those with FEV1 or forced vital capacity (FVC) <60%) showed that those with nonplexiform vasculopathy, predominantly older males with a history of smoking and lower DLCO, had mild emphysema on CT in 46% of cases and, interestingly, significantly more microscopic emphysema on histopathology [36]. In addition to being captured in various registries, a portion of these patients was likely included in prior PAH clinical trials due to lack of CT imaging in the inclusion criteria and a commonly employed exclusion criterion for FEV1 of either <60% or <70% pred, thus potentially allowing patients with mild obstructive disease to be enrolled in the trials [37, 38]. Overall, these observations suggest that the lung function criterion alone is no longer valid in isolation to rule out significant parenchymal lung disease.
PH with moderate-to-severe COPD (GOLD stage 2 and 3) and/or moderate or confluent emphysema
Patients in this group most commonly present with PH of mild-to-moderate severity (mPAP 25–34 mmHg) with slow progression, although some present with severe PH [1, 16, 26, 27, 33]. Patients with mPAP ≥35 mmHg tend to have higher FEV1, lower DLCO and hypocapnia [16, 26, 27, 33]. Patients with higher mPAPs also tend to be more symptomatic, with lower 6MWD, and have higher mortality in comparison to patients with milder vasculopathy [26, 27, 33].
PH with very advanced COPD (GOLD stage 4 and/or advanced emphysema)
Patients with advanced COPD have traditionally been excluded from clinical trials. These patients have a worse prognosis than those with severe airflow obstruction and nonsevere PH or those with severe PH and mild-to-moderate airflow obstruction [16, 25]. Histopathological studies in patients with advanced COPD and severe PH who have undergone lung transplantation demonstrated a more prominent pulmonary vasculopathy than in nonsevere PH [39–41].
PH in the setting of ILD
Prevalence, mortality and predictors of outcomes
Most patients with fibrotic ILDs have mild-to-moderate PH with mPAP 21–34 mmHg, with severe lung disease occurring in 10–15% of patients [3, 6]. In a large cohort of patients with IPF listed for lung transplantation, ∼20% and ∼32% had mild and moderate haemodynamic impairment, respectively, ∼10% had severely increased mPAP >35 mmHg, and the rest had no PH [3]. Patients with ILD-PH are very symptomatic, with increased functional class, decreased 6MWD, and decreased quality of life [42–47]. Overall, the prognosis of patients with ILD-PH is poor, with 1-, 2- and 3-year survival of 73.3%, 51.2% and 34.1%, respectively, based on the data from the COMPERA registry, with similar poor survivals described in other registries [1, 5, 43–46, 48, 49]. In registries and retrospective studies, factors associated with a poor outcome in patients with ILD-PH include a higher mPAP, PVR >5 WU, cardiac index <2.5 L·min−1·m−2, increased arterial elastance, DLCO <35%, systolic (s)PAP >45 mmHg, RV dysfunction demonstrated as RV fractional area change <28% and tricuspid annular plane systolic excursion (TAPSE)/sPAP <0.26 mm·mmHg−1 on transthoracic echocardiography (TTE) and impairment of RV strain on cardiac magnetic resonance imaging (CMR) [1, 3, 5, 45, 48, 50–53].
ILD-associated PH phenotypes
Haemodynamic severity
Most data on ILD-PH emanates from studies of IPF, although some datasets include patients with other fibrotic ILDs including nonspecific interstitial pneumonitis (NSIP) and fibrotic hypersensitivity pneumonitis [3, 4, 54–59].
Based on the most recently updated definition of PH, patients with PVR >2 WU and mPAP >20 mmHg fall under the umbrella of PH. However, given the limited data available [3, 54, 55, 60–63], prospective studies are needed to describe the natural history of disease progression and the impact on exercise capacity and outcomes in patients with milder haemodynamic impairment. PH of any haemodynamic severity based on mPAP measurements has been shown to have a significant impact on morbidity and mortality. An analysis of the COMPERA registry showed that in patients with established ILD-PH, a PVR cut-off >5 WU discriminated outcomes, with a PVR cut-off level of 8 WU showing the best discrimination in survival [1, 5]. However, this dataset included patients with significant PH with median PVR of 7.76 WU with combined pulmonary fibrosis emphysema (CPFE) constituting ∼25% of the cohort, and only included patients with mPAP ≥25 mmHg. In a Japanese study of 80 patients with ILD who underwent right heart catheterisation (RHC) and had a broader range of mPAP values, even mild elevations of mPAP of 21–24 mmHg were associated with increased mortality, decreased exercise tolerance and a greater risk of acute exacerbations [64]. A recent multicentre analysis that included patients with a wider range of mPAP and PVR ≥3 WU demonstrated an increase in mortality for each 1 mmHg change of mPAP, with PVR >5 WU not being a discriminator of outcomes [6].
The predictive role of haemodynamics in ILD-PH has not been stratified based on the degree of RV function, and vice versa, but RV dysfunction has been linked to outcomes in this patient population [50, 53]. In addition, the function of RV as measured by TAPSE/sPAP appears to be similarly impaired in patients with severe and nonsevere PH as defined by PVR >5 WU [53]. Neither mPAP nor PVR cut-offs have been examined as predictors of outcomes when stratified by the severity of parenchymal lung disease. Given this information, it is likely that in ILD, the severity of PH is better conceptualised as a continuum rather than one singular marker of haemodynamic impairment.
Finally, the degree of vascular impairment in ILD may need to have different thresholds when applied to prognostication versus indications for treatment. The concept of severity in the setting of CLD was initially introduced in part to justify an individualised treatment approach in patients with severe PH that appeared to be “out of proportion” to the underlying lung disease. With the recently published data showing efficacy of inhaled treprostinil in patients with ILD with a wider range of PVR impairment, it becomes even more imperative to distinguish the markers of prognostication from the indications for treatment in ILD-PH.
PH in the setting of CTD-ILD
A number of connective tissue diseases (CTDs), including systemic sclerosis (SSc), mixed connective tissue disease (MCTD) and rheumatoid arthritis, can be complicated by fibrotic ILD [65]. A significant proportion of patients with SSc develop some ILD, and up to 30% go on to develop progressive ILD, especially in patients with a UIP pattern. Risk factors for the development of SSc-ILD include male sex, older age at disease onset, Black race, anti-topoisomerase-I antibody positivity and the diffuse cutaneous subtype [66]. Differentiation between SSc-PAH and SSc-ILD-PH can be somewhat arbitrary, as both disease states have pre-capillary PH profiles. The prevalence of SSc-ILD-PH is poorly characterised and ranges from 6.1% to 31.2% [67, 68].
The outcome data are also discrepant. A meta-analysis published in 2013 reported a 3-year survival of only 35% for SSc-ILD-PH compared to 56% in SSc-PAH [69]. In an analysis of a Spanish registry of patients with PAH, the presence of ILD did not affect the 5-year transplant-free survival with poor outcomes in both groups (SSc-ILD-PH 35% versus SSc-PAH 43.5%) [70]. Conversely, an analysis of 3257 German patients reported a 5-year survival of 79.1% for SSc-ILD-PH, compared to SSc-PAH (85%) and SSc-ILD without PH (92.8%) [67]. Another analysis of 200 patients with SSc described several clinical phenotypes in patients with ILD and PH with the highest 3-year mortality of 49.9% in patients with extensive ILD and any degree of PH. Patients with severe PH and either limited ILD or no ILD with low DLCO also had a poor 3-year survival of 61.9% [71].
In patients with MCTD, ILD with the fibrosing NSIP and UIP patterns is the most common pulmonary complication, with an estimated 50–70% of patients having CT evidence of parenchymal involvement [72]. While patients with MCTD-ILD may develop group 3 PH, the prevalence is poorly defined. In patients with rheumatoid arthritis, ILD occurs in 10–15% of cases [73, 74]. While pre-capillary PH can be caused by vasculitis, chronic thromboembolic pulmonary hypertension (CTEPH) and, very rarely, PAH, ILD is the most common cause of PH, but data are very limited [75].
In contrast to CTD-ILD conditions with predominantly inflammatory pathology (such as lupus) in which immunosuppression can improve underlying parenchymal lung disease and associated PH [76], there are no data showing that antifibrotics used in some CTD-ILDs have any effect on the development or progression of vasculopathy. Assessment of the response to treatment with PH medications comes from either registry or case report data and is rarely stratified by clinical phenotypes. One study compared treatment response between patients with SSc-ILD-PH and SSc-PAH and found that although functional class did not improve in patients with ILD-PH, they had a similar haemodynamic response to treatment as those without ILD [77]. Another paper found that in the group of patients with extensive pulmonary fibrosis and severe pre-capillary PH defined by mPAP ≥35 mmHg treated with parenteral prostanoids, the presence of autoimmune disease (mostly SSc) was associated with better transplant-free survival in comparison to those with other fibrotic lung diseases [58]. Another analysis similarly reported that treated patients with CTD-ILD-PH had better survival than patients with IPF-PH, with 5-year survival rates of 56% versus 15% [48].
Patients with CTD-ILD-PH represent a varied and heterogeneous group of patients and is distinct from other forms of ILD-PH in its epidemiology, natural history, response to treatment and outcomes. This patient population should be studied prospectively to gain better understanding of how particular diseases behave clinically, as this will have a potential impact on the design and outcomes of future clinical trials.
PH in patients with mild ILD
Similarly to patients with mild COPD, based on the data from the COMPERA/ASPIRE registries, patients with mild ILD and significant PH are likely to be older, predominantly male, often current/ex-smokers with lower body mass index in comparison to patients with IPAH. Physiologically, these patients have either preserved or mild restriction, severely decreased DLCO <45% pred and lower 6MWD compared to patients with IPAH [4, 35, 78, 79]. When available, CT findings often show either no interstitial lung abnormalities or minimal to mild ILD, but in most registries CT data were not collected. One study showed that 19% of patients with nonsevere ILD-PH and 9% of those with severe PH had <20% fibrosis on chest imaging [80]. Histopathological studies of patients classified as “IPAH with lung phenotype” demonstrate microscopic changes of ILD, further indicating that these patients represent a separate clinical phenotype from IPAH patients [38, 81, 82]. Registry data also suggest that the presence of any degree of interstitial fibrosis has significant prognostic impact in patients classified as IPAH. PH in mild ILD is associated with worse survival compared to patients with classic IPAH and has similar outcomes to those diagnosed with group 3 ILD-PH [78, 79, 83].
ILD-PH with predominantly post-capillary PH
Patients with ILD tend to be older and are likely to have cardiovascular comorbidities (such as obesity, obstructive sleep apnoea, systemic hypertension, atrial fibrillation and coronary artery disease) that puts them at risk of developing post-capillary PH or combined pre- and post-capillary PH [8, 84]. Post-capillary PH is found in 5–20% of patients with ILD and portends a better prognosis than pre-capillary PH [8, 85, 86]. Incorrect phenotyping of these patients may lead to both undertreating and potentially worsening underlying heart failure.
CPFE-associated PH
CPFE is a tobacco-related ILD characterised by emphysema predominantly in the upper lobes and often paraseptal regions (≥5% of total lung volume) and fibrosis in the lower zones of the lungs on HRCT imaging. It is present in up to 25–50% of patients with idiopathic interstitial pneumonias (IIPs) [59]. Fibrosis can be due to UIP, fibrotic NSIP, smoking-related interstitial fibrosis and desquamative interstitial pneumonia. It can also be seen in CTDs (including in never-smokers), fibrotic hypersensitivity pneumonitis and ILD due to occupational and inhalational exposures. CPFE is characterised by male predominance, severe dyspnoea and exercise limitation, relatively preserved lung volumes and airflow, severely altered DLCO and profound desaturation on exercise and poor prognosis [59, 87].
PH in the setting of CPFE occurs in 30–50% of patients, has a poor prognosis and tends to be more severe in comparison to those with either fibrosis or emphysema alone [88, 89]. For a given extent of fibrosis, presence of emphysema contributes to the risk of development of PH compared to those with fibrosis alone, and may increase mortality [90]. However, the likelihood of PH does not differ between patients with CPFE and those with fibrosis alone when matched based on the extent of disease on HRCT [88, 89]. Patients with CPFE-PH are more symptomatic, have higher oxygen requirements and markedly impaired cardiopulmonary exercise testing (CPET) results [91].
Diagnosis of PH in chronic lung disease
There is a significant overlap in symptomatology of CLD and PH, which may lead to delay in diagnosis of the pulmonary vascular component [33, 92]. The 6th WSPH laid the groundwork for the diagnosis of PH in the setting of CLDs using the suspect/support/confirm/stratify strategy [3]. Subsequently, many authors, including the taskforce for the most recent 2022 ESC/ERS guidelines, have published algorithms for how to monitor and assess these patients for development of PH [1, 93, 94].
PH in the setting of CLDs may coexist with other PH aetiologies. Therefore, patients with PH should be evaluated for concomitant PAH risk factors, such as HIV infection, CTD, portal hypertension or CTEPH [95]. Phenotyping such patients can be especially complicated and depends on the extent of each respective contributing factor. These patients should be referred to PH centres for further workup and management [96].
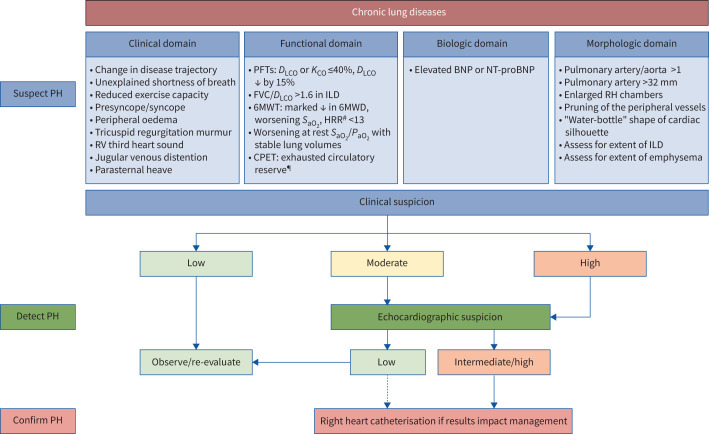
Assessment for PH in the setting of CLDs should be made when the patient is clinically stable, as acute disease exacerbations can significantly raise pulmonary arterial pressures. In addition, other comorbidities, such as thyroid disease and anaemia, that can cause hyperdynamic circulation, should be treated. Key parts of evaluating suspected PH in lung disease include integrating clinical features and available diagnostic modalities from the following list to assess for the need for RHC (figure 2).
1) Clinical features, including disease trajectory (e.g. rapid recent deterioration versus gradual change over years), increased oxygen requirements and the presence or absence of risk factors for PAH (i.e. autoimmunity, specific drug exposure, HIV), CTEPH (history of venothromboembolism), or HFpEF risk factors.
2) Pulmonary function tests, including DLCO, 6-min walk test (6MWT) variables such as distance and degree of desaturation, assessment of oxygenation and ventilation by arterial blood gases and CPET.
3) Findings on cross-sectional imaging with HRCT, CT pulmonary angiogram (CTPA) and in selected cases, CMR.
4) Brain natriuretic peptide (BNP)/N-terminal pro-BNP (NT-proBNP) measurements.
5) TTE.
FIGURE 2.
Diagnostic algorithm for pulmonary hypertension (PH) in the setting of COPD and interstitial lung disease (ILD). The decision to perform a right heart catheterisation should be predicated by a clinical or research need that would impact management and/or provide prognostic information, such as considerations of disease phenotyping and therapeutic interventions, clinical trial enrolment, pre-operative clearance/assistance with perioperative management, evaluation for lung transplantation and, in COPD, assessment for lung volume reduction therapy. A multimodal assessment, including lung function, functional, radiographic and echocardiographic data is recommended to determine when to proceed with haemodynamic assessment. Given that in patients with chronic lung diseases the performance characteristics of transthoracic echocardiography are worse due to poor windows, altered location of the heart in the chest cavity and interference due to the lung tissue overlying the heart, in patients with moderate or high clinical suspicion for PH, right heart catheterisation can be considered irrespective of the transthoracic echocardiography findings (as indicated by the dotted line). RV: right ventricular; PFTs: pulmonary function tests; DLCO: diffusion capacity of the lung for carbon monoxide; KCO: carbon monoxide transfer coefficient; FVC: forced vital capacity; 6MWT: 6-min walk test; 6MWD: 6-min walk distance; SaO2: arterial haemoglobin oxygen saturation; HRR: heart rate recovery; PaO2: arterial partial pressure of oxygen; CPET: cardiopulmonary exercise testing; BNP: brain natriuretic peptide; NT-proBNP: N-terminal pro-BNP; RH: right heart. #: in idiopathic pulmonary fibrosis; ¶: especially in severe PH.
