Abstract
A simple new technique was developed for the rapid purification of either the membrane-bound or the released forms of the variant surface glycoprotein of Trypanosoma brucei in high yield. Whole cells were used as the source of the membrane-bound form, and the supernatant of benzyl alcohol-treated cells was used as the source of the released form. The technique was based on extraction of the acid-treated protein into chloroform/methanol, followed by selective re-partition into aqueous salt solution. The yield of purified protein was found to be dependent critically on a low pH during the extraction/re-partition stages. This finding and the ability to cycle the protein repeatedly through organic and aqueous phases in a strictly pH-dependent manner suggested that the protein could undergo fully reversible denaturation/renaturation only while in an extensively protonated form. The yield was independent of the polarity of the organic phase and the protein concentration over a wide range. After purification, both forms retain their ability to react with specific antibody raised against the authentic native protein purified by conventional means. The amino acid composition and the identity of the N-terminal amino acid was the same for both forms of the protein. In addition, both forms had blocked C-terminal residues. There were determined to be 1.13 X 10(7) copies of the variant surface glycoprotein per cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbet A. F., McGuire T. C. Purification of Trypanosoma brucei variable surface glycoproteins: analysis of degradation occurring during isolation. Parasitology. 1982 Dec;85(Pt 3):511–522. doi: 10.1017/s0031182000056298. [DOI] [PubMed] [Google Scholar]
- Bowles D. J., Voorheis H. P. Release of the surface coat from the plasma membrane of intact bloodstream forms of Trypanosoma brucei requires Ca2+. FEBS Lett. 1982 Mar 8;139(1):17–21. doi: 10.1016/0014-5793(82)80477-8. [DOI] [PubMed] [Google Scholar]
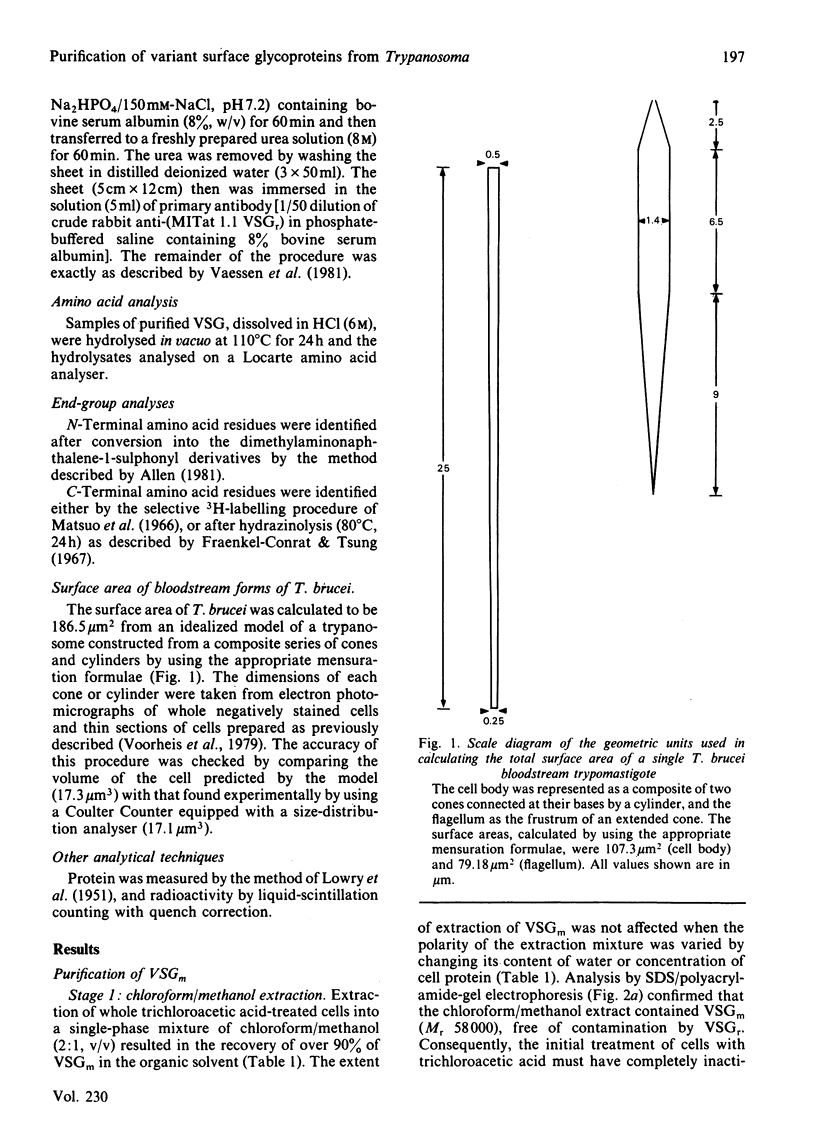
