Abstract
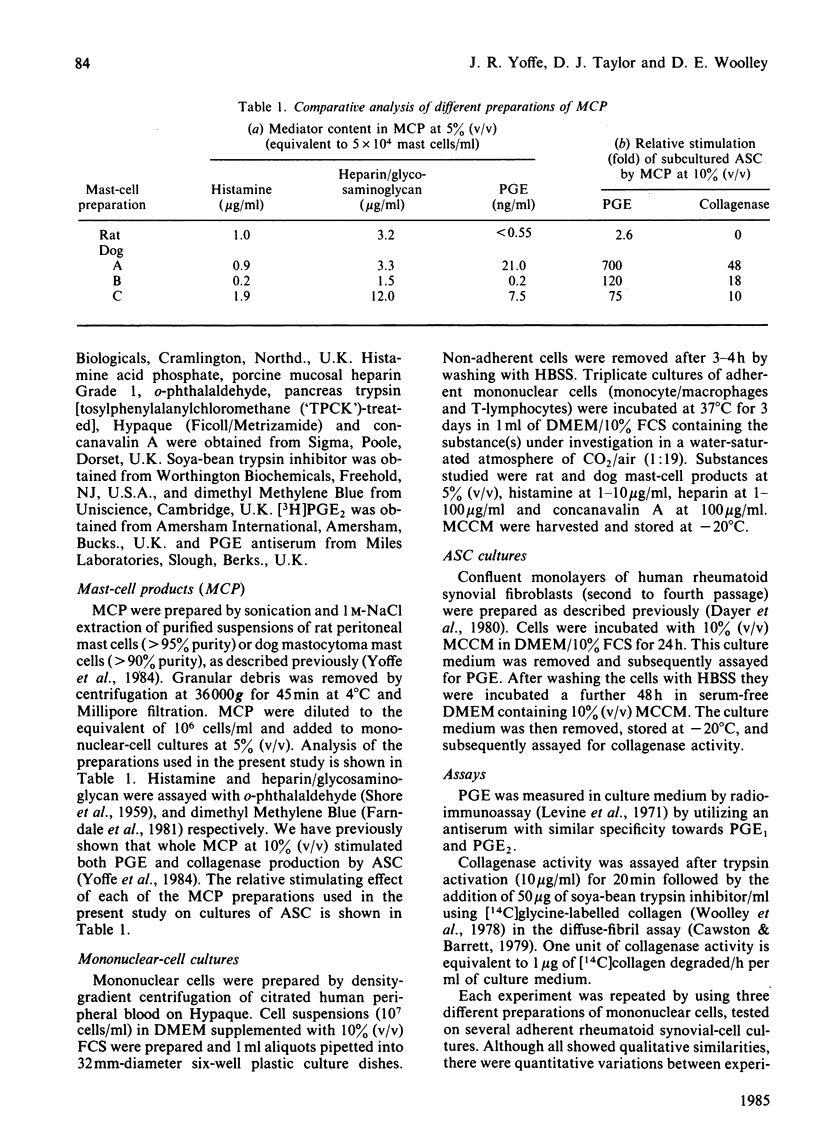
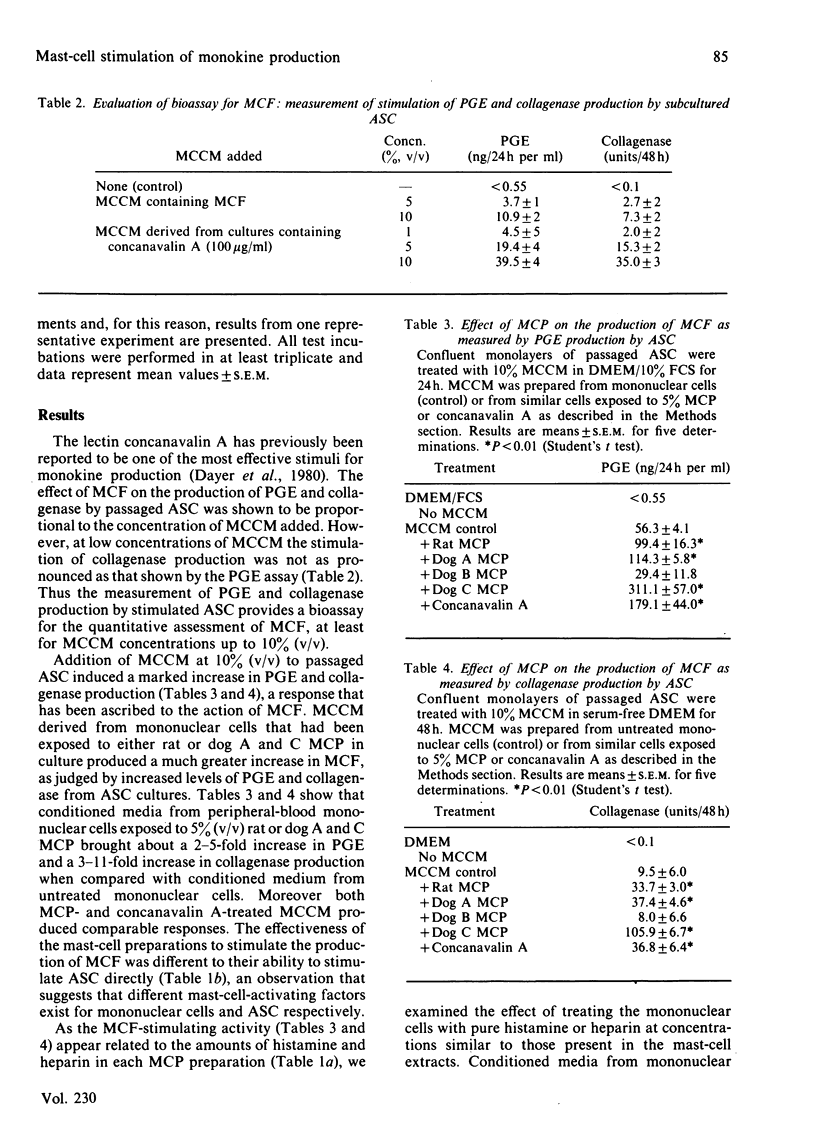
Purified mast cells derived from rat peritoneal fluids and dog mastocytomas were extracted with 1 M-NaCl and sonication techniques. The mast-cell products increased the production of mononuclear cell factor from human peripheral blood mononuclear cells in culture, as judged by the enhanced stimulation of prostaglandin E (2-5 fold) and collagenase (3-11-fold) production by cultured adherent synovial cells. Heparin alone (1-10 micrograms/ml) induced a similar stimulation of mononuclear-cell-factor production by monocyte cultures, whereas histamine (1-10 micrograms/ml) had no effect. The stimulatory effect of mast-cell products and heparin represented a direct effect on mononuclear cells; they did not potentiate the effect of monokine on the synovial cells. These results suggest that mast-cell-macrophage interactions may play a significant role in the pathogenesis of inflammation and connective-tissue degradation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amento E. P., Kurnick J. T., Epstein A., Krane S. M. Modulation of synovial cell products by a factor from a human cell line: T lymphocyte induction of a mononuclear cell factor. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5307–5311. doi: 10.1073/pnas.79.17.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Horisberger U., Martin U. Phagocytosis of mast cell granules by mononuclear phagocytes, neutrophils and eosinophils during anaphylaxis. Int Arch Allergy Appl Immunol. 1982;67(3):219–226. doi: 10.1159/000233022. [DOI] [PubMed] [Google Scholar]
- Barratt M. D., Parsons J. F. Binding of a spin label analogue of compound 48/80 to rat peritoneal mast cells: correlation of binding properties with surface topography. Biochem Pharmacol. 1984 Aug 15;33(16):2563–2568. doi: 10.1016/0006-2952(84)90625-7. [DOI] [PubMed] [Google Scholar]
- Bleiberg I., MacGregor I., Aronson M. Heparin receptors on mouse macrophages. Thromb Res. 1983 Jan 1;29(1):53–61. doi: 10.1016/0049-3848(83)90125-1. [DOI] [PubMed] [Google Scholar]
- Bromley M., Fisher W. D., Woolley D. E. Mast cells at sites of cartilage erosion in the rheumatoid joint. Ann Rheum Dis. 1984 Feb;43(1):76–79. doi: 10.1136/ard.43.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley M., Woolley D. E. Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion. Arthritis Rheum. 1984 Aug;27(8):857–863. doi: 10.1002/art.1780270804. [DOI] [PubMed] [Google Scholar]
- Camussi G., Tetta C., Bussolino F., Masera C., Emanuelli G., Ragni R., Porcellini G. Platelet-activating factor (PAF) release from rat peritoneal cells, evidence for mastocyte-macrophage cooperation. Panminerva Med. 1980 Jul-Sep;22(3):117–124. [PubMed] [Google Scholar]