In many CLDs, PH is associated with diminished exercise capacity and a greater than expected impairment of gas exchange at rest or during exercise based on ventilatory limitation alone. Suggestive findings include a reduction in 6MWD, pronounced exertional desaturation, discrepancy between pulmonary function tests and symptoms, and functional class 3–4 dyspnoea [3, 22, 27, 33, 44, 64, 97, 98].
Pulmonary function tests are an essential part of the workup and evaluation of a patient with CLD. Decreased DLCO, especially in a setting of stable parenchymal disease, has been shown to be associated with PH and has prognostic significance in patients with various ILDs and COPD [65, 99]. In addition, in both COPD-PH and ILD-PH, a decreased DLCO correlates with worsened survival [33, 48, 100, 101]. In fibrotic hypersensitivity pneumonitis, patients also have a higher FVC/DLCO ratio [102]. In most CLDs, the diagnosis of PH is associated with a decreased distance and worsening desaturation during the 6MWT. In addition, in IPF heart rate recovery (a difference between the heart rate at 1 min of recovery and the heart rate at the end of the 6MWT) <13 is associated with both presence of PH and decreased survival [3]. CPET may be helpful in assessing whether the effort dyspnoea and exercise limitation are due to ventilatory or cardiovascular limitation, although data are lacking regarding its clinical use in patients with CLD-PH [103, 104].
While the literature supports the correlation of BNP/NT-proBNP with right atrial pressure on RHC, and as a predictor of mortality in patients with established PH, their role in screening for CLD-PH is less defined, especially as they can be elevated in left heart disease pathology, renal failure and in acute pulmonary embolism and coronary syndrome [105].
In patients with CLDs, the performance characteristics of TTE are often affected by poor windows, altered location of the heart in the chest cavity, and interference due to the lung tissue overlying the heart [106]. Use of an sPAP on TTE ≥45 mmHg, or abnormal RV findings, including hypertrophy, dilatation or RV systolic dysfunction in the absence of an elevated sPAP, has been shown to predict the presence of PH on RHC in patients with ILDs and in mixed cohorts of CLD [107, 108]. In COPD-PH, a low TAPSE/sPAP ratio (a surrogate of RV–pulmonary artery (PA) uncoupling) has been shown to be associated with disease severity, exacerbations of COPD and survival [109]. In an IPF cohort both without PH and mild-to-moderate PH, the TAPSE/sPAP ratio was shown to be associated with all-cause mortality and hospitalisations [51]. In addition, a cut-off of 0.26 mm·mmHg−1 for TAPSE/sPAP discriminated between severe and nonsevere PH based on the 6th WSPH definition and predicted mortality in a cohort of patients with ILD and COPD [53]. Another study suggests that a stepwise composite TTE score can identify patients with severe PH, with and without an estimate of tricuspid regurgitant velocity, using other echocardiographic features including right atrial area, RV:left ventricular (LV) ratio, and LV eccentricity index [80]. TTE also provides assessment of the left heart as patients with CLD are at risk for HFpEF [6, 8, 30, 84, 110, 111]. Machine learning methods by TTE to identify the presence of PH promises to be the way of the future in patients with various forms of PH [112, 113].
Findings on CT imaging have been used as potential predictors of ILD-PH and COPD-PH [3]. HRCT imaging allows for the characterisation and extent of parenchymal disease. It may also enable morphological phenotyping of patients, as well as evaluation of other potential causes of PH such as pulmonary veno-occlusive disease (PVOD) [79]. In both ILD and COPD, the ratio of the PA diameter to the diameter of the ascending aorta >1 on CT angiogram is associated with both the presence of PH and increased mortality [114–119]. In IPF, segmental PA to segmental bronchus diameter also correlates well to PH measured on RHC [120]. In ILD, main PA diameter ≥29 mm has a strong correlation with haemodynamic diagnosis of PH and is not influenced by severity of underlying ILD [118, 120, 121]. In ILD, an increased RV:LV ratio has high correlation with survival in patients with ILD-PH [80]. In addition, CT-measured RV and LV volumes have been shown to have an inverse correlation with TTE-based estimates of sPAP [122]. Measurements of small vessels surface area and bronchial wall thickness are emerging CT markers and might be incorporated in predicting and phenotyping PH in COPD in the future [123, 124]. Automated artificial intelligence derived RV/LV ratios have been shown to have a higher sensitivity for PH in comparison to a manually measured PA/A ratio and correlate with survival [125]. CTPA can also be valuable to detect signs of acute pulmonary embolism or chronic thromboembolic pulmonary disease, given the higher risk of venothromboembolism in both ILD and COPD [126–129]. More work needs to be done to determine which CT characteristics might be useful in combination.
In COPD, CMR appears to have a promising role in the early noninvasive assessment of PH. Recent studies demonstrated that parameters of PA stiffness such as pulse wave velocity have high sensitivity and specificity for identifying PH [130]. In addition, RV mass index and various PA measurements correlate with prognosis [131]. In a cohort with varied CLDs, several computational models using CMR were shown to detect severe PH and correlated with survival [124].
Scoring systems
A number of scoring systems to aid in risk stratification for ILD-PH and COPD-PH have been published recently, with several validated in external cohorts (table 1). The ongoing Pulmonary Hypertension Screening in Patients with Interstitial Lung Disease for Earlier Detection (PHINDER) study of patients with ILD is prospectively collecting data with the goal of deriving a scoring system to help identify those with a high likelihood of concomitant PH [137]. Finally, a modified Delphi consensus statement recommended a stepwise approach using a combination of symptoms, signs of RV dysfunction, low DLCO, findings on chest CT, elevation in NT-proBNP/BNP and TTE RV abnormalities to guide the timing of RHC in patients with CLD-PH [94].
TABLE 1.
Scoring systems for detection of pulmonary hypertension (PH) in interstitial lung disease (ILD) and COPD
| First author, year [reference] | Subjects n | Scoring variables | Outcomes |
|---|---|---|---|
| ILD | |||
| Nathan, 2024# [132] | Derivation cohort: 481 IPF Validation cohort: 204 IPF |
FORD scoring system and FORD index: FVC%/DLCO% ratio Oxygenation nadir during 6MWT Race 6MWD |
PH (2022 ESC/ERS) AUC 0.75 Score ≥33 sensitivity 70% specificity 73% Score ≥53 specificity 98% |
| Joseph, 2023 [133] | 66 ILD | Gas exchange-derived PVC ΔETCO2 (CO measure) TTE sPAP Elevated FVC/DLCO |
PH (2022 ESC/ERS) AUC 0.94 sensitivity 0.86 specificity 0.93 |
| Parikh, 2022#
[134] Parikh, 2023 [135] |
Derivation cohort: 154 ILD Validation cohort: 161 ILD |
History Exam 6MWD DLCO CTA PA/A ratio BNP/NT-proBNP |
PH (6th WSPH) Score ≥6 (0–12) AUC 0.920 sensitivity 86.5% specificity 86.3% |
| Refini, 2021 [120] | 37 IPF | TTE sPAP PA area Diameter of the segmental artery to that of the adjacent bronchus in the apicoposterior segment of the left upper lobe ratio |
mPAP sensitivity 100% specificity 53% |
| Sobiecka, 2020 [136] | 93 ILD | Age 6MWD TLC/DLCO ratio |
Echocardiography sPAP AUC ROC 0.867 |
| Tello, 2019# [53] | 172 CLD 94 ILD 78 COPD |
TAPSE/sPAP ratio | Severe PH (6th WSPH) TAPSE/sPAP ratio 0.26 mm·mmHg−1 sensitivity 80.6% specificity 71.2% |
| Sonti, 2019 [42] | 105 IPF | sPAP FVC/DLCO PA/A ratio |
PH (6th WSPH) NPV 87.2% |
| Bax, 2018# [80] | Derivation cohort: 210 ILD Validation cohort: 61 ILD |
Stepwise echocardiographic algorithm | Severe PH (6th WSPH) Score ≥7 sensitivity 89% specificity 71% PPV 68% NPV 90% AUC 84.8% |
| Furukawa, 2018 [99] | 273 IPF |
DLCO PA/A ratio on CT ≥0.9 PaO2 <80 Torr |
mPAP AUC ROC 0.757 (95% CI 0.682–0.833) |
| COPD | |||
| Kovacs, 2022 [25] | 142 | TTE NT-proBNP PA/A diameter ratio |
Haemodynamically defined pre-capillary severe PH (6th WSPH) PPV 94% NPV 94% |
| Coste, 2019 [123] | 24 | CT-measured bronchial wall thickness % of cross-sectional area of PV <5 mm2 PaO2 |
Sensitivity of 87.5% for severe PH (6th WSPH) |
IPF: idiopathic pulmonary fibrosis; FORD: forced vital capacity (FVC)/diffusing capacity for of the lung for carbon monoxide (DLCO) ratio (F), oxygen saturation nadir during 6-min walk test (6MWT) (O), race (R) and distance ambulated during 6MWT (D); 6MWD: 6-min walk distance; ESC: European Society of Cardiology; ERS: European Respiratory Society; AUC: area under the curve; PVC: pulmonary vascular compliance; ΔETCO2: change in end-tidal carbon dioxide; CO: cardiac output; TTE: transthoracic echocardiography; sPAP: pulmonary artery systolic pressure; CTA: computed tomography angiogram; PA: pulmonary artery; A: aorta; BNP: brain natriuretic peptide; NT-proBNP: N-terminal pro-BNP; WSPH: World Symposium on Pulmonary Hypertension; mPAP: mean pulmonary arterial pressure; TLC: total lung capacity; ROC: receiver operating characteristic; CLD: chronic lung disease; TAPSE: tricuspid annular plane systolic excursion; NPV: negative predictive value; PPV: positive predictive value; CT: computed tomography; PaO2: arterial partial pressure of oxygen; PV: pulmonary volume. #: includes a validation cohort.
Right heart catheterisation
RHC is essential for the diagnosis and phenotyping of PH in CLD. RHC should be considered in instances where the findings will provide prognostic information and result in the change of management such as:
1) pre-operative clearance/assistance with perioperative management;
2) phenotyping of disease and consideration of therapeutic PH interventions;
3) clinical trial enrolment;
4) evaluation for lung transplantation;
5) assessment for lung volume reduction therapy in COPD.
Haemodynamic assessment should be conducted when patients are euvolemic, clinically stable, and the treatment of the underlying lung disease and comorbid conditions have been optimised (including oxygen therapy where indicated). Consideration should be given to where in the respiratory cycle haemodynamic measurements should be made, due to large respirophasic changes, especially in COPD and obese patients. We concur with current recommendations to average all the pressure measurements over several respiratory cycles (instead of using end expiration measurements), obtain pulmonary artery wedge pressure (PAWP) saturation, and consider directly measuring left ventricular end-diastolic pressure (LVEDP) if the PAWP waveform and saturation cannot be obtained, or the values are implausible [1]. In addition, cardiac output should be measured by direct Fick or thermodilution output methods if direct Fick is not available. In patients with risk factors for and TTE features of HFpEF, post-capillary PH should be strongly considered and provocative manoeuvres such as fluid challenge or exercise RHC should be performed in patients with PAWP or LVEDP ≤15 mmHg [138].
Treatment
Holistic approach to management
The treatment of PH associated with CLD is based on a holistic approach that addresses management of the underlying disease, adjunctive general measures and potentially drugs approved for PAH [1, 139]. Optimal treatment of the underlying parenchymal lung disease is the cornerstone of the holistic approach in the management of CLD-PH [1, 59, 140–143]. To what extent treating the underlying respiratory disease has an impact on the trajectory of associated PH is an area for future studies. Adjunctive general measures aim at managing comorbidities such as sleep disordered breathing, comorbid cardiac disease, nutritional status and other factors that may contribute to the pathogenesis and severity of PH, such as hypoxaemia, smoking and acute/chronic thromboembolism. Smoking cessation and avoidance of environmental irritants should be advised if applicable. Exposure to air pollution is a risk factor for acute exacerbations in IPF and COPD, and there is also an association between PH and acute exacerbations in ILD [144]. Furthermore, a population study suggests an association between air pollution and the incidence and mortality of PH [145]. Although this is not based on clinical trial data, it would seem prudent to suggest limiting any inhalational exposures to a minimum in patients. Patients should be vaccinated against influenza, coronavirus disease 2019, Pneumococcus and respiratory syncytial virus if applicable, as most exacerbations of both COPD and ILD are thought to be triggered by respiratory tract infections.
Patients with CLDs are frequently deconditioned, and consistent exercise is recommended to maintain regular adapted activity. Pulmonary rehabilitation has not been evaluated in CLD-PH and the recommendation for pulmonary rehabilitation participation is inferred from the data on PAH, COPD and ILD [146, 147]. In COPD, pulmonary rehabilitation improves exercise capacity and quality of life, and reduces symptoms and hospitalisations [148]. In ILD, pulmonary rehabilitation has a positive effect on functional status and quality of life, including in the subgroups with PH [149, 150]. In patients with PH, pulmonary rehabilitation results in clinically relevant improvements in exercise capacity [151, 152].
PH is associated with exertional hypoxia in most CLDs. Based on the possible contribution of hypoxaemia to the pathogenesis of CLD-PH, we suggest treating hypoxaemia at rest and exertional and nocturnal hypoxaemia. In COPD, a recent meta-analysis suggested that long-term oxygen therapy resulted in mild reductions in mPAP, slowed the progression of PH and reduced mortality [153]. In addition, in patients with COPD, oxygen use for ≥15 h·day−1 may prevent a progressive increase in mPAP [154, 155]. In patients with alveolar hypoventilation, noninvasive ventilation has been shown to improve pulmonary haemodynamics, RV function and exercise capacity [156, 157].
Management of comorbidities is an integral part of the care of patients with CLD-PH. Heart failure with preserved ejection fraction can be an associated cause of dyspnoea and requires optimisation of both risk factors and fluid status [158]. Treatment of sleep disordered breathing may lead to improvement in hypoxic vasoconstriction, pulmonary endothelial dysfunction and, potentially, haemodynamics [159, 160].
Symptoms such as dyspnoea should be managed optimally according to the most recent international guidelines for each chronic respiratory disease [59, 140, 142, 143]. Palliative care should be considered early to help manage symptoms and provide emotional assistance through the course of the patient's illness [161]. Discussion about advanced directives should be conducted with patients in accordance with their individual treatment plans. Finally, PH in CLDs is an indicator for referral for evaluation lung transplantation for potentially eligible patients [162].
Treatment of patients with PH and mild COPD or mild ILD
Based on worldwide registry data, patients with mild COPD and ILD and/or a history of smoking with severely decreased DLCO and associated PH who historically have been classified as PAH are frequently treated with PH monotherapy in the real-world setting and have probably been included in clinical trials. None of the clinical trials of PH medications have included DLCO or imaging in their inclusion criteria. Analyses of various registries strongly suggest that the response to therapy is reduced in this group in comparison to those with a classic IPAH phenotype [4, 79, 83].
