Abstract
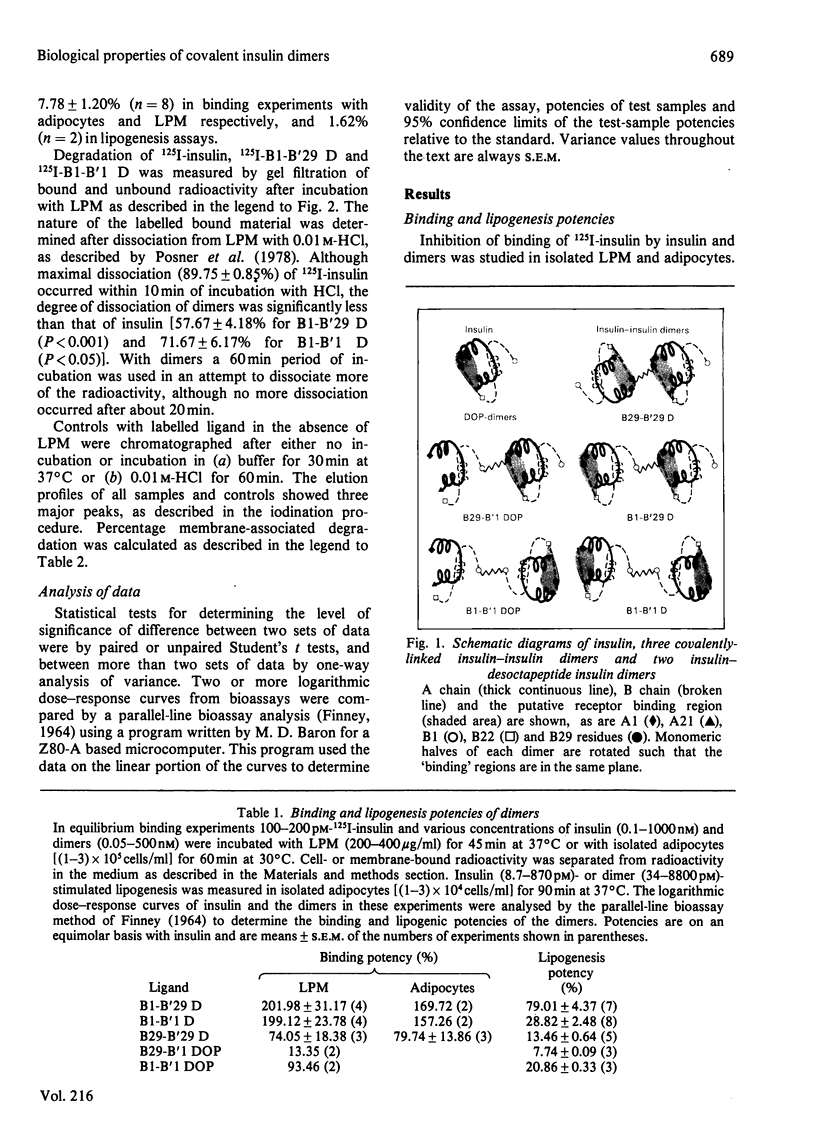
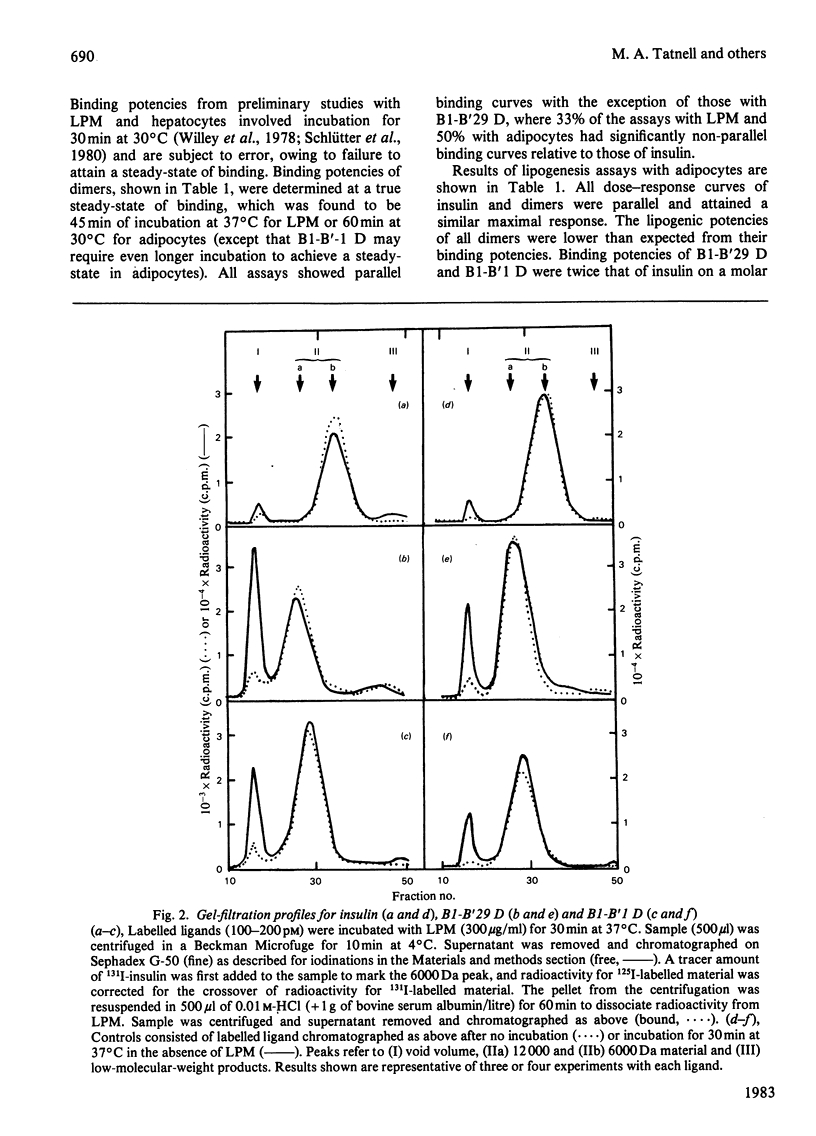
Covalently linked insulin dimers have been prepared by cross-linking two insulin monomers with a flexible suberoyl chain at either the B1 phenylalanine or the B29 lysine residue. Binding potencies of dimers determined by inhibition of binding of 125I-insulin to isolated rat liver plasma membranes or adipocytes were 2.5-7-fold greater than their abilities to stimulate lipogenesis in adipocytes. Rates of liver plasma-membrane-associated degradation of labelled insulin and dimers, measured by gel filtration, were similar at 37 degrees C. Binding and lipogenesis potencies of dimers prepared by substitution of each monomeric half of an asymmetrical dimer with desoctapeptide insulin, an almost inactive derivative, implicated the B1-cross-linked monomeric half as predominantly interacting with the insulin receptor. These results suggest that (1) dimers bind univalently to a bivalent insulin-receptor complex, in which the two individual binding subunits are arranged with anti-parallel symmetry and (2) the mechanism by which insulin binds and initiates its biological responses requires a conformational change within the insulin-receptor complex and/or in the insulin molecule for full biological expression.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke G. T., Chanley J. D., Okada Y., Cosmatos A., Ferderigos N., Katsoyannis P. G. Divergence of the in vitro biological activity and receptor binding affinity of a synthetic insulin analogue, [21-asparaginamide-A]insulin. Biochemistry. 1980 Sep 30;19(20):4547–4556. doi: 10.1021/bi00561a002. [DOI] [PubMed] [Google Scholar]
- Cudworth A. G. Type I diabetes mellitus. Diabetologia. 1978 May;14(5):281–291. doi: 10.1007/BF01223018. [DOI] [PubMed] [Google Scholar]
- Cutfield J. F., Cutfield S. M., Dodson E. J., Dodson G. G., Emdin S. F., Reynolds C. D. Structure and biological activity of hagfish insulin. J Mol Biol. 1979 Jul 25;132(1):85–100. doi: 10.1016/0022-2836(79)90497-2. [DOI] [PubMed] [Google Scholar]
- Cutfield J., Cutfield S., Dodson E., Dodson G., Hodgkin D., Reynolds C. Evidence concerning insulin activity from the structure of a cross-linked derivative. Hoppe Seylers Z Physiol Chem. 1981 Jun;362(6):755–761. doi: 10.1515/bchm2.1981.362.1.755. [DOI] [PubMed] [Google Scholar]
- Dodson E. J., Dodson G. G., Hodgkin D. C., Reynolds C. D. Structural relationships in the two-zinc insulin hexamer. Can J Biochem. 1979 Jun;57(6):469–479. doi: 10.1139/o79-060. [DOI] [PubMed] [Google Scholar]