- Cawston T. E., Barrett A. J. A rapid and reproducible assay for collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979 Nov 1;99(2):340–345. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- Crisp A. J., Chapman C. M., Kirkham S. E., Schiller A. L., Krane S. M. Articular mastocytosis in rheumatoid arthritis. Arthritis Rheum. 1984 Aug;27(8):845–851. doi: 10.1002/art.1780270802. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Passwell J. H., Schneeberger E. E., Krane S. M. Interactions among rheumatoid synovial cells and monocyte-macrophages: production of collagenase-stimulating factor by human monocytes exposed to concanavalin A or immunoglobulin Fc fragments. J Immunol. 1980 Apr;124(4):1712–1720. [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- GOLDHABER P. HEPARIN ENHANCEMENT OF FACTORS STIMULATING BONE RESORPTION IN TISSUE CULTURE. Science. 1965 Jan 22;147(3656):407–408. doi: 10.1126/science.147.3656.407. [DOI] [PubMed] [Google Scholar]
- Godfrey H. P., Ilardi C., Engber W., Graziano F. M. Quantitation of human synovial mast cells in rheumatoid arthritis and other rheumatic diseases. Arthritis Rheum. 1984 Aug;27(8):852–856. doi: 10.1002/art.1780270803. [DOI] [PubMed] [Google Scholar]
- Gowen M., Meikle M. C., Reynolds J. J. Stimulation of bone resorption in vitro by a non-prostanoid factor released by human monocytes in culture. Biochim Biophys Acta. 1983 Jun 2;762(3):471–474. doi: 10.1016/0167-4889(83)90014-9. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Hartveit F. Mast cells and metachromasia in human breast cancer: their occurrence, significance and consequence: a preliminary report. J Pathol. 1981 May;134(1):7–11. doi: 10.1002/path.1711340103. [DOI] [PubMed] [Google Scholar]
- Henry N., Eeckhout Y., van Lamsweerde A. L., Vaes G. Co-operation between metastatic tumor cells and macrophages in the degradation of basement membrane (type IV) collagen. FEBS Lett. 1983 Sep 19;161(2):243–246. doi: 10.1016/0014-5793(83)81017-5. [DOI] [PubMed] [Google Scholar]
- Jacques L. B. Heparin: an old drug with a new paradigm. Science. 1979 Nov 2;206(4418):528–533. doi: 10.1126/science.386509. [DOI] [PubMed] [Google Scholar]
- Krane S. M. Heberden Oration 1980: aspects of the cell biology of the rheumatoid synovial lesion. Ann Rheum Dis. 1981 Oct;40(5):433–448. doi: 10.1136/ard.40.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine L., Gjtierrez Cernosek R. M., Van Vunakis H. Specificities of prostaglandins B 1 , F 1 , and F 2 antigen-antibody reactions. J Biol Chem. 1971 Nov 25;246(22):6782–6785. [PubMed] [Google Scholar]
- Lindahl U., Pertoft H., Seljelid R. Uptake and degradation of mast-cell granules by mouse peritoneal macrophages. Biochem J. 1979 Jul 15;182(1):189–193. doi: 10.1042/bj1820189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencia-Huerta J. M., Benveniste J. Platelet-activating factor and macrophages. I. Evidence for the release from rat and mouse peritoneal macrophages and not from mastocytes. Eur J Immunol. 1979 May;9(5):409–415. doi: 10.1002/eji.1830090512. [DOI] [PubMed] [Google Scholar]