A single-centre study showed that patients with emphysema and preserved spirometry (mean±sd FEV1 99±19%, FEV1/FVC ratio 65±10%) with severe PH had a worse response to therapy (primarily with phosphodiesterase-5 inhibitors (PDE-5i)) as assessed by 6MWD, NT-proBNP and functional class change compared to patients with moderate spirometric impairment, although the former group remained stable [45]. An analysis of patients in the ASPIRE database who fulfilled the criteria of IPAH (PVR of ∼11 WU) with evidence of mild emphysematous (66% of cohort) or mild fibrotic (20% of cohort) changes on CT scans and preserved spirometric indices (COPD mean FEV1/FVC 66±8%, FEV1 88±16%; fibrotic ILD mean FVC 97±19%) was performed [79]. The analysis showed that this group had both poorer survival and decreased response to PH therapies compared to “classic” IPAH patients as measured by the incremental shuttle test and emPHasis-10 quality-of-life score [79]. Patients with no radiographic disease, but a DLCO <45%, had worse survival in comparison to patients with DLCO >45%, although they did demonstrate improved exercise capacity with treatment [79]. One study suggested that patients with isolated low DLCO may have an undiagnosed PVOD as an aetiology of PAH [163].
Pending more data, these patients should be referred to a PH centre for further phenotyping and management. While PH therapies may be of benefit to a subset of patients, harm was demonstrated in patients with mild IPF (FVC 68.7±13.1%) (with 15% of patients diagnosed with PH) in the ARTEMIS trial of ambrisentan, which showed an increased risk for disease progression and respiratory hospitalisations, and in the RISE-IIP trial of riociguat in IIP-PH, which was stopped early for a signal of increased mortality in the treatment arm [164]. If treated, an initial monotherapy is recommended with close monitoring for both response to therapy and adverse effects. Finally, eligible patients should be referred for lung transplant evaluation due to their poor prognosis.
COPD-PH
Recent registry data show that PH medications are frequently used in patients with COPD-PH characterised by moderate and severe obstruction. Characteristics of the populations included in the registries are heterogeneous and include patients with various degrees of haemodynamic impairment, as well as pooled cohorts with other CLDs [25, 27, 33, 44, 97, 165, 166]. Studies evaluating the use of PH therapies (PDE-5i in the majority of cases) have generally included small numbers of patients with severely impaired haemodynamics and have yielded conflicting results (table 2) [1, 3]. Several studies showed that treated and untreated patients had similar survival, despite the former group having worse clinical and haemodynamic profiles, thus suggesting that PH therapy may have a stabilising effect [27, 34]. A meta-analysis that included 23 randomised controlled trials (RCTs) (1159 patients) and 23 non-RCTs (1187 patients) published through 2020 showed that PDE-5i, but not endothelin receptor antagonists, resulted in significantly improved sPAP (pooled treatment effect −5.9 mmHg, 95% CI −10.3– −1.6 mmHg), but with inconsistent clinical benefits [153].
TABLE 2.
Clinical trials and selected retrospective studies targeting COPD, interstitial lung disease (ILD) and ILD-pulmonary hypertension (PH) with PH medications#
| First author, year [reference] | Lung disease | Study design | Subjects n | Therapy | Results | Comments |
|---|---|---|---|---|---|---|
| COPD trials | ||||||
| Vitulo, 2017 [167] | COPD-PH | RCT | 28 | Sildenafil (n=18) | Decrease in PVR, improvement in BODE, DLCO and quality of life | No adverse effect on oxygenation |
| Maron, 2022 [168] | COPD-PH | RCT | 42 | Tadalafil (n=28) | No change in PVR or mPAP at 6 months Improvement in shortness-of-breath questionnaire |
No adverse effect on oxygenation |
| Nathan, 2024 [169] | COPD-PH | RCT | 136 | Inhaled treprostinil (n=66) | Decrease in 6MWD at 12 weeks | Study terminated due to increased SAE in the treated group |
| ILD/IIP/IPF trials | ||||||
| Kolb, 2018 [170] | IPF | RCT | 274 | Nintedanib+sildenafil (n=137) | Primary end-point of change in SGRQ was not met | |
| Behr, 2021 [171] | IPF | RCT | 177 | Pirfenidone+sildenafil (n=88) | No difference in disease progression (composite end-point) | Composite end-point of decline in 6MWD and hospitalisation or all-cause mortality |
| Nathan, 2020 [ 172] | Fibrotic ILD |
RCT | 45 | iNO (n=23) | Improvement in moderate to vigorous and overall activity | Patients on supplemental oxygen |
| Bellerophon Pulse Technologies [173] | Fibrotic ILD |
RCT | 145 | iNO (n=73) | Did not improve moderate to vigorous activity | Patients on supplemental oxygen |
| PH associated with ILD/IIP/IPF trials | ||||||
| Faria-Urbina, 2018 [174] | ILD-PH | Retrospective | 22 | Treprostinil (inhaled) (n=22) |
Improvement in FC and 6MWD No change in resting oxygen requirements |
|
| Nathan, 2019 [62] | IIP-PH | RCT | 147 | Riociguat (n=73) | Terminated early for unfavourable risk/benefit profile | Post hoc analysis of CT scans suggested that advanced CPFE phenotype with emphysema >> fibrosis may have contributed to the negative signal [87] |
| Waxman, 2021 [61] | ILD-PH | RCT | 326 | Treprostinil (inhaled) (n=163) |
Improved 6MWD, NT-proBNP, clinical worsening and FVC | |
| Dawes, 2023 [175] | ILD-PH | Retrospective | 60 | PDE-5i (n=50) ERAs (n=10) |
Patients treated with sildenafil showed longer survival | No effect on V′/Q′ matching |
IIP: idiopathic interstitial pneumonia; IPF: idiopathic pulmonary fibrosis; RCT: randomised controlled trial; PVR: pulmonary vascular resistance; BODE: body mass index, airflow obstruction, dyspnoea and exercise capacity; DLCO: diffusion capacity of the lung for carbon monoxide; mPAP: mean pulmonary artery pressure; 6MWD: 6-min walk distance; SAE: serious adverse effects; SGRQ: St George's Respiratory Questionnaire; iNO: inhaled nitric oxide; FC: functional class; CT: computed tomography; CPFE: combined pulmonary fibrosis emphysema; NT-proBNP: N-terminal pro-brain natriuretic peptide; FVC: forced vital capacity; PDE-5i: phosphodiesterase-5 inhibitor; ERA: endothelin receptor antagonist; V′/Q′: ventilation/perfusion ratio. #: published after the 6th World Symposium on Pulmonary Hypertension.
Data from several registries identified a subset of patients with COPD and severe PH treated primarily with PDE-5i that had beneficial response from therapy as demonstrated by increase in 6MWD by ≥30 m, or functional class improvement or PVR decrease >20% from the baseline [27, 165]. In addition, a meta-analysis focusing on 308 patients from 13 studies with severe PH as defined by PVR >5 WU or mPAP ≥35 mmHg treated with a range of PH medications also showed a significant reduction in mPAP (−3.68 mmHg, 95% CI −2.03–−5.32 mmHg) and PVR (−1.40 WU, 95% CI −1.97–−0.82 WU) [176]. Although there were fewer treated patients who had persistent New York Heart Association functional class III/IV symptoms, there were no significant differences in post-treatment 6MWD or BNP/NT-proBNP [176]. No increased hypoxaemia was noted with treatment [176].
The largest RCT to date (PERFECT) of patients with pre-capillary PH with mPAP ≥30 mmHg and PVR ≥4 WU, in which a total of 66 COPD patients were exposed to inhaled treprostinil, was stopped early at the behest of the data safety monitoring committee for increasing the risk of serious adverse effects, as well as suggestive evidence of an increased risk of mortality [169]. All the patients had radiographic evidence of emphysema, but CT scans were not centrally adjudicated. Patients with a diagnosis of CPFE were excluded from the study. Only a minority of patients were able to achieve a goal dose of 12 puffs four times daily, and treated patients had a numeric decline in 6MWD. The most common serious adverse effects in the treatment group were COPD exacerbation and acute respiratory failure. In the post hoc analysis, patients with DLCO <25% in particular had a reduced likelihood of benefit and appeared to be at an increased risk of mortality [169]. Therefore, inhaled treprostinil is not recommended for future studies or as an off-label treatment for COPD-PH.
Taken together, these data suggest that patients with haemodynamically severe PH should be targeted in future therapeutic studies, with several trials currently in progress (table 3).
TABLE 3.
Investigational therapies for COPD-pulmonary hypertension (PH) and interstitial lung disease (ILD)-PH
| Compound, clinicaltrials.gov identifier [reference] | Pathway/mechanism | Trial | End-points | Company/institution |
|---|---|---|---|---|
| COPD-PH | ||||
| MK-5475-03, NCT05612035 [177] | Daily inhaled sGC | Phase 2a INSIGNIA-PH-COPD |
Efficacy (6MWD) and safety | Merck |
| Tadalafil, NCT05844462 [178] | Daily PDE-5i | Phase 3 ERASE PH-COPD |
Efficacy (6MWD) and safety in severe PH | Assistance Publique Hôpitaux de Paris |
| ILD-PH | ||||
| Treprostinil palmitil, NCT05176951 [179] | Daily DPI formulation of inhaled treprostinil (prostanoid) prodrug | Phase 2 extension |
Safety and tolerability | Insmed |
| Treprostinil, NCT06129240 [180] | Four times daily DPI formulation of inhaled prostanoid | Phase 3 ASCENT extension |
Safety and tolerability | Liquidia |
| Inhaled treprostinil, NCT04691154 [181] | Twice daily aerosolised liposomal prostanoid | Phase 2 open label | Safety and tolerability | Liquidia/Pharmosa |
| Hymecromone, NCT05128929 [182] | Twice daily oral coumarin derivative (inhibitor of hyaluronan synthesis) | Phase 2 SATURN study |
Safety and tolerability | Stanford University |
| Bardoxolone methyl, NCT03068130 [183] | Antioxidant (acts via Nrf2 pathway) | Phase 2 LARIAT and RANGER studies |
6 IPF-PH, 4 IIP-PH 17 CTD ILD-PH patients 6MWD change of +38 m in the IPF-PH cohort |
Reata/Biogen |
sGC: soluble guanylate cyclase; 6MWD: 6-min walk distance; PDE-5i: phosphodiesterase-5 inhibitor; DPI: dry powder inhaler; IPF: idiopathic pulmonary fibrosis; IIP: idiopathic interstitial pneumonia; CTD: connective tissue disease.
ILD-PH
Until recently, most large clinical trials targeting ILD with PH drugs have focused on ILD without documented PH, with disappointing and sometimes harmful results (tables 2 and 3) [3, 139]. In haemodynamically diagnosed ILD-PH, a meta-analysis published in 2017 investigated the effect of PH therapy on 6MWT in two RCTs and four single-arm studies [184]. The meta-analysis of the single-arm studies which included treatment mostly with PDE-5i and subcutaneous treprostinil showed a significant improvement in 6MWD (46.2 m, 95% CI 27.9–64.4 m). However, the collated RCTs did not demonstrate a statistically significant improvement in the 6MWD (21.6 m, 95% CI −17.8–61.0 m). Notably, treatment with PH drugs did not worsen hypoxaemia. One of the largest trials in haemodynamically diagnosed IIP-PH of riociguat (RISE-IIP) was stopped early for a signal of increased mortality in treated patients [62]. In addition, in the ARTEMIS trial of ambrisentan, the drug was not effective in treating IPF and was associated with an increased risk of disease progression and respiratory hospitalisations in treated patients [85]. Based on these data, and in keeping with prior guidelines, the use of riociguat in patients with IIP-PH and ambrisentan in patients with IPF is contraindicated [61, 96].
Clinical trial data for sildenafil are somewhat mixed and have been summarised elsewhere [3, 61, 139]. A Bayesian retrospective analysis of a cohort of ILD-PH patients showed that patients treated with PDE-5i had a longer survival and the survival difference was larger in patients with normal RV function [175]. Based on these data, PDE-5i may be considered in patients with PH associated with ILD on an individual basis, especially in those with severe haemodynamic impairment [96].
Prostanoids have also been evaluated in ILD-PH patients and are summarised elsewhere [3, 139]. The INCREASE trial, a 16-week RCT of inhaled treprostinil delivered by an ultrasound pulse nebuliser, examined the effect of the drug on patients with various forms of ILD-PH (including IPF, non-IPF IIPs, CPFE, CTD-ILD) with mPAP ≥25 mmHg and PVR >3 WU. The study met its primary end-point of change in 6MWD and secondary end-points including change in NT-proBNP and time to clinical worsening [61]. Inhaled treprostinil was found to be well tolerated with expected side-effects of throat irritation, cough, headache and diarrhoea. The therapeutics benefits of the drug appear to be dose-related, as patients who reached a dose of nine or more breaths per session four times per day had both a lower rate of clinical worsening and a higher rate of clinical improvement in a post hoc analysis [185]. Inhaled treprostinil was approved for treatment of ILD-PH in the USA in 2021.
A post hoc analysis of the INCREASE trial showed that treated patients across all the ILD aetiologies were less likely to experience multiple disease progression events compared to placebo [186]. A post hoc win ratio analysis of the original trial data showed that the treated group had a better ratio for mortality, cardiopulmonary hospitalisations, 6MWD decline and clinical improvement [187]. The INCREASE long-term open-label extension data which included a majority of the original cohort (74%) demonstrated minimal decline of exercise capacity over time [188]. Another analysis of the open-label extension dataset suggested that patients without a treatment delay had improved exercise capacity as measured by 6MWD after 1 year of treatment compared with those with a 16-week treatment delay (22.1 m versus −10.3 m) [189]. In addition, in the open-label extension dataset analysis without a treatment delay had a longer time to hospitalisation, exacerbation of lung disease and death in comparison to the original placebo group [189]. Finally, two statistical prediction analyses that account for switching in open-label extension dataset analysis suggested that treatment might be associated with a significant reduction in death [190].
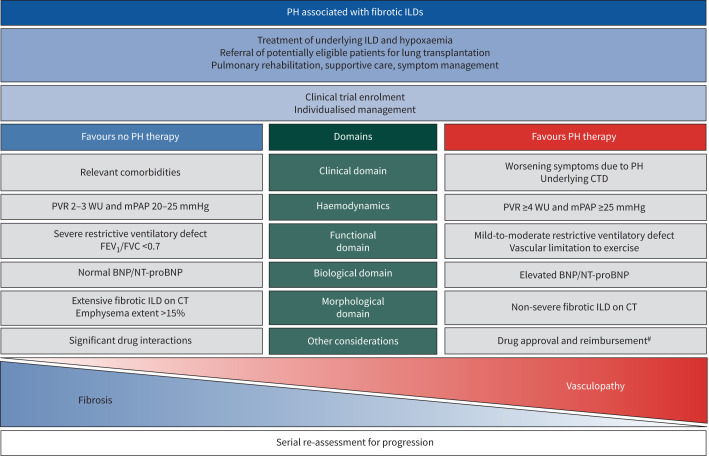
In a pre-specified analysis of the study, patients with milder PH defined by PVR <4 WU, who constituted 20% of the cohort, did not have improvement in 6MWD [61]. Another post hoc analysis examined whether inhaled treprostinil had any clinical benefit in this group of patients [189]. Although not powered for statistical significance, the study showed numerically positive effect of treatment on acute lung disease exacerbation, clinical disease progression (defined as hospitalisation due to a cardiopulmonary indication, >15% 6MWD decline from baseline, lung transplantation or death) and disease progression (defined as time to acute exacerbation or clinical disease progression) [189]. Several new formulations of inhaled treprostinil are currently being studied for ILD-PH. These trials, along with several other studies, are summarised in table 3. A proposed framework on how to approach treatment of ILD-PH is presented in figure 3.
FIGURE 3.
Framework for management of patients with interstitial lung disease (ILD)-associated pulmonary hypertension (PH). This figure represents a framework for management of PH in patients with ILD. The columns “Favours PH therapy” and “Does not favour PH therapy” are not prescriptive and are meant to guide providers in selecting appropriate patients for treatment. Each patient needs to be evaluated in the context of their disease and their treatment needs to be individualised. Please refer to the text for further details. PVR: pulmonary vascular resistance; WU: Wood units; mPAP: mean pulmonary arterial pressure; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; BNP: brain natriuretic peptide; NT-proBNP: N-terminal pro-BNP; CT: computed tomography; CTD: connective tissue disease. #: inhaled treprostinil where approved; off-label use of other PH drugs such as phosphodiesterase-5 inhibitors in an individualised manner (ambrisentan is contraindicated in idiopathic pulmonary fibrosis and riociguat is contraindicated in idiopathic interstitial pneumonia-PH).
