Abstract
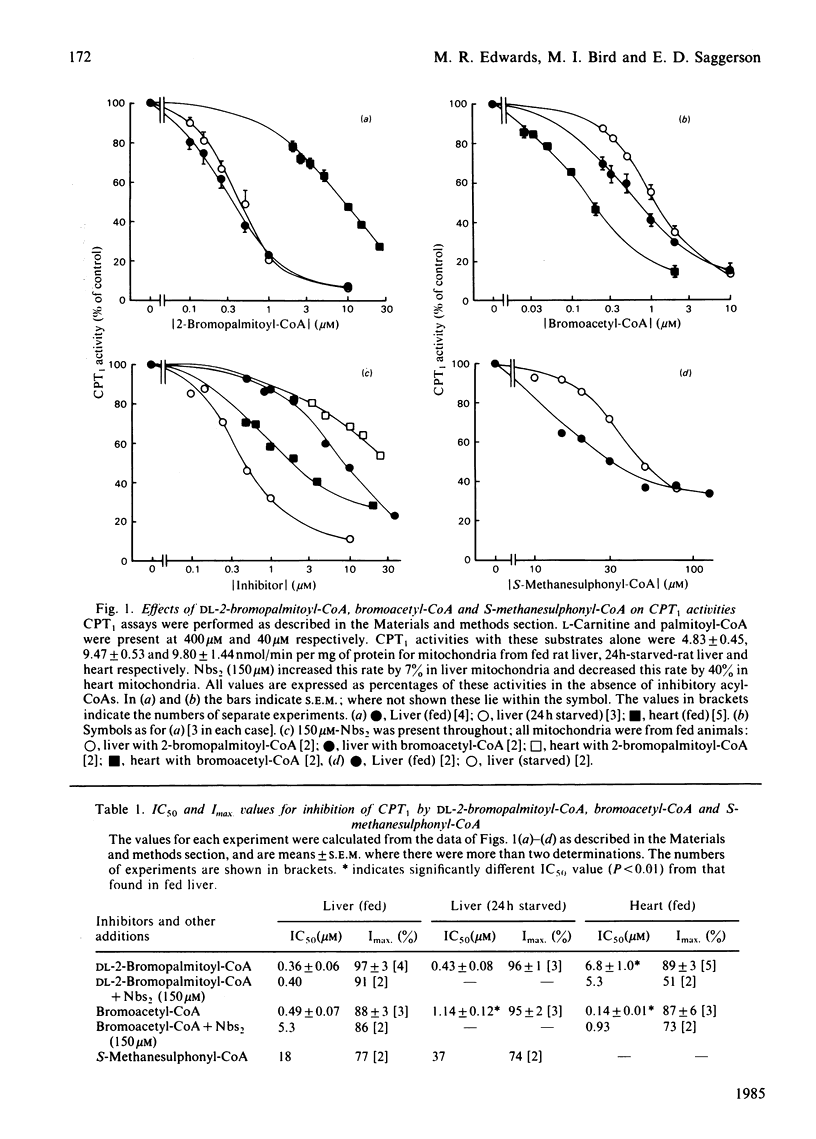
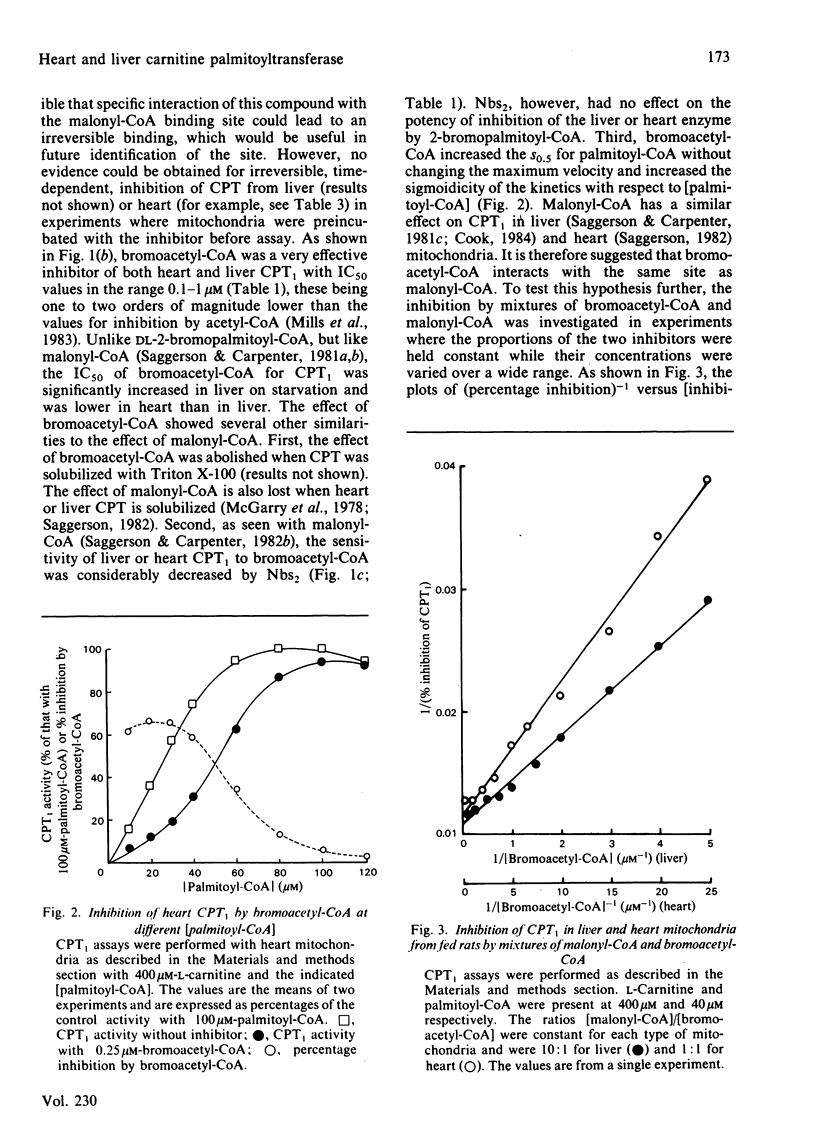
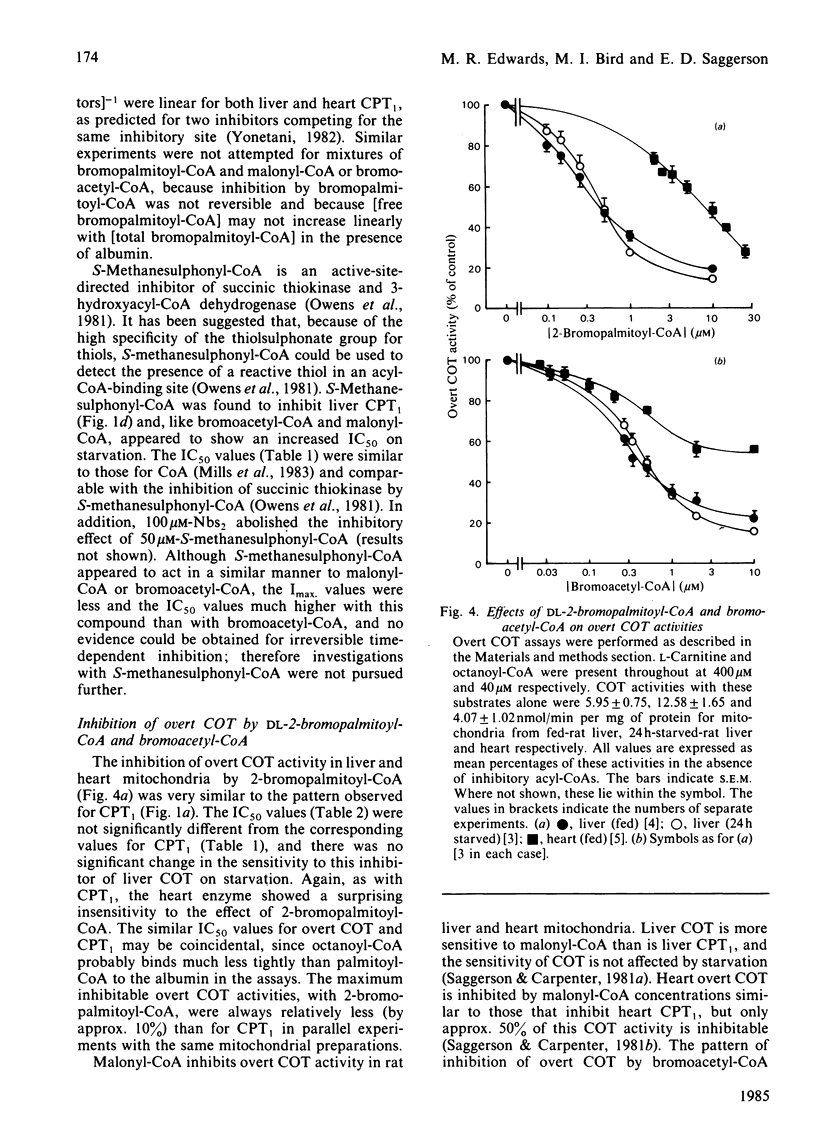
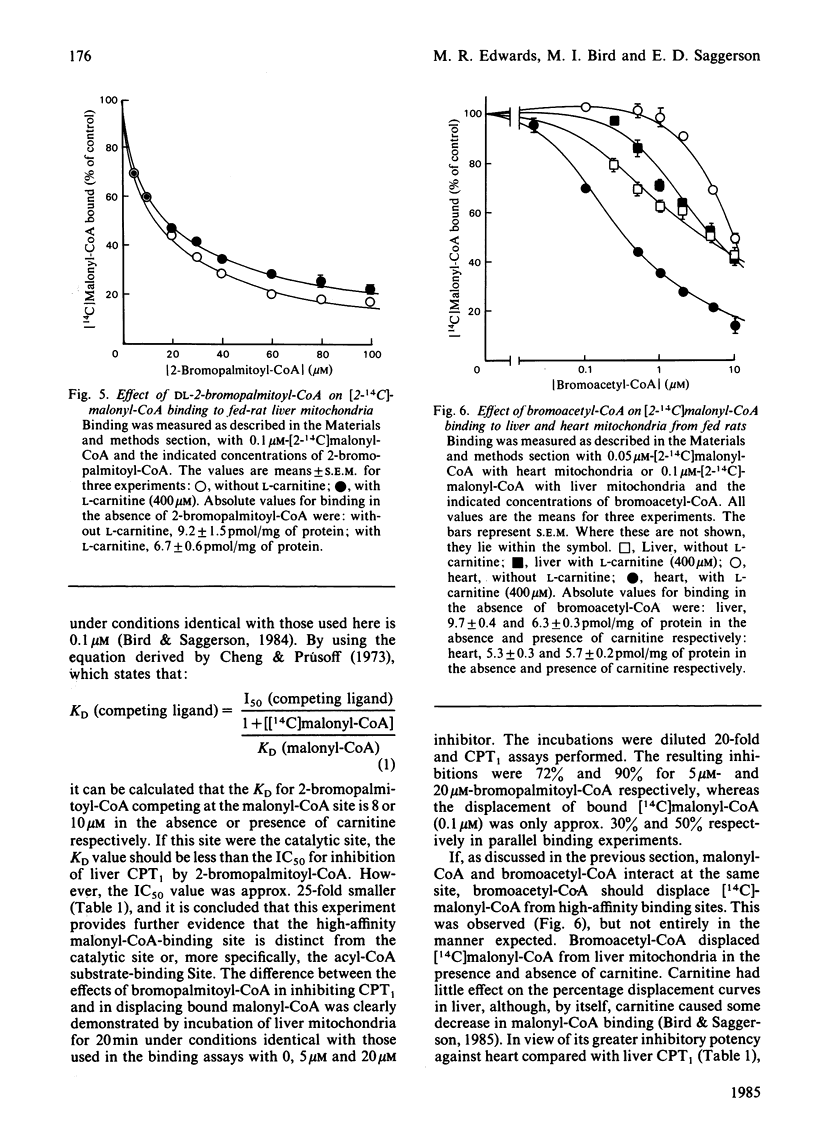
The overt form of carnitine palmitoyltransferase (CPT1) in rat liver and heart mitochondria was inhibited by DL-2-bromopalmitoyl-CoA and bromoacetyl-CoA. S-Methanesulphonyl-CoA inhibited liver CPT1. The inhibitory potency of DL-2-bromopalmitoyl-CoA was 17 times greater with liver than with heart CPT1. Inhibition of CPT1 by DL-2-bromopalmitoyl-CoA was unaffected by 5,5'-dithiobis-(2-nitrobenzoic acid) or (in liver) by starvation. In experiments in which DL-2-bromopalmitoyl-CoA displaced [14C]malonyl-CoA bound to liver mitochondria, the KD (competing) was 25 times the IC50 for inhibition of CPT1 providing evidence that the malonyl-CoA-binding site is unlikely to be the same as the acyl-CoA substrate site. Bromoacetyl-CoA inhibition of CPT1 was more potent in heart than in liver mitochondria and was diminished by 5,5'-dithiobis-(2-nitrobenzoic acid) or (in liver) by starvation. Bromoacetyl-CoA displaced bound [14C]malonyl-CoA from heart and liver mitochondria. In heart mitochondria this displacement was competitive with malonyl-CoA and was considerably facilitated by L-carnitine. In liver mitochondria this synergism between carnitine and bromoacetyl-CoA was not observed. It is suggested that bromoacetyl-CoA interacts with the malonyl-CoA-binding site of CPT1. L-Carnitine also facilitated the displacement by DL-2-bromopalmitoyl-CoA of [14C]malonyl-CoA from heart, but not from liver, mitochondria. DL-2-Bromopalmitoyl-CoA and bromoacetyl-CoA also inhibited overt carnitine octanoyl-transferase in liver and heart mitochondria. These findings are discussed in relation to inter-tissue differences in (a) the response of CPT1 activity to various inhibitors and (b) the relationship between high-affinity malonyl-CoA-binding sites and those sites for binding of L-carnitine and acyl-CoA substrates.