- Menninger H., Putzier R., Mohr W., Wessinghage D., Tillmann K. Granulocyte elastase at the site of cartilage erosion by rheumatoid synovial tissue. Z Rheumatol. 1980 May-Jun;39(5-6):145–156. [PubMed] [Google Scholar]
- Mizel S. B., Dayer J. M., Krane S. M., Mergenhagen S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc Natl Acad Sci U S A. 1981 Apr;78(4):2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Stevens R. L., Austen K. F., Caulfield J. P., Hein A., Liu F. T., Clabby M., Nabel G., Cantor H., Friedman S. Cloned mouse mast cells derived from immunized lymph node cells and from foetal liver cells exhibit characteristics of bone marrow-derived mast cells containing chondroitin sulphate E proteoglycan. Immunology. 1984 Jul;52(3):563–575. [PMC free article] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Schorlemmer H. U., Burger R., Hylton W., Allison A. C. Induction of lysosomal enzyme release from cultured macrophages by dextran sulfate. Clin Immunol Immunopathol. 1977 Jan;7(1):88–96. doi: 10.1016/0090-1229(77)90033-2. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Papamatheakis J. D., Chirigos M. A. Interferon: an inducer of macrophage activation by polyanions. Science. 1977 Aug 12;197(4304):674–676. doi: 10.1126/science.877584. [DOI] [PubMed] [Google Scholar]
- Sugawara I., Ishizaka S. Polysaccharides with sulfate groups are human T-cell mitogens and murine polyclonal B-cell activators (PBAs). I. Fucoidan and heparin. Cell Immunol. 1982 Nov 15;74(1):162–171. doi: 10.1016/0008-8749(82)90016-8. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Brinckerhoff C. E., Mainardi C. L., Vater C. A., Evanson J. M., Harris E. D., Jr Collagenase production by rheumatoid synovial cells: morphological and immunohistochemical studies of the dendritic cell. Ann Rheum Dis. 1979 Jun;38(3):262–270. doi: 10.1136/ard.38.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley D. E., Crossley M. J., Evanson J. M. Collagenase at sites of cartilage erosion in the rheumatoid joint. Arthritis Rheum. 1977 Jul-Aug;20(6):1231–1239. doi: 10.1002/art.1780200612. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Glanville R. W., Roberts D. R., Evanson J. M. Purification, characterization and inhibition of human skin collagenase. Biochem J. 1978 Feb 1;169(2):265–276. doi: 10.1042/bj1690265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoffe J. R., Taylor D. J., Wooley D. E. Mast cell products stimulate collagenase and prostaglandin E production by cultures of adherent rheumatoid synovial cells. Biochem Biophys Res Commun. 1984 Jul 18;122(1):270–276. doi: 10.1016/0006-291x(84)90470-4. [DOI] [PubMed] [Google Scholar]


