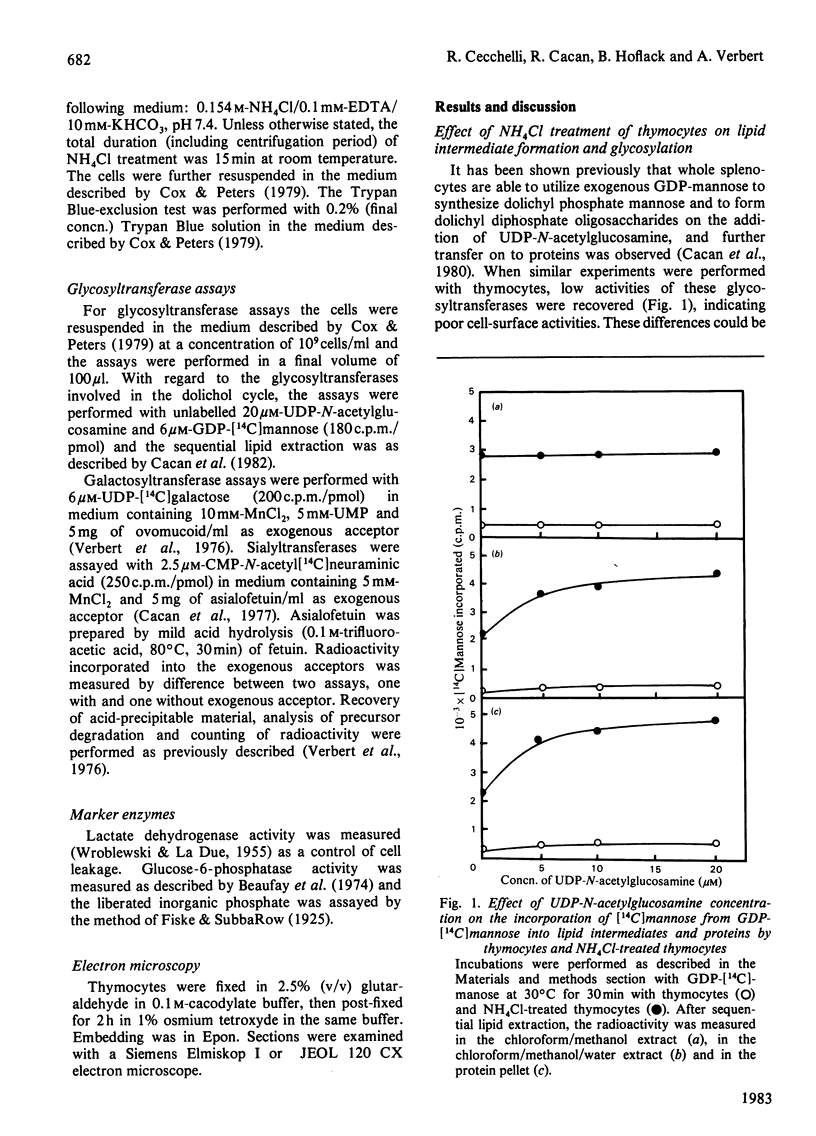
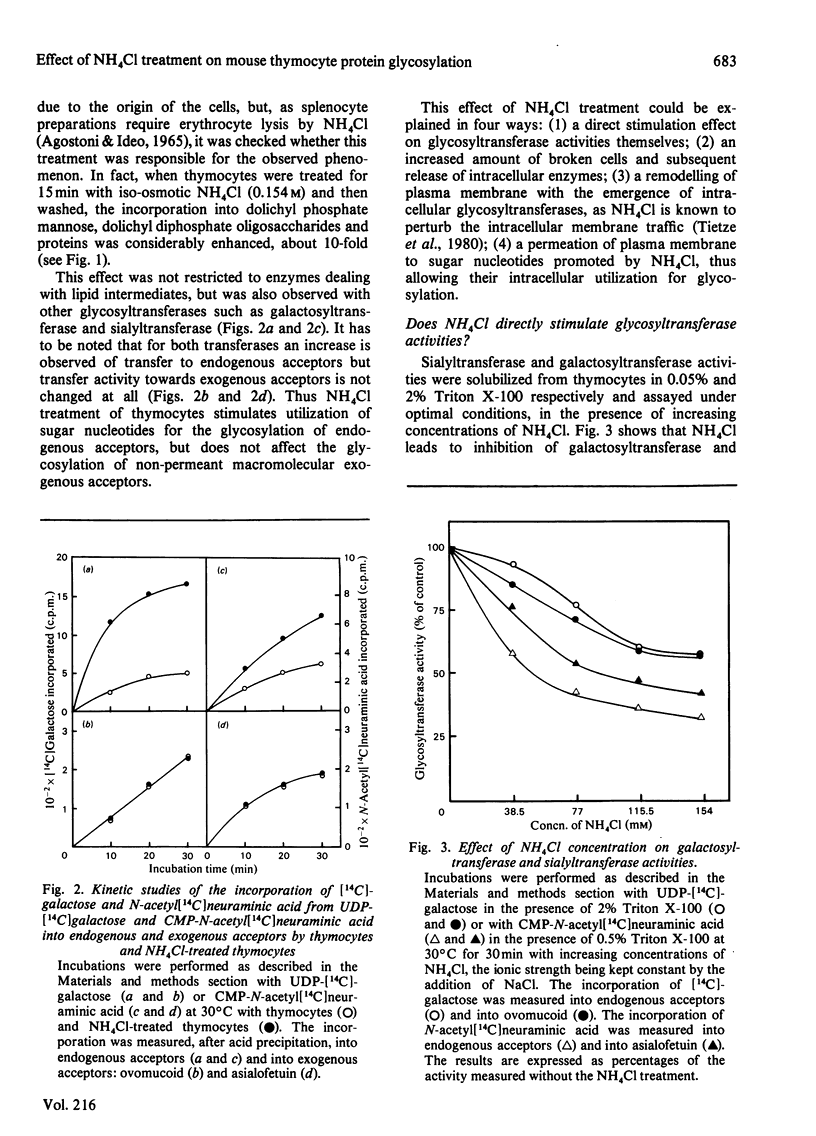
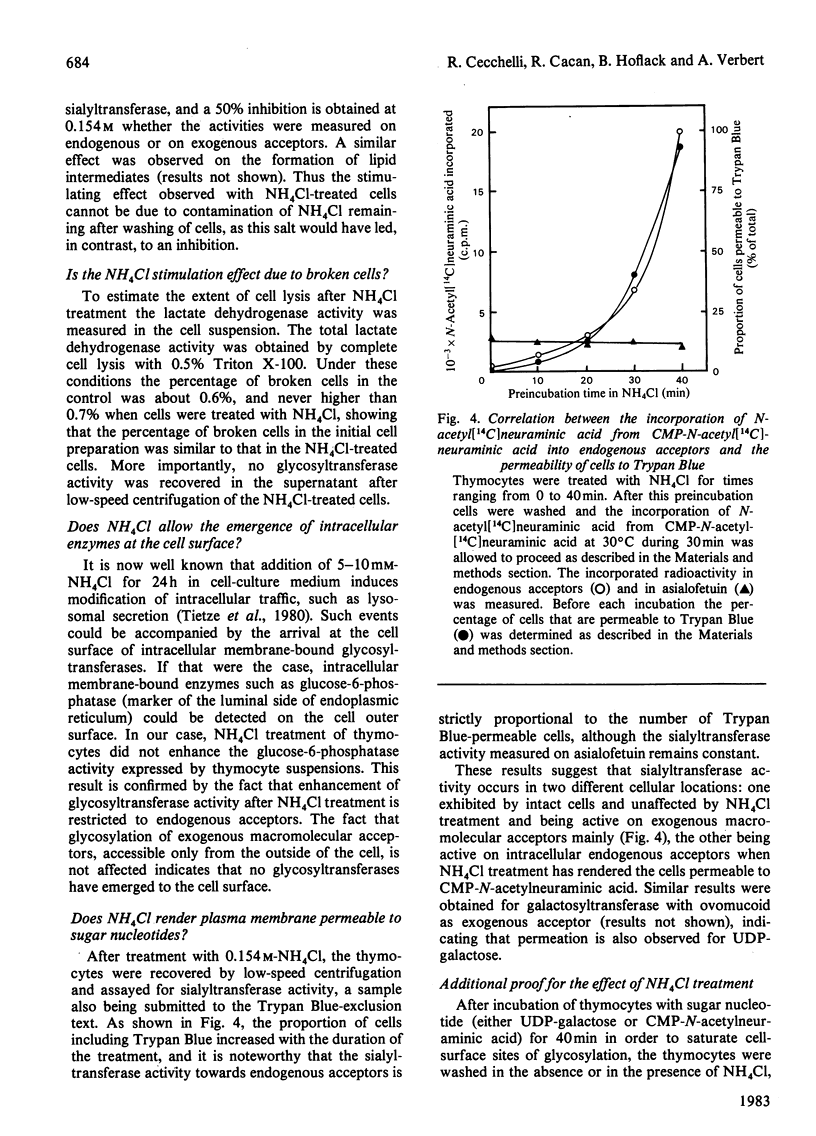
Abstract
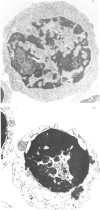
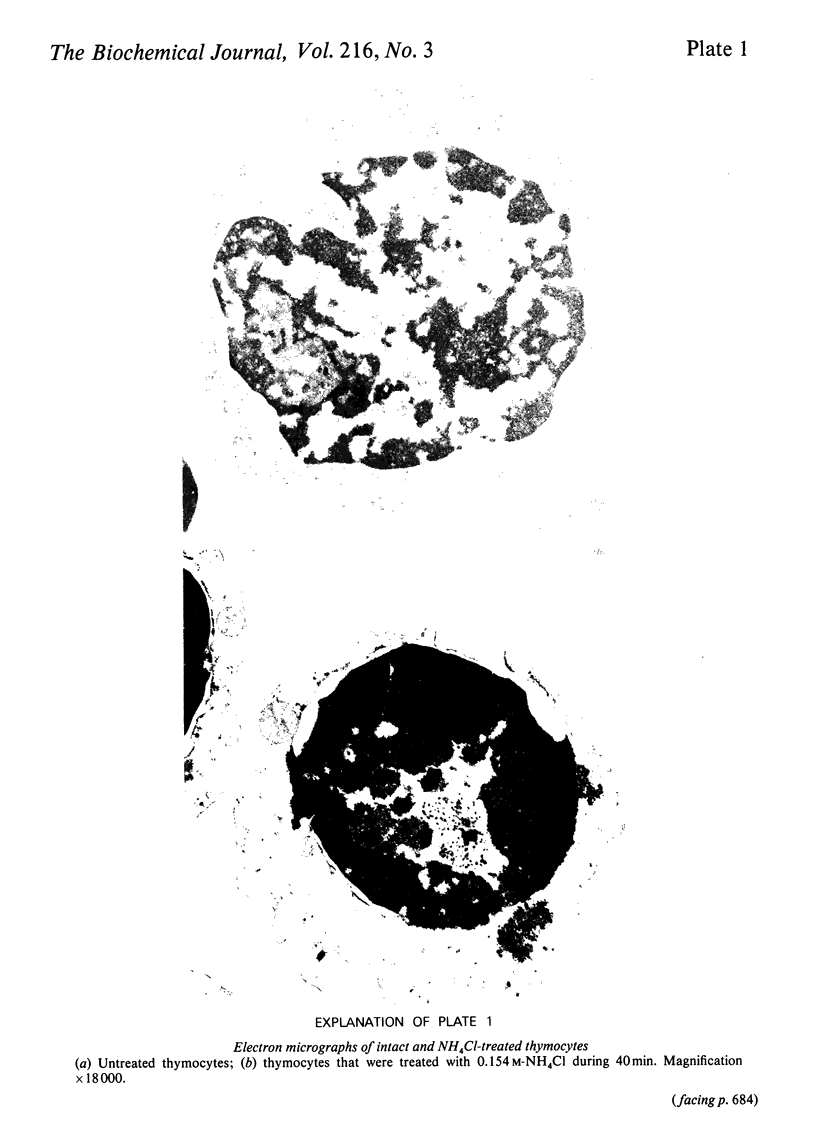
When thymocytes are treated with iso-osmotic NH4Cl, the sugar incorporation into endogenous acceptors from labelled sugar nucleotides is largely increased compared with that in control thymocytes. This effect was obtained with labelled GDP-mannose, UDP-galactose and CMP-N-acetylneuraminic acid. The stimulation observed with NH4Cl-treated thymocytes does not involve the glycosylation of exogenous acceptors, and it was proved that the NH4Cl treatment (1) does not stimulate glycosyltransferase activities themselves, (2) does not lead to the release of soluble glycosyltransferases as the result of an extensive lysis of the thymocytes and (3) does not cause the emergence of glycosyltransferases at the cell surface. In fact, electron-microscopy observations showed that, although marked changes had occurred in the cytoplasm, the plasma membrane is sufficiently maintained to allow the cell to keep roughly its original