Full text
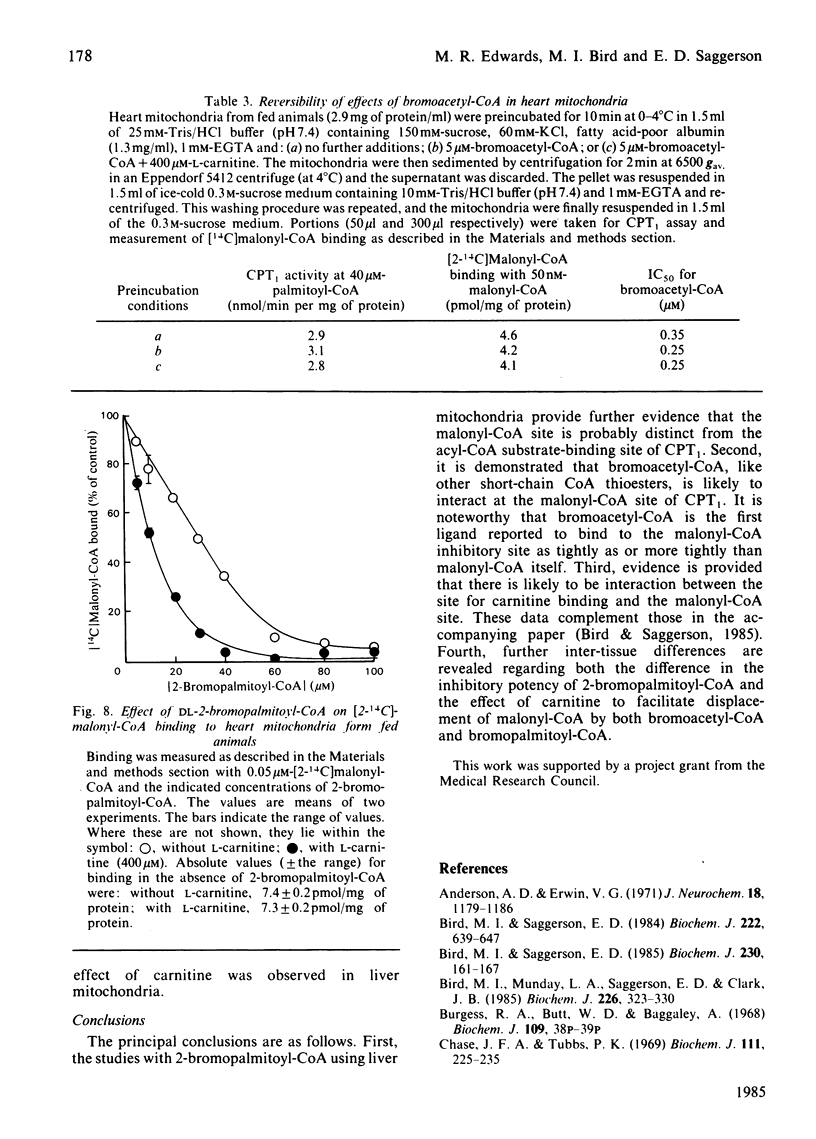
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. D., Erwin V. G. Brain acyl-coenzyme A hydrolase: distribution, purification and properties. J Neurochem. 1971 Jul;18(7):1179–1186. doi: 10.1111/j.1471-4159.1971.tb00216.x. [DOI] [PubMed] [Google Scholar]
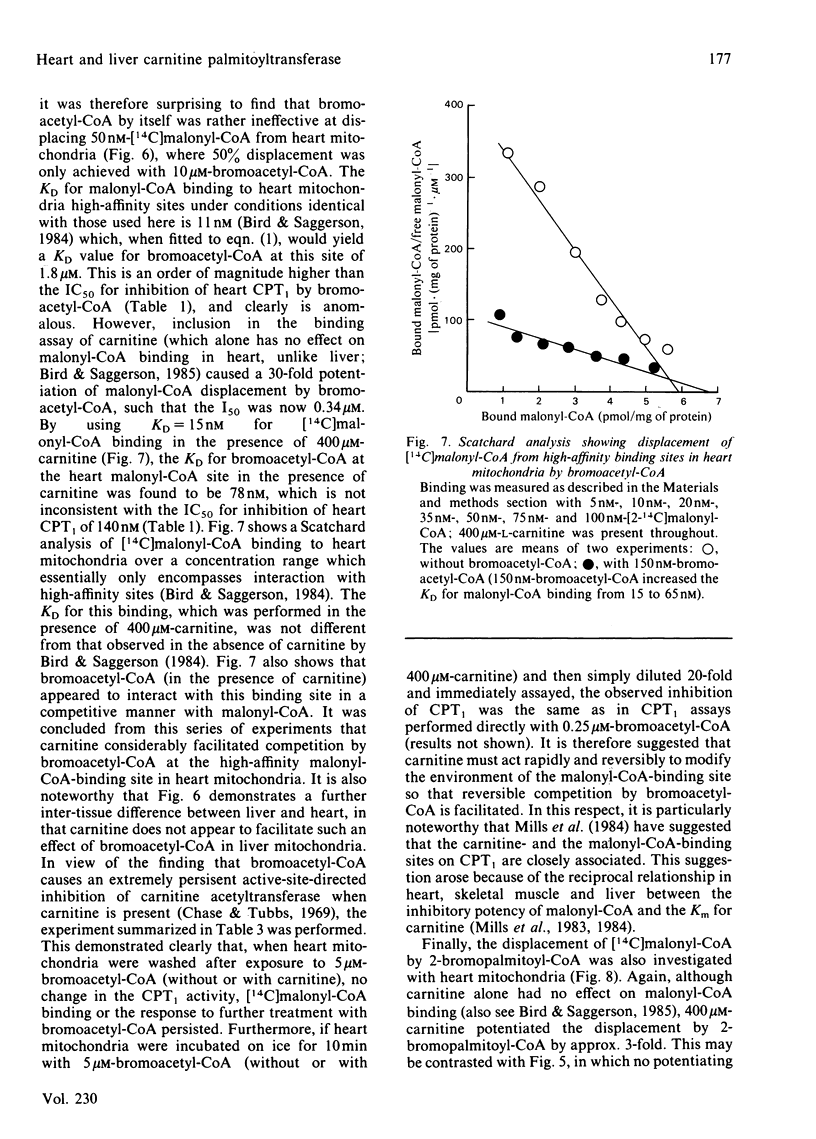
- Bird M. I., Munday L. A., Saggerson E. D., Clark J. B. Carnitine acyltransferase activities in rat brain mitochondria. Bimodal distribution, kinetic constants, regulation by malonyl-CoA and developmental pattern. Biochem J. 1985 Feb 15;226(1):323–330. doi: 10.1042/bj2260323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird M. I., Saggerson E. D. Binding of malonyl-CoA to isolated mitochondria. Evidence for high- and low-affinity sites in liver and heart and relationship to inhibition of carnitine palmitoyltransferase activity. Biochem J. 1984 Sep 15;222(3):639–647. doi: 10.1042/bj2220639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird M. I., Saggerson E. D. Interacting effects of L-carnitine and malonyl-CoA on rat liver carnitine palmitoyltransferase. Biochem J. 1985 Aug 15;230(1):161–167. doi: 10.1042/bj2300161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Conditions for the self-catalysed inactivation of carnitine acetyltransferase. A novel form of enzyme inhibition. Biochem J. 1969 Jan;111(2):225–235. doi: 10.1042/bj1110225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Specific inhibition of mitochondrial fatty acid oxidation by 2-bromopalmitate and its coenzyme A and carnitine esters. Biochem J. 1972 Aug;129(1):55–65. doi: 10.1042/bj1290055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Choi Y. R., Fogle P. J., Clarke P. R., Bieber L. L. Quantitation of water-soluble acylcarnitines and carnitine acyltransferases in rat tissues. J Biol Chem. 1977 Nov 25;252(22):7930–7931. [PubMed] [Google Scholar]
- Cook G. A. Differences in the sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA are due to differences in Ki values. J Biol Chem. 1984 Oct 10;259(19):12030–12033. [PubMed] [Google Scholar]