CPFE-PH
In CPFE-PH, the extent of emphysema may affect how patients respond to treatment with PH therapy. A single study examining the effect of treatment with PH drugs (PDE-5i in ∼70% of patients) showed significant decrease in NT-proBNP and absence of deterioration in other parameters documented by stability in functional class distribution and numerical improvement in 6MWD [45]. However, a post hoc analysis of the RISE IIP trial of riociguat in ILD-PH which was stopped early for increased mortality in the treated group suggested that patients whose CT scans had more emphysema than fibrosis may have contributed to the negative outcomes of the trial [87]. Therefore, based on the data from RISE-IIP and PERFECT trials, future studies for CPFE-PH should target patients with predominantly fibrotic and not emphysematous phenotype. In addition, future registries should collect high-quality HRCT scan data to better understand if there is a difference in response to treatment based on a particular phenotype of CPFE.
PH in other parenchymal lung diseases
PH in the setting of other parenchymal lung diseases with mixed restrictive/obstructive physiology, such as lymphangioleiomyomatosis (LAM) and bronchiectasis, may have features unique to that parenchymal disease or group of diseases.
PH associated with LAM (group 3.4)
Both the previous WSPH and the latest ESC/ERS guidelines re-classified LAM-PH from group 5 PH to group 3 PH, as it is thought to be mainly linked to parenchymal destruction [1, 2].
LAM-PH is infrequent, with cohort studies showing a low prevalence (∼7–8%) with mild haemodynamic impairment [191, 192]. A small retrospective cohort of patients with LAM-PH showed that while most patients had mild PH, the lung transplantation-free survival was 87% at 1 and 56% at 3 years, suggesting that mortality is mainly driven by parenchymal involvement [191]. However, the presence of PH does appear to affect symptoms and exercise capacity, as measured by 6MWD [193].
It has been speculated that activation of the mammalian target of rapamycin (mTOR) pathway is involved in the development of LAM-PH [193, 194]. Sirolimus, an mTORC1 inhibitor, is a recommended treatment for LAM with parenchymal involvement with salutary effects on the rate of decline in FEV1 as well as improved quality of life [195]. A single-centre study in 162 LAM patients supports the long-term efficacy of sirolimus, with stabilisation of lung function among patients treated for >1 year [196]. It is conceivable that sirolimus, by stabilising and improving parenchymal disease, could positively impact the development and/or severity of PH, as suggested by a study with TTE data which showed an improvement in sPAP in 10 treated patients [193]. Off-label use of PH medications has been shown to improve haemodynamics, but has only been reported in case series [191].
PH associated with bronchiectasis (group 3.4)
PH may also complicate the course of advanced bronchiectasis and cystic fibrosis. The prevalence of PH in bronchiectasis has not been characterised extensively, with particularly few studies reporting haemodynamic confirmation of PH; these data derive mostly from pre-transplant literature [197, 198]. PH is usually mild to moderate in severity and primarily diagnosed in patients with advanced parenchymal involvement [199, 200]. In cystic fibrosis, PH is associated with increased rate of acute exacerbations [201]. Furthermore, the presence of severe PH is associated with increased risk of waitlist mortality in patients listed for lung transplantation [197, 198]. In non-cystic fibrosis bronchiectasis, PH is also associated with increased mortality with a 5-year survival of ∼65% [202, 203]. Data on treatment of PH associated with bronchiectasis are very limited and come from either very small case series or single case reports [202]. In appropriate candidates, patients with bronchiectasis and PH should be referred for lung transplantation workup [162].
PH in other parenchymal lung diseases with multiple pathogenic mechanisms
PH associated with pulmonary sarcoidosis (group 5.2)
The prevalence of sarcoidosis-associated PH (SAPH) is reported to be 3–5% in the general sarcoidosis population, but significantly higher (up to 98%) in patients with dyspnoea or referred for lung transplantation [204–207]. Although SAPH can occur without parenchymal involvement, the destruction of the vascular bed by parenchymal fibrosis appears to be the leading cause, as most patients have Scadding stage 4 sarcoidosis [206–209]. However, the pathogenesis of SAPH is multifactorial and may include fibrosis-associated remodelling and obliteration of pulmonary vessels, extrinsic compression of central pulmonary vessels by lymphadenopathy or mediastinal fibrosis, PVOD-like lesions, granulomatous involvement of pulmonary vessels, left heart dysfunction and portopulmonary hypertension. Therefore, SAPH remains classified in group 5 PH [2]. The diagnostic workup of SAPH should focus on determining the leading pathogenic mechanism of PH, as it may have a direct impact on the treatment approach [204].
SAPH is associated with significant symptomatology and morbidity, clinically evidenced by worsening World Health Organization functional class, decreased 6MWD, increased desaturation and oxygen use [204, 210]. The presence of PH is an independent predictor of mortality with a 5-year transplant-free survival of 66.3% and is an indication for lung transplant workup referral [208, 211]. Analyses of a large SAPH cohort (RESAPH registry) showed that a PVR ≥5 WU, mPAP ≥40 mmHg and 6MWD <300 m were associated with a decreased transplant-free survival, while a low FEV1/FVC ratio was associated with improved survival, suggesting that patients with obstruction may represent a subset with predominantly airway involvement and possibly less likelihood for contiguous vascular disease [208, 212].
A small (n=16) double-blind placebo-controlled trial of riociguat in patients with parenchymal SAPH showed improvement in 6MWD and decrease in time to clinical worsening [213]. A small trial of inhaled treprostinil is currently enrolling patients [214]. Due to lack of robust RCT data, patients with SAPH should be referred to PH centres for phenotyping and individualised approach to therapy.
PH associated with pulmonary Langerhans cell histiocytosis (group 5.2)
Langerhans cell histiocytosis is a disease characterised by clonal expansion of myeloid precursors that infiltrate organs and differentiate into dendritic Langerhans cells [215]. PH is a frequent complication of pulmonary Langerhans cell histiocytosis (PLCH), with a prevalence of up 41% and cumulative incidence of 4.5% at 5 years in incident patients [216, 217]. There is discrepancy between the haemodynamic severity and the degree of parenchymal lung involvement. Thus, PLCH remains classified in group 5 PH due to a multifactorial pathogenesis including diffuse proliferative vasculopathy of the small-to-medium-sized pulmonary vessels, especially pulmonary arterioles and venules with direct infiltration with Langerhans cells and PA remodelling seen in the vasculature away from the active PLCH lesions [218, 219]. Exercise intolerance is multifactorial, with both ventilatory and cardiocirculatory limitations [220]. With limited data available, PLCH-PH is associated with significant functional limitation, as assessed by CPET and 6MWT, and appears to impact mortality [220, 221].
Data on the use of PAH therapies in the treatment of PLCH-PH remain limited and are based on retrospective cohorts of monotherapy, with the suggestion of clinical and haemodynamic improvement [222–224]. However, management of these patients should be undertaken in PH centres, as some studies reported severe acute pulmonary oedema with intravenous epoprostenol, possibly due to venous involvement by the Langerhans cells [223]. In patients with advanced disease, with or without PH, lung transplantation remains the only possible option.
PH associated with neurofibromatosis 1 (group 5.2)
PH associated with neurofibromatosis type 1 (NF-1) is often associated with lung disease, characterised by cysts and ground-glass opacities in 75% of cases [225]. Haemodynamic impairment is usually severe, even in the setting of mild parenchymal disease. Histopathological data demonstrate significant pulmonary vascular remodelling with both arterial and venous thickening and features consistent with nonspecific interstitial pneumonia [225]. PH in NF-1 is associated with severe functional limitation and prognosis is poor, with transplant-free survival at 5 years of 42% [225].
Post-tuberculosis PH
Many patients with tuberculosis (TB) develop long-term pulmonary complications, including PH [226, 227]. However, the frequency of post-TB PH is not well known, as TB is more common in low- and middle-income countries, where RHC is not routinely available. In a recent meta-analysis of 14 studies reporting the prevalence of PH in patients with post-TB lung disease, only four papers reported haemodynamically diagnosed data, while the others reported TTE-based results [227]. The prevalence of PH ranged from 35% to 95% in patients with chronic respiratory failure, from 27% to 54% in symptomatic hospitalised patients, and up to 9% in asymptomatic outpatients. Post-TB PH seems to affect a younger age group compared to other parenchymal disease related-PH, with the most affected age-category being 40–60 years. Among the patients with post-TB lung disease, males are almost three times more likely to develop PH [228].
The mechanisms of PH in post-TB lung disease are thought to be mainly due to the destruction of the vascular bed, although the data remain limited. One study demonstrated a significant association between the number of previous episodes of TB and the development of PH, with each episode found to increase the odds of developing PH by 2.13-fold [229]. In addition, hypoxic vasoconstriction that can induce pulmonary vascular remodelling, chronic inflammation due to tobacco, biomass and environmental exposures, chronic thromboembolic pulmonary disease, concomitant small airway disease or emphysema as well as cofactors such as HIV infection and viral hepatitis-induced liver disease may be contributory [227]. TB is a known cause of mediastinal fibrosis, another potential mechanism or contributor to the development of PH [230]. Finally, TB increases the risk of cardiovascular disease, thus evoking post-capillary PH as a potential aetiology or contributor to post-TB PH [231].
As in other lung diseases, PH portends a poor prognosis. Patients with post-TB PH have longer hospital stays and increased re-admission rates compared to those without PH [232]. Additionally, the presence of PH may increase the risk of acute exacerbations of respiratory symptoms, thus further decreasing functional capacity and worsening quality of life [233]. The only data on the efficacy and tolerability of specific PH treatments for post-TB PH come from case reports. Therefore, further research is needed to understand the pathophysiology and prevalence of post-TB PH, to phenotype this disease and to determine effective treatment modalities.
Future directions and clinical trial design
Research on treatments for PH associated with CLDs has been mostly marked by “negative” clinical trials, in which a particular treatment was not shown to be beneficial. Existing gaps in knowledge are summarised in table 4. To increase the likelihood of success in the treatment development in group 3 PH, we propose the following.
TABLE 4.
Existing gaps in evidence on treatments for pulmonary hypertension (PH) associated with chronic lung disease (CLD)
| Gaps in the evidence | What is needed |
|---|---|
| Data on the incidence and prevalence of CLD-PH (and its subtypes) using the updated definition of PH | Multinational prospective registries with accurate and consistent use of diagnostic criteria and haemodynamic definitions |
| Phenotyping of PH in CLD | High-quality imaging and blood biobanking for omics and genetics studies |
| Accurate confirmation of the type and extent of parenchymal disease. Why are some CLDs associated with more severe PH than others? | Systematic collection and adjudication of both HRCT description and imaging data More sophisticated imaging modalities as well as use of AI and deep learning HRCT parenchymal imaging should be incorporated in the inclusion and exclusion criteria for group 1 PAH studies |
| Demographic, epidemiologic and clinical data on CLD-PH in patients with PVR 2–3 WU and mPAP 20–25 mmHg | This patient group needs to be studied prospectively to better understand disease progression and impact on outcomes, as well as the role of PH therapy on outcomes |
| Uniform definition or understanding of CPFE-PH, which is a distinct phenotype both in clinical behaviour and response to therapy | Pre-specified subgroup analyses of CPFE-PH patients in future clinical trials |
| Validated multimodal risk assessment scores to predict outcomes in the CLD-PH population | Prospective studies to identify such scoring systems |
| Validated predictive scoring systems to predict the presence of PH on CLD | Prospective studies to derive scores with subsequent validation cohorts |
| Delineation of pathogenetic and pathophysiologic pathways leading to PH in rare parenchymal lung diseases | Continued development of more precise animal models Procurement of human tissue or cells (e.g. explanted lung, other novel methods to acquire pulmonary vascular cells) Identification of circulating biomarkers that may be relevant to understanding the pathobiology of these diseases |
| Knowledge about the effect and safety of specific treatments for pulmonary vasculopathy, and impact of specific treatments for underlying respiratory diseases on the development and clinical behaviour of PH. Prognostic and assessment criteria and end-points to judge the efficacy of treatments for these diseases | Expansion of secondary and exploratory end-points in future clinical trials to understand which end-points are most relevant to CLD-PH patients Implementation of novel, adaptive trial designs in future CLD-PH trials |
| Lack of CLD-PH-specific patient-reported outcomes | Collection and validation of data to develop CLD-PH-specific patient-reported outcomes |
PVR: pulmonary vascular resistance; WU: Wood units; mPAP: mean pulmonary arterial pressure; HRCT: high-resolution computed tomography; AI: artificial intelligence; PAH: pulmonary arterial hypertension; CPFE: combined pulmonary fibrosis and emphysema.
Patient phenotyping
It remains fundamental to properly identify and phenotype underlying lung diseases and vasculopathy for the purpose of clinical trials, as the effect and tolerance of treatments can differ according to the underlying phenotype [234]. We recommend collecting and centrally adjudicating the CT scans in the studies for ILD-PH and COPD-PH. Furthermore, development of objective digital imaging scoring systems to quantify and assess distribution of the underlying parenchymal patterns is recommended.
Trial end-points and duration of the treatment trials
A robust clinical end-point should measure a physiologically relevant variable, improvement or deterioration of the clinical condition, disease specific biomarker and/or “how a patient feels, functions, and/or survives” with only the latter currently accepted by the US Food and Drug Administration for approval in the USA. Changes in haemodynamics, especially PVR, are best suited to phase 2 clinical trials. In addition to 6MWT, the most employed end-point in PAH trials and the INCREASE study of ILD-PH [185], composite end-points with two or more distinct efficacy outcomes with recognised clinically meaningful implications may offer advantages, including increased number of events with a smaller sample size. However, composite end-points can also be dominated by frequently occurring and less clinically meaningful events (6MWD decrease rather than hospitalisation). Therefore, the choice of variables in a composite of end-points is open to some debate. The elements of these end-points should consist of variables that measure how patients feel, function and/or survive. In addition, a composite of events that show improvement in the patients’ clinical status may also be considered. Ultimately, new therapies should result in improved activity levels. Changes in moderate activity assessed by accelerometry and actigraphy have been proposed as a new clinically relevant end-point in cardiopulmonary diseases; however, further refinement and validation is required [235].
Trial designs
Novel trial designs are needed to maximise the likelihood of success in finding new treatments for PH due to parenchymal lung diseases. For example, reducing the number of patients in the placebo arm is a major challenge, and can be achieved by “pooling” placebo-arm data, i.e. an approach in which several treatments are compared with one placebo arm or in which the placebo arms from phases 2 and 3 clinical trials are combined, perhaps as part of a seamless transition from phase 2 to 3. Prioritising components of composite end-points by utilising hierarchical end-points, analysed with statistical methodologies such as the win ratio, facilitates differentiation of the clinical impact of parameters included in a composite criterion. For example, the win ratio was used in a post hoc analysis of the INCREASE trial and suggested a beneficial impact of inhaled treprostinil on mortality, hospitalisation, a composite assessment of disease exacerbation and clinical improvement [187]. However, the win ratio, like all composites, is typically driven by effects of the intervention on measurements of greatest frequency that often have lesser clinical importance, like changes in a biomarker [236]. Therefore, the elements of the composite end-point to be analysed using the win ratio must be defined in a pre-specified manner, chosen because of their clinical relevance, and ranked according to clinical significance [237]. Finally, adaptive trial designs such as randomised discontinuation trials in which all patients are started on active treatment with subsequent randomisation to either treatment versus converting to placebo once safety has been documented may result in an overall decreased trial time [234]. Furthermore, adaptive multi-interventional trial platforms where different interventions are conducted in parallel to one placebo arm and possibly embedded into clinical practice and/or registry data collection may optimise resources, increase trial accessibility for the patients and ultimately accelerate the discovery of new, effective therapies [238].