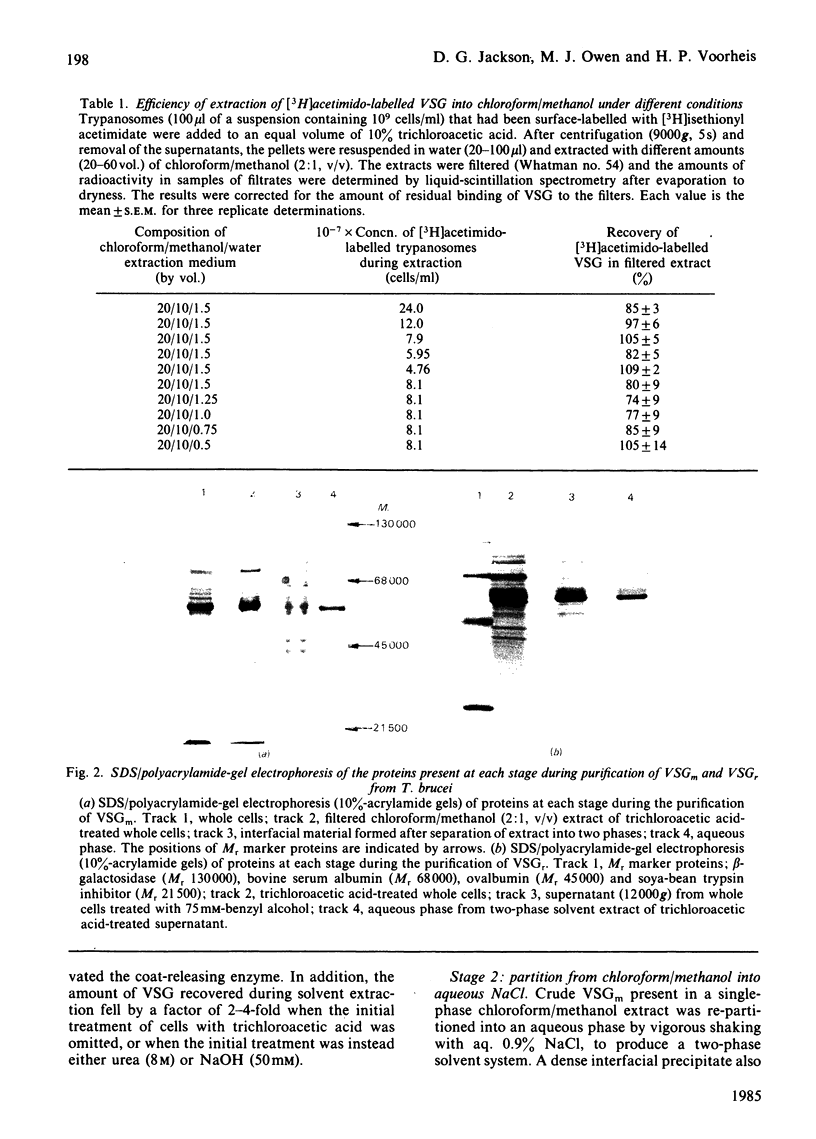
- Cardoso de Almeida M. L., Turner M. J. The membrane form of variant surface glycoproteins of Trypanosoma brucei. Nature. 1983 Mar 24;302(5906):349–352. doi: 10.1038/302349a0. [DOI] [PubMed] [Google Scholar]
- Cohen C., Reinhardt B., Parry D. A., Roelants G. E., Hirsch W., Kanwé B. Alpha-helical coiled-coil structures of Trypanosoma brucei variable surface glycoproteins. Nature. 1984 Sep 13;311(5982):169–171. doi: 10.1038/311169a0. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Cross G. A. Myristylation of the membrane form of a Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1984 Mar 10;259(5):3011–3015. [PubMed] [Google Scholar]
- Freymann D. M., Metcalf P., Turner M., Wiley D. C. 6 A-resolution X-ray structure of a variable surface glycoprotein from Trypanosoma brucei. Nature. 1984 Sep 13;311(5982):167–169. doi: 10.1038/311167a0. [DOI] [PubMed] [Google Scholar]
- Holder A. A. Carbohydrate is linked through ethanolamine to the C-terminal amino acid of Trypanosoma brucei variant surface glycoprotein. Biochem J. 1983 Jan 1;209(1):261–262. doi: 10.1042/bj2090261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Cross G. A. Glycopeptides from variant surface glycoproteins of Trypanosoma Brucei. C-terminal location of antigenically cross-reacting carbohydrate moieties. Mol Biochem Parasitol. 1981 Feb;2(3-4):135–150. doi: 10.1016/0166-6851(81)90095-5. [DOI] [PubMed] [Google Scholar]
- Jackson D. G., Voorheis H. P. Release of the variable surface coat glycoprotein from Trypanosoma brucei requires the cleavage of a phosphate ester. J Biol Chem. 1985 Apr 25;260(8):5179–5183. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsuo H., Fujimoto Y., Tatsuno T. A novel method for the determination of C-terminal amino acid in polypeptides by selective tritium labelling. Biochem Biophys Res Commun. 1966 Jan 4;22(1):69–74. doi: 10.1016/0006-291x(66)90604-8. [DOI] [PubMed] [Google Scholar]