shape and to retain the intracellular vesicles. We thus demonstrate that this stimulation is due to an enhancement of the entry of sugar nucleotides into the cell. As demonstrated by the inclusion of Trypan Blue within the cells, and the non-stimulation of glycosylation of exogenous large-molecular-mass acceptors, the effect of NH4Cl seems to be limited to the penetration of small-molecular-sized compounds through the plasma membrane. Thus NH4Cl treatment allows the labelled sugar nucleotides to penetrate the cell and to behave as the cellular pool to be utilized for glycosylation by intracellular vesicles.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGOSTONI A., IDEO G. SEPARATION OF LARGE NUMBERS OF LYMPHOCYTES FROM HUMAN BLOOD. Experientia. 1965 Feb 15;21:82–83. doi: 10.1007/BF02144754. [DOI] [PubMed] [Google Scholar]
- Cacan R., Hoflack B., Verbert A. Effect of bis-(p-nitrophenyl) phosphate on the biosynthesis and the utilization of lipid-intermediates. Biochem J. 1982 Sep 1;205(3):603–609. doi: 10.1042/bj2050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacan R., Hoflack B., Verbert A. Fate of oligosaccharide-lipid intermediates synthesized by resting rat-spleen lymphocytes. Eur J Biochem. 1980 May;106(2):473–479. doi: 10.1111/j.1432-1033.1980.tb04594.x. [DOI] [PubMed] [Google Scholar]
- Cacan R., Verbert A., Hoflack B., Montreuil J. Occurrence of an intracellular inhibitor of ectosialyltransferase in lymphocytes. FEBS Lett. 1977 Sep 1;81(1):53–56. doi: 10.1016/0014-5793(77)80926-5. [DOI] [PubMed] [Google Scholar]
- Carey D. J., Sommers L. W., Hirschberg C. B. CMP-N-acetylneuraminic acid: isolation from and penetration into mouse liver microsomes. Cell. 1980 Mar;19(3):597–605. doi: 10.1016/s0092-8674(80)80036-5. [DOI] [PubMed] [Google Scholar]
- Coates S. W., Gurney T., Jr, Sommers L. W., Yeh M., Hirschberg C. B. Subcellular localization of sugar nucleotide synthetases. J Biol Chem. 1980 Oct 10;255(19):9225–9229. [PubMed] [Google Scholar]
- Cox T. M., Peters T. J. The kinetics of iron uptake in vitro by human duodenal mucosa: studies in normal subjects. J Physiol. 1979 Apr;289:469–478. doi: 10.1113/jphysiol.1979.sp012747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B. Orientation of glycoprotein galactosyltransferase and sialyltransferase enzymes in vesicles derived from rat liver Golgi apparatus. J Cell Biol. 1981 May;89(2):246–255. doi: 10.1083/jcb.89.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce M., Turley E. A., Roth S. Cell surface glycosyltransferase activities. Int Rev Cytol. 1980;65:1–47. doi: 10.1016/s0074-7696(08)61958-0. [DOI] [PubMed] [Google Scholar]
- Sommers L. W., Hirschberg C. B. Transport of sugar nucleotides into rat liver Golgi. A new Golgi marker activity. J Biol Chem. 1982 Sep 25;257(18):10811–10817. [PubMed] [Google Scholar]
- Tietze C., Schlesinger P., Stahl P. Chloroquine and ammonium ion inhibit receptor-mediated endocytosis of mannose-glycoconjugates by macrophages: apparent inhibition of receptor recycling. Biochem Biophys Res Commun. 1980 Mar 13;93(1):1–8. doi: 10.1016/s0006-291x(80)80237-3. [DOI] [PubMed] [Google Scholar]
- Verbert A., Cacan R., Montreuil J. Ectogalactosyltransferase. Presence of enzyme and acceptors on the rat lymphocyte cell surface. Eur J Biochem. 1976 Nov 1;70(1):49–53. doi: 10.1111/j.1432-1033.1976.tb10954.x. [DOI] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]