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01200-2024.Supplement (150.2KB, pdf)
Shareable PDF
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.01222-2024
Conflict of interest: O.A. Shlobin reports consultancy fees from United Therapeutics, Merck, Janssen and Aerami, payment or honoraria for lectures, presentations, manuscript writing or educational events from Ferrera and United Therapeutics, participation on a data safety monitoring board or advisory board with Janssen, and leadership roles with ACCP/Chest and World Symposium on Pulmonary Hypertension task force. Y. Adir reports consultancy fees and payment or honoraria for lectures, presentations, manuscript writing or educational events from MSD Israel and Bayer Israel, support for attending meetings from BI Israel and RAFA Israel, and participation on a data safety monitoring board or advisory board with MSD, GB and Acceleron. J.A. Barbera reports grants from Merck Sharp & Dome and Ferrer International, consultancy fees from Merck Sharp & Dome, Janssen-Cilag and Ferrer International, payment or honoraria for lectures, presentations, manuscript writing or educational events from Merck Sharp & Dome, Janssen-Cilag, Ferrer International and AOP Orphan, support for attending meetings from Merck Sharp & Dome and Janssen-Cilag, and a leadership role with the World Symposium on Pulmonary Hypertension task force. V. Cottin reports consultancy fees and payment or honoraria for lectures, presentations, or educational events from Ferrer/United Therapeutics. S. Harari reports consultancy fees from Roche-Boehringer Ingelheim, payment or honoraria for lectures, presentations, manuscript writing or educational events from Boehringer Ingelheim, participation on a data safety monitoring board or advisory board “Prise en charge pragmatique de la fibrose pulmonaire idiopathique en progression: essai randomisé PROGRESSION-IPF”, and leadership roles with the World Symposium on Pulmonary Hypertension task force, FERS of the ERS and officer of the society, and FATS of the ATS. E-M. Jutant reports consultancy fees from Chiesi, payment or honoraria for lectures, presentations, manuscript writing or educational events from Chiesi, GSK, MSD and AstraZeneca, support for attending meetings from Janssen and MSD, and a leadership role with the World Symposium on Pulmonary Hypertension task force. J. Pepke-Zaba reports grants from MSD, consultancy fees from MSD, Janssen, Gossamer and Ferrer, support for attending meetings from Janssen, and a leadership role with the World Symposium on Pulmonary Hypertension task force. H-A. Ghofrani reports consultancy fees from Gossamer Bio, Inc., Aerovate, Altavant, Bayer AG, Attgeno, Janssen/Actelion, MSD/Acceleron, Pfizer, Liquidia, Morphic and Keros, payment or honoraria for lectures, presentations, manuscript writing or educational events from Bayer AG, Janssen/Actelion, Gossamer Bio, Keros and MSD/Acceleron, participation on a data safety monitoring board or advisory board with Aerovate, Altavant, Attgeno, Janssen/Actelion, Insmed, MSD/Acceleron, Pfizer and Bayer AG, and the following financial (or non-financial) interests: the author's spouse is and employee of Liquidia. R. Channick reports consultancy fees from Bayer, Merck, Gossamer, Respira and Janssen, payment or honoraria for lectures, presentations, manuscript writing or educational events from Bayer and Janssen, and participation on a data safety monitoring board or advisory board with Altavant.
References
- 1.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023; 61: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53: 1801914. doi: 10.1183/13993003.01914-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoeper MM, Dwivedi K, Pausch C, et al. Phenotyping of idiopathic pulmonary arterial hypertension: a registry analysis. Lancet Respir Med 2022; 10: 937–948. doi: 10.1016/S2213-2600(22)00097-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsson KM, Hoeper MM, Pausch C, et al. Pulmonary vascular resistance predicts mortality in patients with pulmonary hypertension associated with interstitial lung disease: results from the COMPERA registry. Eur Respir J 2021; 58: 2101483. doi: 10.1183/13993003.01483-2021 [DOI] [PubMed] [Google Scholar]
- 6.Piccari L, Wort SJ, Meloni F, et al. The effect of borderline pulmonary hypertension on survival in chronic lung disease. Respiration 2022; 101: 717–727. doi: 10.1159/000524263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikkho SM, Richter MJ, Shen E, et al. Clinical significance of pulmonary hypertension in interstitial lung disease: a consensus statement from the Pulmonary Vascular Research Institute's innovative drug development initiative – group 3 pulmonary hypertension. Pulm Circ 2022; 12: e12127. doi: 10.1002/pul2.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan SD, Barnett SD, King CS, et al. Impact of the new definition for pulmonary hypertension in patients with lung disease: an analysis of the United Network for Organ Sharing database. Pulm Circ 2021; 11: 2045894021999960. doi: 10.1177/2045894021999960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovacs G, Bartolome S, Denton CP, et al. Definition, classification and diagnosis of pulmonary hypertension. Eur Respir J 2024; 64: 2401324. Doi: 10.1183/13993003.01324-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landis SH, Muellerova H, Mannino DM, et al. Continuing to Confront COPD International Patient Survey: methods, COPD prevalence, and disease burden in 2012–2013. Int J Chron Obstruct Pulmon Dis 2014; 9: 597–611. doi: 10.2147/COPD.S61854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabbri LM, Celli BR, Agustí A, et al. COPD and multimorbidity: recognising and addressing a syndemic occurrence. Lancet Respir Med 2023; 11: 1020–1034. doi: 10.1016/S2213-2600(23)00261-8 [DOI] [PubMed] [Google Scholar]
- 12.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for the Diagnosis, Management and Prevention of COPD. 2023. Available from: https://goldcopd.org/2023-gold-report-2/ Date last accessed: April 2022.
- 13.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010; 182: 598–604. doi: 10.1164/rccm.200912-1843CC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med 2015; 175: 1539–1549. doi: 10.1001/jamainternmed.2015.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Kaddouri B, Strand MJ, Baraghoshi D, et al. Fleischner Society visual emphysema CT patterns help predict progression of emphysema in current and former smokers: results from the COPDGene Study. Radiology 2021; 298: 441–449. doi: 10.1148/radiol.2020200563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Liu Y, Zhao S, et al. The incidence and prevalence of pulmonary hypertension in the COPD population: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2022; 17: 1365–1379. doi: 10.2147/COPD.S359873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilde JM, Skjørten I, Hansteen V, et al. Haemodynamic responses to exercise in patients with COPD. Eur Respir J 2013; 41: 1031–1041. doi: 10.1183/09031936.00085612 [DOI] [PubMed] [Google Scholar]
- 18.Portillo K, Torralba Y, Blanco I, et al. Pulmonary hemodynamic profile in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015; 10: 1313–1320. doi: 10.2147/COPD.S78180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen KH, Iversen M, Kjaergaard J, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant 2012; 31: 373–380. doi: 10.1016/j.healun.2011.11.020 [DOI] [PubMed] [Google Scholar]
- 20.Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 189–194. doi: 10.1164/rccm.200401-006OC [DOI] [PubMed] [Google Scholar]
- 21.Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186: 155–161. doi: 10.1164/rccm.201201-0034OC [DOI] [PubMed] [Google Scholar]
- 22.Torres-Castro R, Gimeno-Santos E, Vilaró J, et al. Effect of pulmonary hypertension on exercise tolerance in patients with COPD: a prognostic systematic review and meta-analysis. Eur Respir Rev 2021; 30: 200321. doi: 10.1183/16000617.0321-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klinger JR, Wu B, Morland K, et al. Burden of pulmonary hypertension due to chronic obstructive pulmonary disease: analysis of exacerbations and healthcare resource utilization in the United States. Respir Med 2023; 219: 107412. doi: 10.1016/j.rmed.2023.107412 [DOI] [PubMed] [Google Scholar]
- 24.Weiss T, Near AM, Zhao X, et al. Healthcare resource utilization in patients with pulmonary hypertension associated with chronic obstructive pulmonary disease (PH-COPD): a real-world data analysis. BMC Pulm Med 2023; 23: 455. doi: 10.1186/s12890-023-02698-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovacs G, Avian A, Bachmaier G, et al. Severe pulmonary hypertension in COPD: impact on survival and diagnostic approach. Chest 2022; 162: 202–212. doi: 10.1016/j.chest.2022.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeder K, Avian A, Bachmaier G, et al. Elevated pulmonary vascular resistance predicts mortality in COPD patients. Eur Respir J 2021; 58: 2100944. doi: 10.1183/13993003.00944-2021 [DOI] [PubMed] [Google Scholar]
- 27.Vizza CD, Hoeper MM, Huscher D, et al. Pulmonary hypertension in patients with COPD: results from the comparative, prospective registry of newly initiated therapies for pulmonary hypertension (COMPERA). Chest 2021; 160: 678–689. doi: 10.1016/j.chest.2021.02.012 [DOI] [PubMed] [Google Scholar]
- 28.Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62: 25 Suppl., D109–D116. doi: 10.1016/j.jacc.2013.10.036 [DOI] [PubMed] [Google Scholar]
- 29.Roversi S, Fabbri LM, Sin DD, et al. Chronic obstructive pulmonary disease and cardiac diseases. An urgent need for integrated care. Am J Respir Crit Care Med 2016; 194: 1319–1336. doi: 10.1164/rccm.201604-0690SO [DOI] [PubMed] [Google Scholar]
- 30.Piccari L, Del Pozo R, Blanco I, et al. Association between systemic and pulmonary vascular dysfunction in COPD. Int J Chron Obstruct Pulmon Dis 2020; 15: 2037–2047. doi: 10.2147/COPD.S257679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labaki WW, Gu T, Murray S, et al. Causes of and clinical features associated with death in tobacco cigarette users by lung function impairment. Am J Respir Crit Care Med 2023; 208: 451–460. doi: 10.1164/rccm.202210-1887OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest 2005; 127: 1531–1536. doi: 10.1378/chest.127.5.1531 [DOI] [PubMed] [Google Scholar]
- 33.Dauriat G, Reynaud-Gaubert M, Cottin V, et al. Severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a prospective French multicenter cohort. J Heart Lung Transplant 2021; 40: 1009–1018. doi: 10.1016/j.healun.2021.04.021 [DOI] [PubMed] [Google Scholar]
- 34.Kovacs G, Agusti A, Barberà JA, et al. Pulmonary vascular involvement in chronic obstructive pulmonary disease. Is there a pulmonary vascular phenotype? Am J Respir Crit Care Med 2018; 198: 1000–1011. doi: 10.1164/rccm.201801-0095PP [DOI] [PubMed] [Google Scholar]
- 35.Hoeper MM, Pausch C, Grünig E, et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant 2020; 39: 1435–1444. doi: 10.1016/j.healun.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 36.Nossent EJ, Smits J, Seegers C, et al. Clinical correlates of a nonplexiform vasculopathy in patients with a diagnosis of idiopathic pulmonary arterial hypertension. Chest 2024; 166: 190–200. doi: 10.1016/j.chest.2024.02.046 [DOI] [PubMed] [Google Scholar]
- 37.Olsson KM, Corte TJ, Kamp JC, et al. Pulmonary hypertension associated with lung disease: new insights into pathomechanisms, diagnosis, and management. Lancet Respir Med 2023; 11: 820–835. doi: 10.1016/S2213-2600(23)00259-X [DOI] [PubMed] [Google Scholar]
- 38.Godinas L, Harari S, Barberà JA, et al. Mild parenchymal lung disease is still lung disease. Eur Respir J 2020; 56: 2003542. doi: 10.1183/13993003.03542-2020 [DOI] [PubMed] [Google Scholar]
- 39.Bunel V, Guyard A, Dauriat G, et al. Pulmonary arterial histologic lesions in patients with COPD with severe pulmonary hypertension. Chest 2019; 156: 33–44. doi: 10.1016/j.chest.2019.02.333 [DOI] [PubMed] [Google Scholar]
- 40.Carlsen J, Hasseriis Andersen K, Boesgaard S, et al. Pulmonary arterial lesions in explanted lungs after transplantation correlate with severity of pulmonary hypertension in chronic obstructive pulmonary disease. J Heart Lung Transplant 2013; 32: 347–354. doi: 10.1016/j.healun.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 41.Zeder K, Marsh LM, Avian A, et al. Compartment-specific remodeling patterns in end-stage chronic obstructive pulmonary disease with and without severe pulmonary hypertension. J Heart Lung Transplant 2024; 43: 1090–1101. doi: 10.1016/j.healun.2024.02.1044 [DOI] [PubMed] [Google Scholar]
- 42.Sonti R, Gersten RA, Barnett S, et al. Multimodal noninvasive prediction of pulmonary hypertension in IPF. Clin Respir J 2019; 13: 567–573. doi: 10.1111/crj.13059 [DOI] [PubMed] [Google Scholar]
- 43.Hoeper MM, Behr J, Held M, et al. Pulmonary hypertension in patients with chronic fibrosing idiopathic interstitial pneumonias. PLoS One 2015; 10: e0141911. doi: 10.1371/journal.pone.0141911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gall H, Felix JF, Schneck FK, et al. The Giessen Pulmonary Hypertension Registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant 2017; 36: 957–967. doi: 10.1016/j.healun.2017.02.016 [DOI] [PubMed] [Google Scholar]
- 45.Brewis MJ, Church AC, Johnson MK, et al. Severe pulmonary hypertension in lung disease: phenotypes and response to treatment. Eur Respir J 2015; 46: 1378–1389. doi: 10.1183/13993003.02307-2014 [DOI] [PubMed] [Google Scholar]
- 46.Chebib N, Mornex JF, Traclet J, et al. Pulmonary hypertension in chronic lung diseases: comparison to other pulmonary hypertension groups. Pulm Circ 2018; 8: 2045894018775056. doi: 10.1177/2045894018775056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piccari L, Kovacs G, Jones S, et al. The European Voice of the Patient living with pulmonary hypertension associated with interstitial lung disease: diagnosis, symptoms, impacts, and treatments. Pulm Circ 2024; 14: e12405. doi: 10.1002/pul2.12405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alhamad EH, Cal JG, Alrajhi NN, et al. Predictors of mortality in patients with interstitial lung disease-associated pulmonary hypertension. J Clin Med 2020; 9: 3828. doi: 10.3390/jcm9123828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hemnes AR, Leopold JA, Radeva MK, et al. Clinical characteristics and transplant-free survival across the spectrum of pulmonary vascular disease. J Am Coll Cardiol 2022; 80: 697–718. doi: 10.1016/j.jacc.2022.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prins KW, Rose L, Archer SL, et al. Clinical determinants and prognostic implications of right ventricular dysfunction in pulmonary hypertension caused by chronic lung disease. J Am Heart Assoc 2019; 8: e011464. doi: 10.1161/JAHA.118.011464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonaglioni A, Caminati A, Nicolosi GL, et al. Incremental prognostic value of arterial elastance in mild-to-moderate idiopathic pulmonary fibrosis. Int J Cardiovasc Imaging 2022; 38: 1473–1485. doi: 10.1007/s10554-022-02541-y [DOI] [PubMed] [Google Scholar]
- 52.Kamide H, Kato S, Hayakawa K, et al. Impairment of right ventricular strain evaluated by cardiovascular magnetic resonance feature tracking in patients with interstitial lung disease. Int J Cardiovasc Imaging 2021; 37: 1073–1083. doi: 10.1007/s10554-020-02079-x [DOI] [PubMed] [Google Scholar]
- 53.Tello K, Ghofrani HA, Heinze C, et al. A simple echocardiographic estimate of right ventricular-arterial coupling to assess severity and outcome in pulmonary hypertension on chronic lung disease. Eur Respir J 2019; 54: 1802435. doi: 10.1183/13993003.02435-2018 [DOI] [PubMed] [Google Scholar]