- McConnell J., Turner M., Rovis L. Biosynthesis of Trypanosoma brucei variant surface glycoproteins - analysis of carbohydrate heterogeneity and timing of post-translational modifications. Mol Biochem Parasitol. 1983 Jun;8(2):119–135. doi: 10.1016/0166-6851(83)90004-x. [DOI] [PubMed] [Google Scholar]
- Reinwald E., Rautenberg P., Risse H. J. Purification of the variant antigens of Trypanosoma congolense: a new approach to the isolation of glycoproteins. Biochim Biophys Acta. 1981 Mar 27;668(1):119–131. doi: 10.1016/0005-2795(81)90155-0. [DOI] [PubMed] [Google Scholar]
- Strickler J. E., Mancini P. E., Patton C. L. Trypanosoma brucei brucei: isolation of the major surface coat glycoprotein by lectin affinity chromatography. Exp Parasitol. 1978 Dec;46(2):262–276. doi: 10.1016/0014-4894(78)90140-6. [DOI] [PubMed] [Google Scholar]
- Strickler J. E., Patton C. L. Trypanosoma brucei brucei: inhibition of glycosylation of the major variable surface coat glycoprotein by tunicamycin. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1529–1533. doi: 10.1073/pnas.77.3.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Vaessen R. T., Kreike J., Groot G. S. Protein transfer to nitrocellulose filters. A simple method for quantitation of single proteins in complex mixtures. FEBS Lett. 1981 Feb 23;124(2):193–196. doi: 10.1016/0014-5793(81)80134-2. [DOI] [PubMed] [Google Scholar]
- Voorheis H. P., Bowles D. J., Smith G. A. Characteristics of the release of the surface coat protein from bloodstream forms of Trypanosoma brucei. J Biol Chem. 1982 Mar 10;257(5):2300–2304. [PubMed] [Google Scholar]
- Voorheis H. P. Fatty acid uptake by bloodstream forms of Trypanosoma brucei and other species of the kinetoplastida. Mol Biochem Parasitol. 1980 Jun;1(3):177–186. doi: 10.1016/0166-6851(80)90016-x. [DOI] [PubMed] [Google Scholar]
- Voorheis H. P., Gale J. S., Owen M. J., Edwards W. The isolation and partial characterization of the plasma membrane from Trypanosoma brucei. Biochem J. 1979 Apr 15;180(1):11–24. doi: 10.1042/bj1800011. [DOI] [PMC free article] [PubMed] [Google Scholar]