- Hoppel C. L., Tomec R. J. Carnitine palmityltransferase. Location of two enzymatic activities in rat liver mitochondria. J Biol Chem. 1972 Feb 10;247(3):832–841. [PubMed] [Google Scholar]
- Knauer T. E. Factors affecting the activity and stability of the palmitoyl-coenzyme A hydrolase of rat brain. Biochem J. 1979 Jun 1;179(3):515–523. doi: 10.1042/bj1790515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markwell M. A., Bieber L. L., Tolbert N. E. Differential increase of hepatic peroxisomal, mitochondrial and microsomal carnitine acyltransferases in clofibrate-fed rats. Biochem Pharmacol. 1977 Sep 15;26(18):1697–1702. doi: 10.1016/0006-2952(77)90147-2. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Mills S. E., Long C. S., Foster D. W. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983 Jul 15;214(1):21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Effects of pH on the interaction of substrates and malonyl-CoA with mitochondrial carnitine palmitoyltransferase I. Biochem J. 1984 Apr 15;219(2):601–608. doi: 10.1042/bj2190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and related compounds with mitochondria from different rat tissues. Relationship between ligand binding and inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 Jul 15;214(1):83–91. doi: 10.1042/bj2140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa S., Ozasa H., Osumi T., Hashimoto T. Purification and properties of carnitine octanoyltransferase and carnitine palmitoyltransferase from rat liver. J Biochem. 1983 Aug;94(2):529–542. doi: 10.1093/oxfordjournals.jbchem.a134384. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D. Carnitine acyltransferase activities in rat liver and heart measured with palmitoyl-CoA and octanoyl-CoA. Latency, effects of K+, bivalent metal ions and malonyl-CoA. Biochem J. 1982 Feb 15;202(2):397–405. doi: 10.1042/bj2020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Malonyl CoA inhibition of carnitine acyltransferase activities: effects of thiol-group reagents. FEBS Lett. 1982 Jan 11;137(1):124–128. doi: 10.1016/0014-5793(82)80329-3. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Sensitivity of brown-adipose-tissue carnitine palmitoyltransferase to inhibition by malonyl-CoA. Biochem J. 1982 Apr 15;204(1):373–375. doi: 10.1042/bj2040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A., Tselentis B. S. Effects of thyroidectomy and starvation on the activity and properties of hepatic carnitine palmitoyltransferase. Biochem J. 1982 Dec 15;208(3):667–672. doi: 10.1042/bj2080667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer F., Mahadevan S., Erfle J. D. The accumulation of citrate cycle intermediates in rat liver cells oxidizing palmitate. Biochim Biophys Acta. 1971 Jun 8;239(1):26–32. doi: 10.1016/0005-2760(71)90188-3. [DOI] [PubMed] [Google Scholar]
- Veerkamp J. H., Van Moerkerk H. T. The effect of malonyl-CoA on fatty acid oxidation in rat muscle and liver mitochondria. Biochim Biophys Acta. 1982 Feb 15;710(2):252–255. doi: 10.1016/0005-2760(82)90157-6. [DOI] [PubMed] [Google Scholar]
- West D. W., Chase J. F., Tubbs P. K. The separation and properties of two forms of carnitine palmitoyltransferase from ox liver mitochondria. Biochem Biophys Res Commun. 1971 Mar 5;42(5):912–918. doi: 10.1016/0006-291x(71)90517-1. [DOI] [PubMed] [Google Scholar]
- Yonetani T. The Yonetani-Theorell graphical method for examining overlapping subsites of enzyme active centers. Methods Enzymol. 1982;87:500–509. doi: 10.1016/s0076-6879(82)87028-6. [DOI] [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G., Gray S. R. Changes in the ability of malonyl-CoA to inhibit carnitine palmitoyltransferase I activity and to bind to rat liver mitochondria during incubation in vitro. Differences in binding at 0 degree C and 37 degrees C with a fixed concentration of malonyl-CoA. Biochem J. 1984 Sep 1;222(2):335–342. doi: 10.1042/bj2220335. [DOI] [PMC free article] [PubMed] [Google Scholar]