- 54.King CS, Brown AW, Shlobin OA, et al. Prevalence and impact of WHO group 3 pulmonary hypertension in advanced idiopathic nonspecific interstitial pneumonia. Eur Respir J 2018; 52: 1800545. doi: 10.1183/13993003.00545-2018 [DOI] [PubMed] [Google Scholar]
- 55.Girgis RE, Hoeper MM. Pulmonary hypertension in fibrosing idiopathic interstitial pneumonia: uncertainties, challenges and opportunities. J Heart Lung Transplant 2021; 40: 872–881. doi: 10.1016/j.healun.2021.03.004 [DOI] [PubMed] [Google Scholar]
- 56.Piccari L, Allwood B, Antoniou K, et al. Pathogenesis, clinical features, and phenotypes of pulmonary hypertension associated with interstitial lung disease: a consensus statement from the Pulmonary Vascular Research Institute's Innovative Drug Development Initiative – Group 3 Pulmonary Hypertension. Pulm Circ 2023; 13: e12213. doi: 10.1002/pul2.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Launay D, Sobanski V, Hachulla E, et al. Pulmonary hypertension in systemic sclerosis: different phenotypes. Eur Respir Rev 2017; 26: 170056. doi: 10.1183/16000617.0056-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saggar R, Giri PC, Deng C, et al. Significance of autoimmune disease in severe pulmonary hypertension complicating extensive pulmonary fibrosis: a prospective cohort study. Pulm Circ 2021; 11: 20458940211011329. doi: 10.1177/20458940211011329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cottin V, Selman M, Inoue Y, et al. Syndrome of combined pulmonary fibrosis and emphysema: an official ATS/ERS/JRS/ALAT research statement. Am J Respir Crit Care Med 2022; 206: e7–e41. doi: 10.1164/rccm.202206-1041ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oliveira RK, Pereira CA, Ramos RP, et al. A haemodynamic study of pulmonary hypertension in chronic hypersensitivity pneumonitis. Eur Respir J 2014; 44: 415–424. doi: 10.1183/09031936.00010414 [DOI] [PubMed] [Google Scholar]
- 61.Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N Engl J Med 2021; 384: 325–334. doi: 10.1056/NEJMoa2008470 [DOI] [PubMed] [Google Scholar]
- 62.Nathan SD, Behr J, Collard HR, et al. Riociguat for idiopathic interstitial pneumonia-associated pulmonary hypertension (RISE-IIP): a randomised, placebo-controlled phase 2b study. Lancet Respir Med 2019; 7: 780–790. doi: 10.1016/S2213-2600(19)30250-4 [DOI] [PubMed] [Google Scholar]
- 63.Wälscher J, Gross B, Morisset J, et al. Comorbidities and survival in patients with chronic hypersensitivity pneumonitis. Respir Res 2020; 21: 12. doi: 10.1186/s12931-020-1283-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nemoto K, Oh-Ishi S, Akiyama T, et al. Borderline pulmonary hypertension is associated with exercise intolerance and increased risk for acute exacerbation in patients with interstitial lung disease. BMC Pulm Med 2019; 19: 167. doi: 10.1186/s12890-019-0932-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zou RH, Wallace WD, Nouraie SM, et al. Lower DLco% identifies exercise pulmonary hypertension in patients with parenchymal lung disease referred for dyspnea. Pulm Circ 2020; 10: 2045894019891912. doi: 10.1177/2045894019891912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denton CP, Khanna D. Systemic sclerosis. Lancet 2017; 390: 1685–1699. doi: 10.1016/S0140-6736(17)30933-9 [DOI] [PubMed] [Google Scholar]
- 67.Moinzadeh P, Bonella F, Oberste M, et al. Impact of systemic sclerosis-associated interstitial lung disease with and without pulmonary hypertension on survival: a large cohort study of the German Network for Systemic Sclerosis. Chest 2024; 165: 132–145. doi: 10.1016/j.chest.2023.08.013 [DOI] [PubMed] [Google Scholar]
- 68.Young A, Vummidi D, Visovatti S, et al. Prevalence, treatment, and outcomes of coexistent pulmonary hypertension and interstitial lung disease in systemic sclerosis. Arthritis Rheumatol 2019; 71: 1339–1349. doi: 10.1002/art.40862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lefèvre G, Dauchet L, Hachulla E, et al. Survival and prognostic factors in systemic sclerosis-associated pulmonary hypertension: a systematic review and meta-analysis. Arthritis Rheum 2013; 65: 2412–2423. doi: 10.1002/art.38029 [DOI] [PubMed] [Google Scholar]
- 70.Guillén-Del-Castillo A, Meseguer ML, Fonollosa-Pla V, et al. Impact of interstitial lung disease on the survival of systemic sclerosis with pulmonary arterial hypertension. Sci Rep 2022; 12: 5289. doi: 10.1038/s41598-022-09353-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Launay D, Montani D, Hassoun PM, et al. Clinical phenotypes and survival of pre-capillary pulmonary hypertension in systemic sclerosis. PLoS One 2018; 13: e0197112. doi: 10.1371/journal.pone.0197112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamanaka Y, Baba T, Hagiwara E, et al. Radiological images of interstitial pneumonia in mixed connective tissue disease compared with scleroderma and polymyositis/dermatomyositis. Eur J Radiol 2018; 107: 26–32. doi: 10.1016/j.ejrad.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 73.Hyldgaard C, Hilberg O, Pedersen AB, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis 2017; 76: 1700–1706. doi: 10.1136/annrheumdis-2017-211138 [DOI] [PubMed] [Google Scholar]
- 74.Joy GM, Arbiv OA, Wong CK, et al. Prevalence, imaging patterns and risk factors of interstitial lung disease in connective tissue disease: a systematic review and meta-analysis. Eur Respir Rev 2023; 32: 220210. doi: 10.1183/16000617.0210-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Montani D, Henry J, O'Connell C, et al. Association between rheumatoid arthritis and pulmonary hypertension: data from the French Pulmonary Hypertension Registry. Respiration 2018; 95: 244–250. doi: 10.1159/000485631 [DOI] [PubMed] [Google Scholar]
- 76.Sanchez O, Sitbon O, Jaïs X, et al. Immunosuppressive therapy in connective tissue diseases-associated pulmonary arterial hypertension. Chest 2006; 130: 182–189. doi: 10.1378/chest.130.1.182 [DOI] [PubMed] [Google Scholar]
- 77.Chauvelot L, Gamondes D, Berthiller J, et al. Hemodynamic response to treatment and outcomes in pulmonary hypertension associated with interstitial lung disease versus pulmonary arterial hypertension in systemic sclerosis: data from a study identifying prognostic factors in pulmonary hypertension associated with interstitial lung disease. Arthritis Rheumatol 2021; 73: 295–304. doi: 10.1002/art.41512 [DOI] [PubMed] [Google Scholar]
- 78.Peacock AJ, Ling Y, Johnson MK, et al. Idiopathic pulmonary arterial hypertension and co-existing lung disease: is this a new phenotype? Pulm Circ 2020; 10: 2045894020914851. doi: 10.1177/2045894020914851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lewis RA, Thompson AAR, Billings CG, et al. Mild parenchymal lung disease and/or low diffusion capacity impacts survival and treatment response in patients diagnosed with idiopathic pulmonary arterial hypertension. Eur Respir J 2020; 55: 2000041. doi: 10.1183/13993003.00041-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bax S, Bredy C, Kempny A, et al. A stepwise composite echocardiographic score predicts severe pulmonary hypertension in patients with interstitial lung disease. ERJ Open Res 2018; 4: 00124-2017. doi: 10.1183/23120541.00124-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Foris V, Brcic L, Douschan P, et al. Advanced interstitial lung fibrosis with emphysema and pulmonary hypertension with no evidence for interstitial lung disease on high resolution CT. Pulm Circ 2019; 9: 2045894019832214. doi: 10.1177/2045894019832214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El-Kersh K, Nathan SD. Phenotypes of idiopathic pulmonary arterial hypertension. Lancet Respir Med 2022; 10: e88. doi: 10.1016/S2213-2600(22)00304-6 [DOI] [PubMed] [Google Scholar]
- 83.Dwivedi K, Condliffe R, Sharkey M, et al. Computed tomography lung parenchymal descriptions in routine radiological reporting have diagnostic and prognostic utility in patients with idiopathic pulmonary arterial hypertension and pulmonary hypertension associated with lung disease. ERJ Open Res 2022; 8: 00549-2021. doi: 10.1183/23120541.00549-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buonauro A, Santoro C, Galderisi M, et al. Impaired right and left ventricular longitudinal function in patients with fibrotic interstitial lung diseases. J Clin Med 2020; 9: 587. doi: 10.3390/jcm9020587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raghu G, Nathan SD, Behr J, et al. Pulmonary hypertension in idiopathic pulmonary fibrosis with mild-to-moderate restriction. Eur Respir J 2015; 46: 1370–1377. doi: 10.1183/13993003.01537-2014 [DOI] [PubMed] [Google Scholar]
- 86.Teramachi R, Taniguchi H, Kondoh Y, et al. Impact of post-capillary pulmonary hypertension on mortality in interstitial lung disease. Respir Investig 2021; 59: 342–349. doi: 10.1016/j.resinv.2020.12.010 [DOI] [PubMed] [Google Scholar]
- 87.Nathan SD, Cottin V, Behr J, et al. Impact of lung morphology on clinical outcomes with riociguat in patients with pulmonary hypertension and idiopathic interstitial pneumonia: a post hoc subgroup analysis of the RISE-IIP study. J Heart Lung Transplant 2021; 40: 494–503. doi: 10.1016/j.healun.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 88.Jacob J, Bartholmai BJ, Rajagopalan S, et al. Likelihood of pulmonary hypertension in patients with idiopathic pulmonary fibrosis and emphysema. Respirology 2018; 23: 593–599. doi: 10.1111/resp.13231 [DOI] [PubMed] [Google Scholar]
- 89.Jacob J, Bartholmai BJ, Rajagopalan S, et al. Functional and prognostic effects when emphysema complicates idiopathic pulmonary fibrosis. Eur Respir J 2017; 50: 1700379. doi: 10.1183/13993003.00379-2017 [DOI] [PubMed] [Google Scholar]
- 90.Zhao A, Gudmundsson E, Mogulkoc N, et al. Mortality surrogates in combined pulmonary fibrosis and emphysema. Eur Respir J 2024; 63: 2300127. doi: 10.1183/13993003.00127-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Westhoff M, Litterst P, Ewert R. Cardiopulmonary exercise testing in combined pulmonary fibrosis and emphysema. Respiration 2021; 100: 395–403. doi: 10.1159/000513848 [DOI] [PubMed] [Google Scholar]
- 92.Braganza M, Shaw J, Solverson K, et al. A prospective evaluation of the diagnostic accuracy of the physical examination for pulmonary hypertension. Chest 2019; 155: 982–990. doi: 10.1016/j.chest.2019.01.035 [DOI] [PubMed] [Google Scholar]
- 93.King CS, Shlobin OA. The trouble with group 3 pulmonary hypertension in interstitial lung disease: dilemmas in diagnosis and the conundrum of treatment. Chest 2020; 158: 1651–1664. doi: 10.1016/j.chest.2020.04.046 [DOI] [PubMed] [Google Scholar]
- 94.Rahaghi FF, Kolaitis NA, Adegunsoye A, et al. Screening strategies for pulmonary hypertension in patients with interstitial lung disease: a multidisciplinary Delphi study. Chest 2022; 162: 145–155. doi: 10.1016/j.chest.2022.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kerr KM, Elliott CG, Chin K, et al. Results from the United States chronic thromboembolic pulmonary hypertension registry: enrollment characteristics and 1-year follow-up. Chest 2021; 160: 1822–1831. doi: 10.1016/j.chest.2021.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 97.Dauriat G, Reynaud-Gaubert M, Cottin V, et al. Severe pulmonary hypertension associated with COPD: long-term results of a prospective French multicentre cohort. Eur Respir J 2022; 60: 2102897. doi: 10.1183/13993003.02897-2021 [DOI] [PubMed] [Google Scholar]
- 98.Yoo DK, Zompatori M, Barrile A, et al. Associated pulmonary hypertension is an independent contributor to exercise intolerance in chronic fibrosing interstitial pneumonias. Respiration 2018; 96: 543–551. doi: 10.1159/000491095 [DOI] [PubMed] [Google Scholar]
- 99.Furukawa T, Kondoh Y, Taniguchi H, et al. A scoring system to predict the elevation of mean pulmonary arterial pressure in idiopathic pulmonary fibrosis. Eur Respir J 2018; 51: 1701311. doi: 10.1183/13993003.01311-2017 [DOI] [PubMed] [Google Scholar]
- 100.Rose L, Prins KW, Archer SL, et al. Survival in pulmonary hypertension due to chronic lung disease: influence of low diffusion capacity of the lungs for carbon monoxide. J Heart Lung Transplant 2019; 38: 145–155. doi: 10.1016/j.healun.2018.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Balasubramanian A, Kolb TM, Damico RL, et al. Diffusing capacity is an independent predictor of outcomes in pulmonary hypertension associated with COPD. Chest 2020; 158: 722–734. doi: 10.1016/j.chest.2020.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trushenko NV, Suvorova OA, Nekludova GV, et al. Predictors of pulmonary hypertension and right ventricular dysfunction in patients with hypersensitivity pneumonitis. Life 2023; 13: 1348. doi: 10.3390/life13061348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Armstrong HF, Thirapatarapong W, Dussault NE, et al. Distinguishing pulmonary hypertension in interstitial lung disease by ventilation and perfusion defects measured by cardiopulmonary exercise testing. Respiration 2013; 86: 407–413. doi: 10.1159/000350445 [DOI] [PubMed] [Google Scholar]
- 104.Boerrigter BG, Bogaard HJ, Trip P, et al. Ventilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertension. Chest 2012; 142: 1166–1174. doi: 10.1378/chest.11-2798 [DOI] [PubMed] [Google Scholar]
- 105.Tian F, Song W, Wang L, et al. NT-pro BNP in AECOPD-PH: old biomarker, new insights-based on a large retrospective case-controlled study. Respir Res 2021; 22: 321. doi: 10.1186/s12931-021-01917-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sonaglioni A, Cassandro R, Luisi F, et al. Correlation between Doppler echocardiography and right heart catheterisation-derived systolic and mean pulmonary artery pressures: determinants of discrepancies between the two methods. Heart Lung Circ 2021; 30: 656–664. doi: 10.1016/j.hlc.2020.10.009 [DOI] [PubMed] [Google Scholar]
- 107.Keir GJ, Wort SJ, Kokosi M, et al. Pulmonary hypertension in interstitial lung disease: limitations of echocardiography compared to cardiac catheterization. Respirology 2018; 23: 687–694. doi: 10.1111/resp.13250 [DOI] [PubMed] [Google Scholar]
- 108.Nowak J, Hudzik B, Jastrzebski D, et al. Pulmonary hypertension in advanced lung diseases: echocardiography as an important part of patient evaluation for lung transplantation. Clin Respir J 2018; 12: 930–938. doi: 10.1111/crj.12608 [DOI] [PubMed] [Google Scholar]
- 109.Yogeswaran A, Kuhnert S, Gall H, et al. Relevance of cor pulmonale in COPD with and without pulmonary hypertension: a retrospective cohort study. Front Cardiovasc Med 2022; 9: 826369. doi: 10.3389/fcvm.2022.826369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakayama S, Chubachi S, Sakurai K, et al. Characteristics of chronic obstructive pulmonary disease patients with pulmonary hypertension assessed by echocardiography in a three-year observational cohort study. Int J Chron Obstruct Pulmon Dis 2020; 15: 487–499. doi: 10.2147/COPD.S230952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caminati A, Zompatori M, Fuccillo N, et al. Coronary artery calcium score is a prognostic factor for mortality in idiopathic pulmonary fibrosis. Minerva Med 2023; 114: 815–824. doi: 10.23736/S0026-4806.23.08720-7 [DOI] [PubMed] [Google Scholar]
- 112.Anand V, Weston AD, Scott CG, et al. Machine learning for diagnosis of pulmonary hypertension by echocardiography. Mayo Clin Proc 2024; 99: 260–270. doi: 10.1016/j.mayocp.2023.05.006 [DOI] [PubMed] [Google Scholar]
- 113.Hirata Y, Tsuji T, Kotoku J, et al. Echocardiographic artificial intelligence for pulmonary hypertension classification. Heart 2024; 110: 586–593. doi: 10.1136/heartjnl-2023-323320 [DOI] [PubMed] [Google Scholar]