- Dodson E. J., Dodson G. G., Hubbard R. E., Reynolds C. D. Insulin's structural behavior and its relation to activity. Biopolymers. 1983 Jan;22(1):281–291. doi: 10.1002/bip.360220137. [DOI] [PubMed] [Google Scholar]
- Freychet P., Brandenburg D., Wollmer A. Receptor-binding assay of chemically modified insulins. Comparison with in vitro and in vivo bioassays. Diabetologia. 1974 Feb;10(1):1–5. doi: 10.1007/BF00421406. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann J. Assay of insulin-like activity by the isolated fat cell method. I. Factors influencing the response to crystalline insulin. Diabetologia. 1967 Aug;3(4):382–388. doi: 10.1007/BF02342631. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Gammeltoft S. The biological activity and the binding affinity of modified insulins determined on isolated rat fat cells. Diabetologia. 1974 Apr;10(2):105–113. doi: 10.1007/BF01219665. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Osterlind K., Vinten J., Gammeltoft S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim Biophys Acta. 1972 Nov 24;286(1):1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Sonne O. Binding and receptor-mediated degradation of insulin in adipocytes. J Biol Chem. 1978 Nov 10;253(21):7857–7863. [PubMed] [Google Scholar]
- Horuk R., Blundell T. L., Lazarus N. R., Neville R. W., Stone D., Wollmer A. A monomeric insulin from the porcupine (Hystrix cristata), an Old World hystricomorph. Nature. 1980 Aug 21;286(5775):822–824. doi: 10.1038/286822a0. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Hazum E., Cuatrecasas P. The subunit structure of rat liver insulin receptor. Antibodies directed against the insulin-binding subunit. J Biol Chem. 1980 Jul 25;255(14):6937–6940. [PubMed] [Google Scholar]
- Kikuchi K., Larner J., Freer R. J., Day A. R., Morris H., Dell A. Studies on the biological activity of degraded insulins and insulin fragments. J Biol Chem. 1980 Oct 10;255(19):9281–9288. [PubMed] [Google Scholar]
- Massague J., Pilch P. F., Czech M. P. Electrophoretic resolution of three major insulin receptor structures with unique subunit stoichiometries. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7137–7141. doi: 10.1073/pnas.77.12.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton A. P. The bivalent ligand hypothesis. A quantitative model for hormone action. Mol Pharmacol. 1981 Jan;19(1):1–14. [PubMed] [Google Scholar]
- Moody A. J., Stan M. A., Stan M., Gliemann J. A simple free fat cell bioassay for insulin. Horm Metab Res. 1974 Jan;6(1):12–16. doi: 10.1055/s-0028-1093895. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Kobayashi M., Chang H. Interactions between insulin and its receptors after the initial binding event. Functional heterogeneity and relationships to insulin degradation. Diabetes. 1979 May;28(5):460–471. doi: 10.2337/diab.28.5.460. [DOI] [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. Hormone binding alters the conformation of the insulin receptor. Science. 1980 Dec 5;210(4474):1152–1153. doi: 10.1126/science.7003712. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Josefsberg Z., Bergeron J. J. Intracellular polypeptide hormone receptors. Characterization of insulin binding sites in Golgi fractions from the liver of female rats. J Biol Chem. 1978 Jun 10;253(11):4067–4073. [PubMed] [Google Scholar]
- Pullen R. A., Lindsay D. G., Wood S. P., Tickle I. J., Blundell T. L., Wollmer A., Krail G., Brandenburg D., Zahn H., Gliemann J. Receptor-binding region of insulin. Nature. 1976 Feb 5;259(5542):369–373. doi: 10.1038/259369a0. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rösen P., Simon M., Reinauer H. Binding of insulin to bovine liver plasma membrane. Use of insulin analogues modified at the A1 residue. Biochem J. 1980 Mar 15;186(3):945–952. doi: 10.1042/bj1860945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Dockerill S., Adamiak D. A., Tickle I. J., Blundell T. X-ray analysis of glucagon and its relationship to receptor binding. Nature. 1975 Oct 30;257(5529):751–757. doi: 10.1038/257751a0. [DOI] [PubMed] [Google Scholar]
- Schüttler A., Brandenburg D. Preparation and properties of covalently linked insulin dimers. Hoppe Seylers Z Physiol Chem. 1982 Mar;363(3):317–330. doi: 10.1515/bchm2.1982.363.1.317. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., Rossi B., Kowalski A., Gazzano H., Ponzio G. Receptor-mediated phosphorylation of the hepatic insulin receptor: evidence that the Mr 95,000 receptor subunit is its own kinase. Proc Natl Acad Sci U S A. 1983 Feb;80(4):945–949. doi: 10.1073/pnas.80.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelbroeck M., Van Obberghen E., De Meyts P. Thermodynamics of the interaction of insulin with its receptor. J Biol Chem. 1979 Aug 25;254(16):7736–7740. [PubMed] [Google Scholar]
- Wisher M. H., Dron D. I., Sönksen P. H., Thomas J. H. The insulin-degrading activity of plasma-membrane fractions from rat liver. Biochem Soc Trans. 1977;5(1):313–316. doi: 10.1042/bst0050313. [DOI] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip C. C., Moule M. L., Yeung C. W. Subunit structure of insulin receptor of rat adipocytes as demonstrated by photoaffinity labeling. Biochemistry. 1982 Jun 8;21(12):2940–2945. doi: 10.1021/bi00541a021. [DOI] [PubMed] [Google Scholar]