- 114.Yagi M, Taniguchi H, Kondoh Y, et al. CT-determined pulmonary artery to aorta ratio as a predictor of elevated pulmonary artery pressure and survival in idiopathic pulmonary fibrosis. Respirology 2017; 22: 1393–1399. doi: 10.1111/resp.13066 [DOI] [PubMed] [Google Scholar]
- 115.Wu XG, Shi YJ, Wang XH, et al. Diagnostic value of computed tomography-based pulmonary artery to aorta ratio measurement in chronic obstructive pulmonary disease with pulmonary hypertension: a systematic review and meta-analysis. Clin Respir J 2022; 16: 276–283. doi: 10.1111/crj.13485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Choi JS, Lee SH, Leem AY, et al. Prognostic impact of the ratio of the main pulmonary artery to that of the aorta on chest computed tomography in patients with idiopathic pulmonary fibrosis. BMC Pulm Med 2019; 19: 81. doi: 10.1186/s12890-019-0843-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alkukhun L, Wang XF, Ahmed MK, et al. Non-invasive screening for pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med 2016; 117: 65–72. doi: 10.1016/j.rmed.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ratanawatkul P, Oh A, Richards JC, et al. Performance of pulmonary artery dimensions measured on high-resolution computed tomography scan for identifying pulmonary hypertension. ERJ Open Res 2020; 6: 00232-2019. doi: 10.1183/23120541.00232-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheng Y, Li L, Tu X, et al. The main pulmonary artery to the ascending aorta diameter ratio (PA/A) as a predictor of worse outcomes in hospitalized patients with AECOPD. Int J Chron Obstruct Pulmon Dis 2022; 17: 1157–1165. doi: 10.2147/COPD.S357696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Refini RM, Bettini G, Kacerja E, et al. The role of the combination of echo-HRCT score as a tool to evaluate the presence of pulmonary hypertension in idiopathic pulmonary fibrosis. Intern Emerg Med 2021; 16: 941–947. doi: 10.1007/s11739-020-02539-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chin M, Johns C, Currie BJ, et al. Pulmonary artery size in interstitial lung disease and pulmonary hypertension: association with interstitial lung disease severity and diagnostic utility. Front Cardiovasc Med 2018; 5: 53. doi: 10.3389/fcvm.2018.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rahaghi FN, Vegas-Sanchez-Ferrero G, Minhas JK, et al. Ventricular geometry from non-contrast non-ECG-gated CT scans: an imaging marker of cardiopulmonary disease in smokers. Acad Radiol 2017; 24: 594–602. doi: 10.1016/j.acra.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coste F, Benlala I, Dournes G, et al. Quantitative CT assessment of bronchial and vascular alterations in severe precapillary pulmonary hypertension. Int J Chron Obstruct Pulmon Dis 2019; 14: 381–389. doi: 10.2147/COPD.S177638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alkhanfar D, Shahin Y, Alandejani F, et al. Severe pulmonary hypertension associated with lung disease is characterised by a loss of small pulmonary vessels on quantitative computed tomography. ERJ Open Res 2022; 8: 00503-2021. doi: 10.1183/23120541.00503-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Charters PFP, Rossdale J, Brown W, et al. Diagnostic accuracy of an automated artificial intelligence derived right ventricular to left ventricular diameter ratio tool on CT pulmonary angiography to predict pulmonary hypertension at right heart catheterisation. Clin Radiol 2022; 77: e500–e508. doi: 10.1016/j.crad.2022.03.009 [DOI] [PubMed] [Google Scholar]
- 126.Yang R, Liu G, Deng C. Pulmonary embolism with chronic obstructive pulmonary disease. Chronic Dis Transl Med 2021; 7: 149–156. doi: 10.1016/j.cdtm.2021.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boonpheng B, Ungprasert P. Risk of venous thromboembolism in patients with idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 109–114. doi: 10.36141/svdld.v35i2.6213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun H, Liu M, Yang X, et al. Incidence and risk factors of venous thrombotic events in patients with interstitial lung disease during hospitalization. Thromb J 2023; 21: 17. doi: 10.1186/s12959-023-00458-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Le Mao R, Ropars T, Tromeur C, et al. Risk of recurrent venous thromboembolism and bleeding in patients with interstitial lung disease: a cohort study. J Thromb Thrombolysis 2022; 53: 67–73. doi: 10.1007/s11239-021-02518-z [DOI] [PubMed] [Google Scholar]
- 130.Agoston-Coldea L, Lupu S, Mocan T. Pulmonary artery stiffness by cardiac magnetic resonance imaging predicts major adverse cardiovascular events in patients with chronic obstructive pulmonary disease. Sci Rep 2018; 8: 14447. doi: 10.1038/s41598-018-32784-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Johns CS, Rajaram S, Capener DA, et al. Non-invasive methods for estimating mPAP in COPD using cardiovascular magnetic resonance imaging. Eur Radiol 2018; 28: 1438–1448. doi: 10.1007/s00330-017-5143-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nathan SD, Chandel A, Wang Y, et al. Derivation and validation of a noninvasive prediction tool to identify pulmonary hypertension in patients with IPF: evolution of the model FORD. J Heart Lung Transplant 2024; 43: 547–553. doi: 10.1016/j.healun.2023.11.005 [DOI] [PubMed] [Google Scholar]
- 133.Joseph P, Savarimuthu S, Zhao J, et al. Noninvasive determinants of pulmonary hypertension in interstitial lung disease. Pulm Circ 2023; 13: e12197. doi: 10.1002/pul2.12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Parikh R, Konstantinidis I, O'Sullivan DM, et al. Pulmonary hypertension in patients with interstitial lung disease: a tool for early detection. Pulm Circ 2022; 12: e12141. doi: 10.1002/pul2.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Parikh R, O'Sullivan DM, Farber HW. The PH-ILD detection tool: external validation and use in patients with ILD. Pulm Circ 2023; 13: e12273. doi: 10.1002/pul2.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sobiecka M, Lewandowska K, Kober J, et al. Can a new scoring system improve prediction of pulmonary hypertension in newly recognised interstitial lung diseases? Lung 2020; 198: 547–554. doi: 10.1007/s00408-020-00346-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pulmonary Hypertension Screening in Patients with Interstitial Lung Disease for Earlier Detection (PHINDER). https://clinicaltrials.gov/study/NCT05776225?term=phinder&rank=1. Date last accessed: 23 April 2024. Date last updated: 5 July 2024.
- 138.Ewert R, Heine A, Müller-Heinrich A, et al. Exercise and fluid challenge during right heart catheterisation for evaluation of dyspnoea. Pulm Circ 2020; 10: 10.1177_2045894020917887. doi: 10.1177/2045894020917887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shlobin OA, Shen E, Wort SJ, et al. Pulmonary hypertension in the setting of interstitial lung disease: approach to management and treatment. A consensus statement from the Pulmonary Vascular Research Institute's Innovative Drug Development Initiative – Group 3 Pulmonary Hypertension. Pulm Circ 2024; 14: e12310. doi: 10.1002/pul2.12310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2020; 201: e56–e69. doi: 10.1164/rccm.202003-0625ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 2015; 192: e3–19. doi: 10.1164/rccm.201506-1063ST [DOI] [PubMed] [Google Scholar]
- 142.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022; 205: e18–e47. doi: 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wijsenbeek M, Cottin V. Spectrum of fibrotic lung diseases. N Engl J Med 2020; 383: 958–968. doi: 10.1056/NEJMra2005230 [DOI] [PubMed] [Google Scholar]
- 144.Winterbottom CJ, Shah RJ, Patterson KC, et al. Exposure to ambient particulate matter is associated with accelerated functional decline in idiopathic pulmonary fibrosis. Chest 2018; 153: 1221–1228. doi: 10.1016/j.chest.2017.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shi H, Chen L, Zhang S, et al. Dynamic association of ambient air pollution with incidence and mortality of pulmonary hypertension: a multistate trajectory analysis. Ecotoxicol Environ Saf 2023; 262: 115126. doi: 10.1016/j.ecoenv.2023.115126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Blanco I, Santos S, Gea J, et al. Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. Eur Respir J 2013; 42: 982–992. doi: 10.1183/09031936.00176312 [DOI] [PubMed] [Google Scholar]
- 147.Keyser RE, Christensen EJ, Chin LM, et al. Changes in fatigability following intense aerobic exercise training in patients with interstitial lung disease. Respir Med 2015; 109: 517–525. doi: 10.1016/j.rmed.2015.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 2015: CD003793. doi: 10.1002/14651858.CD003793.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nolan CM, Polgar O, Schofield SJ, et al. Pulmonary rehabilitation in idiopathic pulmonary fibrosis and COPD: a propensity-matched real-world study. Chest 2022; 161: 728–737. doi: 10.1016/j.chest.2021.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kataoka K, Nishiyama O, Ogura T, et al. Long-term effect of pulmonary rehabilitation in idiopathic pulmonary fibrosis: a randomised controlled trial. Thorax 2023; 78: 784–791. doi: 10.1136/thorax-2022-219792 [DOI] [PubMed] [Google Scholar]
- 151.Grünig E, MacKenzie A, Peacock AJ, et al. Standardized exercise training is feasible, safe, and effective in pulmonary arterial and chronic thromboembolic pulmonary hypertension: results from a large European multicentre randomized controlled trial. Eur Heart J 2021; 42: 2284–2295. doi: 10.1093/eurheartj/ehaa696 [DOI] [PubMed] [Google Scholar]
- 152.Morris NR, Kermeen FD, Jones AW, et al. Exercise-based rehabilitation programmes for pulmonary hypertension. Cochrane Database Syst Rev 2023; 3: CD011285. doi: 10.1002/14651858.CD011285.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Arif R, Pandey A, Zhao Y, et al. Treatment of pulmonary hypertension associated with COPD: a systematic review. ERJ Open Res 2022; 8: 00348-2021. doi: 10.1183/23120541.00348-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Timms RM, Khaja FU, Williams GW. Hemodynamic response to oxygen therapy in chronic obstructive pulmonary disease. Ann Intern Med 1985; 102: 29–36. doi: 10.7326/0003-4819-102-1-29 [DOI] [PubMed] [Google Scholar]
- 155.Weitzenblum E, Sautegeau A, Ehrhart M, et al. Long-term oxygen therapy can reverse the progression of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1985; 131: 493–498. doi: 10.1164/arrd.1985.131.4.493 [DOI] [PubMed] [Google Scholar]
- 156.Castro-Anon O, Golpe R, Perez-de-Llano LA, et al. Haemodynamic effects of non-invasive ventilation in patients with obesity-hypoventilation syndrome. Respirology 2012; 17: 1269–1274. doi: 10.1111/j.1440-1843.2012.02252.x [DOI] [PubMed] [Google Scholar]
- 157.Held M, Walthelm J, Baron S, et al. Functional impact of pulmonary hypertension due to hypoventilation and changes under noninvasive ventilation. Eur Respir J 2014; 43: 156–165. doi: 10.1183/09031936.00147712 [DOI] [PubMed] [Google Scholar]
- 158.Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: a review. JAMA 2023; 329: 827–838. doi: 10.1001/jama.2023.2020 [DOI] [PubMed] [Google Scholar]
- 159.Adir Y, Humbert M, Chaouat A. Sleep-related breathing disorders and pulmonary hypertension. Eur Respir J 2021; 57: 2002258. doi: 10.1183/13993003.02258-2020 [DOI] [PubMed] [Google Scholar]
- 160.Imran TF, Ghazipura M, Liu S, et al. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: a meta-analysis. Heart Fail Rev 2016; 21: 591–598. doi: 10.1007/s10741-016-9548-5 [DOI] [PubMed] [Google Scholar]
- 161.Janssen DJA, Bajwah S, Boon MH, et al. European Respiratory Society clinical practice guideline: palliative care for people with COPD or interstitial lung disease. Eur Respir J 2023; 62: 2202014. doi: 10.1183/13993003.02014-2022 [DOI] [PubMed] [Google Scholar]
- 162.Leard LE, Holm AM, Valapour M, et al. Consensus document for the selection of lung transplant candidates: an update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2021; 40: 1349–1379. doi: 10.1016/j.healun.2021.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cruz-Utrilla A, Perez-Olivares C, Martinez-Menaca A, et al. Phenotypes of idiopathic pulmonary arterial hypertension. Lancet Respir Med 2022; 10: e87. doi: 10.1016/S2213-2600(22)00290-9 [DOI] [PubMed] [Google Scholar]
- 164.Raghu G, Behr J, Brown KK, et al. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med 2013; 158: 641–649. doi: 10.7326/0003-4819-158-9-201305070-00003 [DOI] [PubMed] [Google Scholar]
- 165.Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J 2013; 41: 1292–1301. doi: 10.1183/09031936.00079512 [DOI] [PubMed] [Google Scholar]
- 166.Tanabe N, Kumamaru H, Tamura Y, et al. Multi-institutional prospective cohort study of patients with pulmonary hypertension associated with respiratory diseases. Circ J 2021; 85: 333–342. doi: 10.1253/circj.CJ-20-0939 [DOI] [PubMed] [Google Scholar]
- 167.Vitulo P, Stanziola A, Confalonieri M, et al. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. J Heart Lung Transplant 2017; 36: 166–174. 10.1016/j.healun.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 168.Maron BA, Choudhary G, Goldstein RL, et al. Tadalafil for veterans with chronic obstructive pulmonary disease – pulmonary hypertension: a multicenter, placebo‐controlled randomized trial. Pulm Circ 2022; 12: e12043. 10.1002/pul2.v12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nathan SD, Argula R, Trivieri MG, et al. Inhaled treprostinil in pulmonary hypertension associated with COPD: PERFECT study results. Eur Respir J 2024; 63: 2400172. 10.1183/13993003.00172-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kolb M, Raghu G, Wells AU, et al. Nintedanib plus sildenafil in patients with idiopathic pulmonary fibrosis. N Engl J Med 2018; 379: 1722–1731. 10.1056/NEJMoa1811737 [DOI] [PubMed] [Google Scholar]
- 171.Behr J, Nathan SD, Wuyts WA, et al. Efficacy and safety of sildenafil added to pirfenidone in patients with advanced idiopathic pulmonary fibrosis and risk of pulmonary hypertension: a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med 2021; 9: 85–95. 10.1016/S2213-2600(20)30356-8 [DOI] [PubMed] [Google Scholar]
- 172.Nathan SD, Flaherty KR, Glassberg MK, et al. A randomized, double-blind, placebo-controlled study of pulsed, inhaled nitric oxide in subjects at risk of pulmonary hypertension associated with pulmonary fibrosis. Chest 2020; 158: 637–645. 10.1016/j.chest.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 173.A Study to Assess Pulsed Inhaled Nitric Oxide in Subjects with Pulmonary Fibrosis at Risk for Pulmonary Hypertension (REBUILD). https://clinicaltrials.gov/study/NCT03267108. Date last updated: 27 July 2023.
- 174.Faria-Urbina M, Oliveira RKF, Agarwal M, et al. Inhaled treprostinil in pulmonary hypertension associated with lung disease. Lung 2018; 196: 139–146. 10.1007/s00408-017-0081-7 [DOI] [PubMed] [Google Scholar]
- 175.Dawes TJW, McCabe C, Dimopoulos K, et al. Phosphodiesterase 5 inhibitor treatment and survival in interstitial lung disease pulmonary hypertension: a Bayesian retrospective observational cohort study. Respirology 2023; 28: 262–272. 10.1111/resp.v28.3 [DOI] [PubMed] [Google Scholar]
- 176.Elkhapery A, Hammami MB, Sulica R, et al. Pulmonary vasodilator therapy in severe pulmonary hypertension due to chronic obstructive pulmonary disease (severe PH-COPD): a systematic review and meta-analysis. J Cardiovasc Dev Dis 2023; 10: 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.MK-5475-013 INSIGNIA-PH-COPD: a study of the efficacy and safety of MK-5475 (an inhaled sGC stimulator) in adults with PH-COPD. https://clinicaltrials.gov/study/NCT05612035. Date last updated: 3 July 2024.
- 178.Tadalafil for severe pulmonary hypertension due to chronic obstructive pulmonary disease (ERASE PH-COPD). https://clinicaltrials.gov/study/NCT05844462. Date last updated: 21 June 2024.
- 179.A study to evaluate the safety and tolerability of treprostinil palmitil inhalation powder in participants with pulmonary hypertension associated with interstitial lung disease. https://clinicaltrials.gov/study/NCT05176951. Date last updated: 25 March 2024.
- 180.An Open-Label ProSpective MultiCENTer Study to Evaluate Safety and Tolerability of Dry Powder Inhaled Treprostinil in PH (ASCENT). https://clinicaltrials.gov/study/NCT06129240. Date last updated: 31 January 2024.
- 181.A phase 3 study to evaluate the safety and tolerability of L606 in subjects with PAH or PH-ILD. https://clinicaltrials.gov/study/NCT04691154. Date last updated: 14 August 2023.
- 182.Investigation of H01 in adults with pulmonary hypertension including interstitial lung disease (the SATURN study). https://clinicaltrials.gov/study/NCT05128929. Date last updated: 4 October 2023.
- 183.Extended access program to assess long-term safety of bardoxolone methyl in patients with pulmonary hypertension RANGER. https://clinicaltrials.gov/study/NCT03068130. Date last updated: 6 February 2024.
- 184.Prins KW, Duval S, Markowitz J, et al. Chronic use of PAH-specific therapy in World Health Organization group III pulmonary hypertension: a systematic review and meta-analysis. Pulm Circ 2017; 7: 145–155. doi: 10.1086/690017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nathan SD, Deng C, King CS, et al. Inhaled treprostinil dosage in pulmonary hypertension associated with interstitial lung disease and its effects on clinical outcomes. Chest 2023; 163: 398–406. doi: 10.1016/j.chest.2022.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nathan SD, Tapson VF, Elwing J, et al. Efficacy of inhaled treprostinil on multiple disease progression events in patients with pulmonary hypertension due to parenchymal lung disease in the INCREASE trial. Am J Respir Crit Care Med 2022; 205: 198–207. doi: 10.1164/rccm.202107-1766OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nathan SD, Medarov B, Ho L, et al. A novel approach to clinical change endpoints: a win ratio analysis of the INCREASE trial. Ann Am Thorac Soc 2023; 20: 1537–1540. doi: 10.1513/AnnalsATS.202303-229RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Waxman A, Restrepo-Jaramillo R, Thenappan T, et al. Long-term inhaled treprostinil for pulmonary hypertension due to interstitial lung disease: INCREASE open-label extension study. Eur Respir J 2023; 61: 2202414. doi: 10.1183/13993003.02414-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Weatherald J, Nathan SD, El-Kersh K, et al. Inhaled treprostinil in patients with pulmonary hypertension associated with interstitial lung disease with less severe haemodynamics: a post hoc analysis of the INCREASE study. BMJ Open Respir Res 2024; 11: e002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nathan SD, Johri S, Joly JM, et al. Survival analysis from the INCREASE study in PH-ILD: evaluating the impact of treatment crossover on overall mortality. Thorax 2024; 79: 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cottin V, Harari S, Humbert M, et al. Pulmonary hypertension in lymphangioleiomyomatosis: characteristics in 20 patients. Eur Respir J 2012; 40: 630–640. doi: 10.1183/09031936.00093111 [DOI] [PubMed] [Google Scholar]
- 192.Freitas CSG, Baldi BG, Jardim C, et al. Pulmonary hypertension in lymphangioleiomyomatosis: prevalence, severity and the role of carbon monoxide diffusion capacity as a screening method. Orphanet J Rare Dis 2017; 12: 74. doi: 10.1186/s13023-017-0626-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wu X, Xu W, Wang J, et al. Clinical characteristics in lymphangioleiomyomatosis-related pulmonary hypertension: an observation on 50 patients. Front Med 2019; 13: 259–266. doi: 10.1007/s11684-018-0634-z [DOI] [PubMed] [Google Scholar]
- 194.Harari S, Spagnolo P, Cocconcelli E, et al. Recent advances in the pathobiology and clinical management of lymphangioleiomyomatosis. Curr Opin Pulm Med 2018; 24: 469–476. doi: 10.1097/MCP.0000000000000502 [DOI] [PubMed] [Google Scholar]
- 195.McCormack FX, Inoue Y, Moss J, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med 2011; 364: 1595–1606. doi: 10.1056/NEJMoa1100391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Harari S, Torre O, Elia D, et al. Improving survival in lymphangioleiomyomatosis: a 16-year observational study in a large cohort of patients. Respiration 2021; 100: 989–999. doi: 10.1159/000516330 [DOI] [PubMed] [Google Scholar]
- 197.Hayes D Jr, Higgins RS, Kirkby S, et al. Impact of pulmonary hypertension on survival in patients with cystic fibrosis undergoing lung transplantation: an analysis of the UNOS registry. J Cyst Fibros 2014; 13: 416–423. doi: 10.1016/j.jcf.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 198.Hayes D Jr, Tobias JD, Mansour HM, et al. Pulmonary hypertension in cystic fibrosis with advanced lung disease. Am J Respir Crit Care Med 2014; 190: 898–905. doi: 10.1164/rccm.201407-1382OC [DOI] [PubMed] [Google Scholar]
- 199.Zouk AN, Gulati S, Xing D, et al. Pulmonary artery enlargement is associated with pulmonary hypertension and decreased survival in severe cystic fibrosis: a cohort study. PLoS One 2020; 15: e0229173. doi: 10.1371/journal.pone.0229173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li D, Wang B, Wang H, et al. Prognostic significance of pulmonary hypertension in patients with cystic fibrosis: a systematic review and meta-analysis. Medicine 2018; 97: e9708. doi: 10.1097/MD.0000000000009708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ratjen F. Pulmonary artery hypertension: an underrated disease manifestation in cystic fibrosis? Lancet Respir Med 2016; 4: 596–598. doi: 10.1016/S2213-2600(16)30107-2 [DOI] [PubMed] [Google Scholar]
- 202.Wang L, Jiang S, Shi J, et al. Clinical characteristics of pulmonary hypertension in bronchiectasis. Front Med 2016; 10: 336–344. doi: 10.1007/s11684-016-0461-z [DOI] [PubMed] [Google Scholar]
- 203.Öcal S, Portakal O, Öcal A, et al. Factors associated with pulmonary hypertension and long-term survival in bronchiectasis subjects. Respir Med 2016; 119: 109–114. doi: 10.1016/j.rmed.2016.08.027 [DOI] [PubMed] [Google Scholar]
- 204.Savale L, Huitema M, Shlobin O, et al. WASOG statement on the diagnosis and management of sarcoidosis-associated pulmonary hypertension. Eur Respir Rev 2022; 31: 210165. doi: 10.1183/16000617.0165-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Le Pavec J, Valeyre D, Gazengel P, et al. Lung transplantation for sarcoidosis: outcome and prognostic factors. Eur Respir J 2021; 58: 2003358. doi: 10.1183/13993003.03358-2020 [DOI] [PubMed] [Google Scholar]
- 206.Huitema MP, Bakker ALM, Mager JJ, et al. Prevalence of pulmonary hypertension in pulmonary sarcoidosis: the first large European prospective study. Eur Respir J 2019; 54: 1900897. doi: 10.1183/13993003.00897-2019 [DOI] [PubMed] [Google Scholar]
- 207.Pabst S, Grohé C, Skowasch D. Prevalence of sarcoidosis-associated pulmonary hypertension: cumulative analysis of two PULSAR studies. Eur Respir J 2020; 55: 1902223. doi: 10.1183/13993003.02223-2019 [DOI] [PubMed] [Google Scholar]
- 208.Shlobin OA, Kouranos V, Barnett SD, et al. Physiological predictors of survival in patients with sarcoidosis-associated pulmonary hypertension: results from an international registry. Eur Respir J 2020; 55: 1901747. doi: 10.1183/13993003.01747-2019 [DOI] [PubMed] [Google Scholar]
- 209.Parikh KS, Dahhan T, Nicholl L, et al. Clinical features and outcomes of patients with sarcoidosis-associated pulmonary hypertension. Sci Rep 2019; 9: 4061. doi: 10.1038/s41598-019-40030-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med 2018; 139: 72–78. doi: 10.1016/j.rmed.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 211.Tiosano S, Versini M, Dar Antaki L, et al. The long-term prognostic significance of sarcoidosis-associated pulmonary hypertension – a cohort study. Clin Immunol 2019; 199: 57–61. doi: 10.1016/j.clim.2018.12.012 [DOI] [PubMed] [Google Scholar]
- 212.Gayen SK, Baughman RP, Nathan SD, et al. Pulmonary hemodynamics and transplant-free survival in sarcoidosis-associated pulmonary hypertension: results from an international registry. Pulm Circ 2023; 13: e12297. doi: 10.1002/pul2.12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Baughman RP, Shlobin OA, Gupta R, et al. Riociguat for sarcoidosis-associated pulmonary hypertension: results of a 1-year double-blind, placebo-controlled trial. Chest 2022; 161: 448–457. doi: 10.1016/j.chest.2021.07.2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Inhaled Treprostinil in Sarcoidosis Patients with Pulmonary Hypertension (SAPPHIRE). www.clinicaltrials.gov/study/NCT03814317. Date last updated: 28 May 2024.
- 215.Rodriguez-Galindo C, Allen CE. Langerhans cell histiocytosis. Blood 2020; 135: 1319–1331. doi: 10.1182/blood.2019000934 [DOI] [PubMed] [Google Scholar]
- 216.Heiden BT, Eaton DB Jr, Chang SH, et al. The impact of persistent smoking after surgery on long-term outcomes after stage I non-small cell lung cancer resection. Chest 2022; 161: 1687–1696. doi: 10.1016/j.chest.2021.12.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Goyal G, Tazi A, Go RS, et al. International expert consensus recommendations for the diagnosis and treatment of Langerhans cell histiocytosis in adults. Blood 2022; 139: 2601–2621. doi: 10.1182/blood.2021014343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fartoukh M, Humbert M, Capron F, et al. Severe pulmonary hypertension in histiocytosis X. Am J Respir Crit Care Med 2000; 161: 216–223. doi: 10.1164/ajrccm.161.1.9807024 [DOI] [PubMed] [Google Scholar]
- 219.Bois MC, May AM, Vassallo R, et al. Morphometric study of pulmonary arterial changes in pulmonary Langerhans cell histiocytosis. Arch Pathol Lab Med 2018; 142: 929–937. doi: 10.5858/arpa.2017-0463-OA [DOI] [PubMed] [Google Scholar]
- 220.Heiden GI, Sobral JB, Freitas CSG, et al. Mechanisms of exercise limitation and prevalence of pulmonary hypertension in pulmonary Langerhans cell histiocytosis. Chest 2020; 158: 2440–2448. doi: 10.1016/j.chest.2020.05.609 [DOI] [PubMed] [Google Scholar]
- 221.Benattia A, Bugnet E, Walter-Petrich A, et al. Long-term outcomes of adult pulmonary Langerhans cell histiocytosis: a prospective cohort. Eur Respir J 2022; 59: 2101017. doi: 10.1183/13993003.01017-2021 [DOI] [PubMed] [Google Scholar]
- 222.Nemoto K, Oh-Ishi S, Inui T, et al. Long-term improvement during tadalafil therapy in a patient with pulmonary hypertension secondary to pulmonary Langerhans cell histiocytosis. Respir Med Case Rep 2016; 18: 54–57. doi: 10.1016/j.rmcr.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Le Pavec J, Lorillon G, Jaïs X, et al. Pulmonary Langerhans cell histiocytosis-associated pulmonary hypertension: clinical characteristics and impact of pulmonary arterial hypertension therapies. Chest 2012; 142: 1150–1157. doi: 10.1378/chest.11-2490 [DOI] [PubMed] [Google Scholar]
- 224.Kiakouama L, Cottin V, Etienne-Mastroïanni B, et al. Severe pulmonary hypertension in histiocytosis X: long-term improvement with bosentan. Eur Respir J 2010; 36: 202–204. doi: 10.1183/09031936.00004810 [DOI] [PubMed] [Google Scholar]
- 225.Jutant EM, Jais X, Girerd B, et al. Phenotype and outcomes of pulmonary hypertension associated with neurofibromatosis type 1. Am J Respir Crit Care Med 2020; 202: 843–852. doi: 10.1164/rccm.202001-0105OC [DOI] [PubMed] [Google Scholar]
- 226.Mocumbi A, Humbert M, Saxena A, et al. Pulmonary hypertension. Nat Rev Dis Primers 2024; 10: 1. doi: 10.1038/s41572-023-00486-7 [DOI] [PubMed] [Google Scholar]
- 227.van Heerden JK, Louw EH, Thienemann F, et al. The prevalence of pulmonary hypertension in post-tuberculosis and active tuberculosis populations: a systematic review and meta-analysis. Eur Respir Rev 2024; 33: 230154. doi: 10.1183/16000617.0154-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bhattacharyya P, Saha D, Bhattacherjee PD, et al. Tuberculosis associated pulmonary hypertension: the revelation of a clinical observation. Lung India 2016; 33: 135–139. doi: 10.4103/0970-2113.177433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Louw E, Baines N, Maarman G, et al. The prevalence of pulmonary hypertension after successful tuberculosis treatment in a community sample of adult patients. Pulm Circ 2023; 13: e12184. doi: 10.1002/pul2.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wu Z, Jarvis H, Howard LS, et al. Post-tuberculous fibrosing mediastinitis: a review of the literature. BMJ Open Respir Res 2017; 4: e000174. doi: 10.1136/bmjresp-2016-000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Salindri AD, Wang JY, Lin HH, et al. Post-tuberculosis incidence of diabetes, myocardial infarction, and stroke: retrospective cohort analysis of patients formerly treated for tuberculosis in Taiwan, 2002–2013. Int J Infect Dis 2019; 84: 127–130. doi: 10.1016/j.ijid.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Maarman GJ, Shaw J, Allwood B. Pulmonary hypertension in majority countries: opportunities amidst challenges. Curr Opin Pulm Med 2020; 26: 373–383. doi: 10.1097/MCP.0000000000000702 [DOI] [PubMed] [Google Scholar]
- 233.Walsh KF, Lui JK. Post-tuberculosis pulmonary hypertension: a case of global disparity in health care. Lancet Glob Health 2022; 10: e476. doi: 10.1016/S2214-109X(22)00042-0 [DOI] [PubMed] [Google Scholar]
- 234.Nathan SD, Fernandes P, Psotka M, et al. Pulmonary hypertension in interstitial lung disease: clinical trial design and endpoints: a consensus statement from the Pulmonary Vascular Research Institute's Innovative Drug Development Initiative – Group 3 Pulmonary Hypertension. Pulm Circ 2022; 12: e12178. doi: 10.1002/pul2.12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Teylan M, Kantorowski A, Homsy D, et al. Physical activity in COPD: minimal clinically important difference for medical events. Chron Respir Dis 2019; 16: 1479973118816424. doi: 10.1177/1479973118816424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Butler J, Stockbridge N, Packer M. Win ratio: a seductive but potentially misleading method for evaluating evidence from clinical trials. Circulation 2024; 149: 1546–1548. doi: 10.1161/CIRCULATIONAHA.123.067786 [DOI] [PubMed] [Google Scholar]
- 237.Redfors B, Gregson J, Crowley A, et al. The win ratio approach for composite endpoints: practical guidance based on previous experience. Eur Heart J 2020; 41: 4391–4399. doi: 10.1093/eurheartj/ehaa665 [DOI] [PubMed] [Google Scholar]
- 238.Kawano-Dourado L, Kulkarni T, Ryerson CJ, et al. Adaptive multi-interventional trial platform to improve patient care for fibrotic interstitial lung diseases. Thorax 2024; 79: 788–795. doi: 10.1136/thorax-2023-221148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01200-2024.Supplement (150.2KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01200-2024.Shareable (680.5KB, pdf)