Abstract
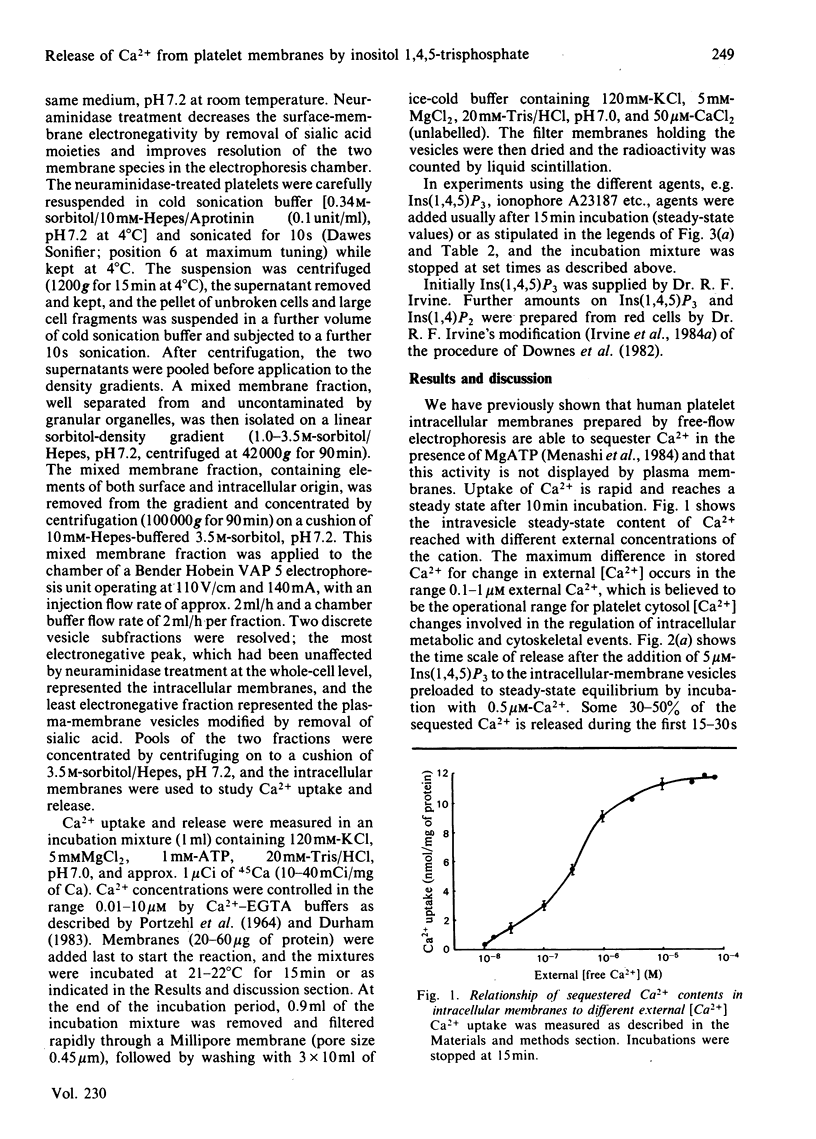
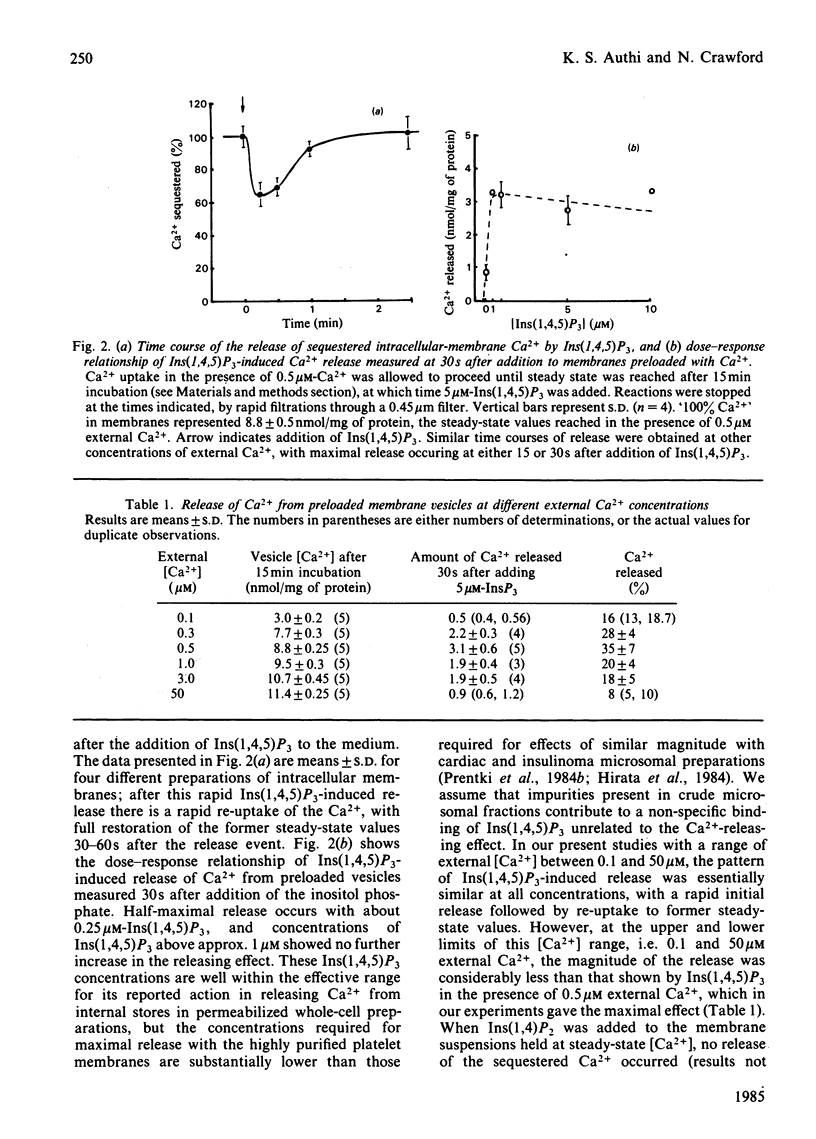
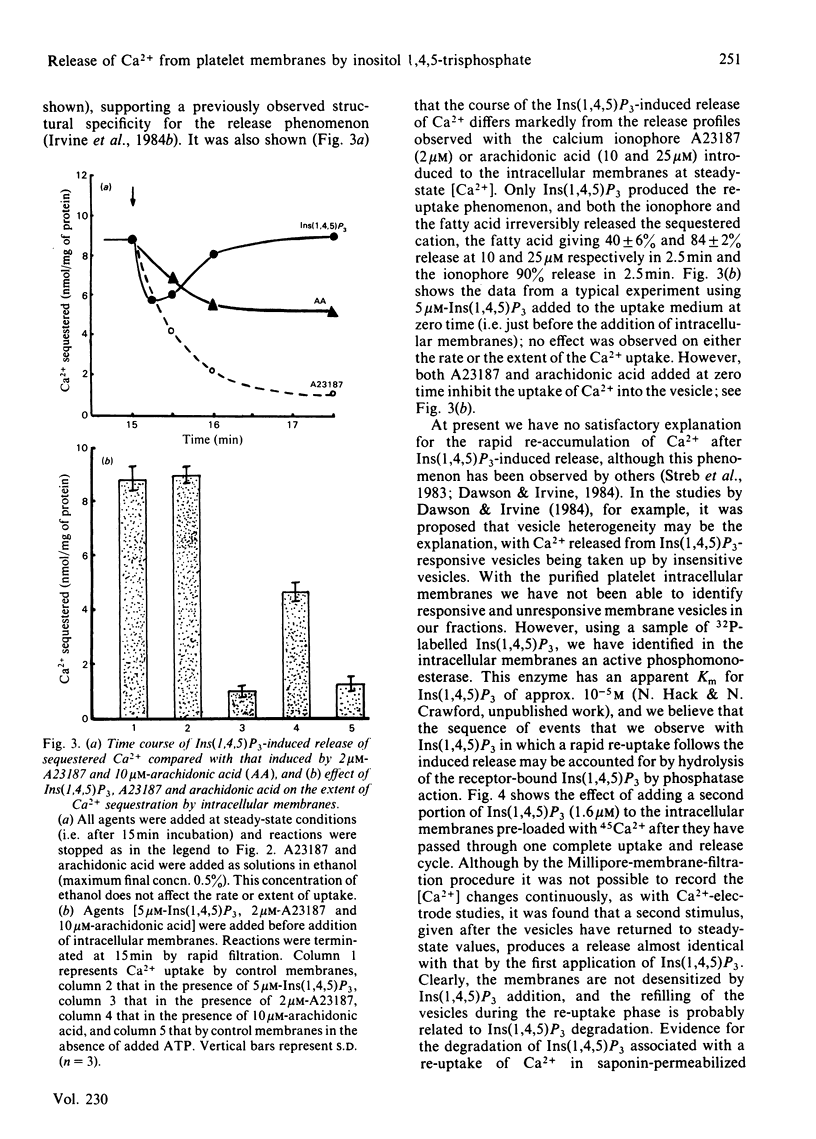
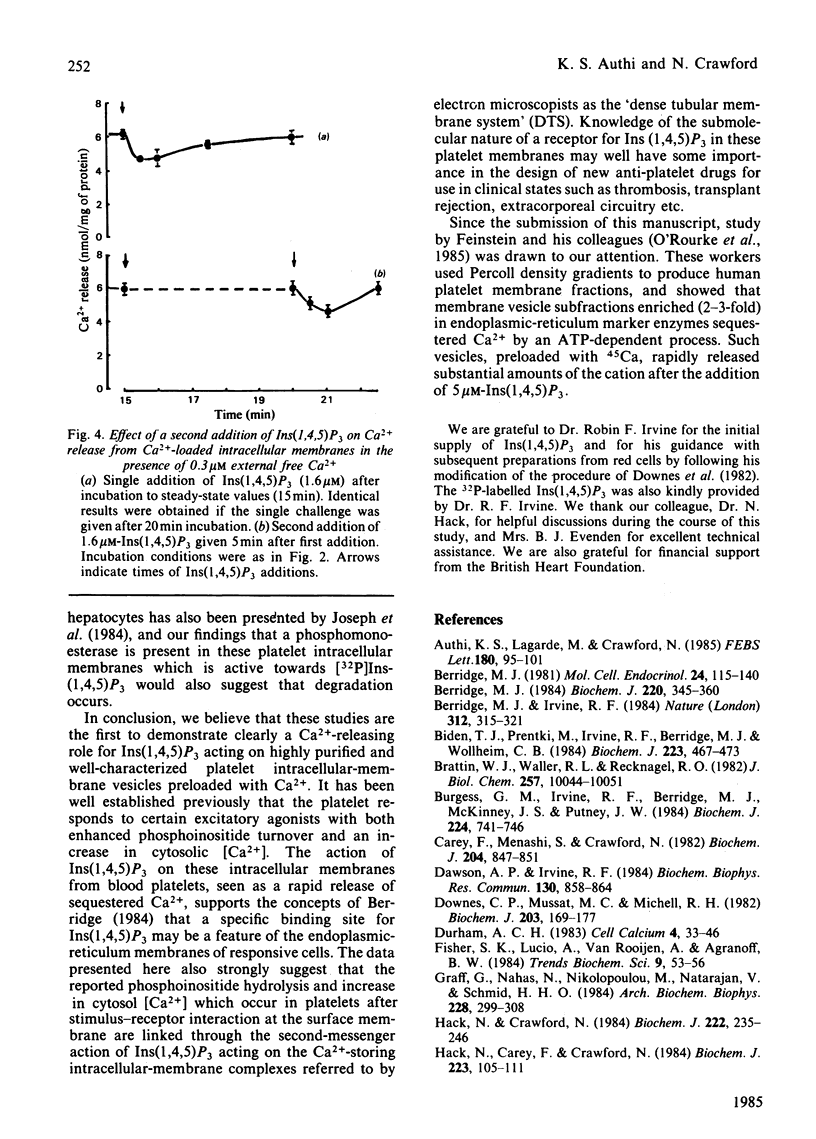
Evidence has accumulated in support of a role for intracellularly generated inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] in raising cytosol [Ca2+] when various hormones, neurotransmitters, growth factors and other stimulants act on cell surfaces. The increase in [Ca2+] that follows stimulant-receptor interaction is accompanied by rapid hydrolysis of phosphoinositides. One product, Ins(1,4,5)P3, arising from the breakdown of phosphatidylinositol 4,5-bisphosphate was shown to promote the release of Ca2+ from non-mitochondrial stores in a variety of cells. Although platelet intracellular membranes have been implicated in the control of cytosol [Ca2+] and we previously characterized a Ca2+-sequestering mechanism associated with them, we have as yet no knowledge of how this Ca2+ store is mobilized after a stimulus-receptor interaction at the platelet surface. Using free-flow electrophoresis, we isolated and purified human platelet intracellular membranes. They show high enrichment and exclusive localization of the endoplasmic-reticulum marker NADH:cytochrome c reductase, and they sequester Ca2+ by an ATP-dependent process, reaching steady-state values in 10-12 min. Saturation with Ca2+ occurs at around 10-30 microM external Ca2+. When Ins(1,4,5)P3 is added to the 45Ca-loaded vesicles, a rapid release of Ca2+ occurs (approx. 35% in 15-30s). The magnitude of the release depends upon external [Ca2+], being maximum in the range 0.3-0.8 microM and low at external [Ca2+] greater than 1 microM. After release there is a rapid re-uptake of Ca2+, with restoration of the former steady-state values within 1 min. Half-maximal release occurs at approx. 0.25 microM-Ins(1,4,5)P3. This release and re-uptake pattern is not observed with ionophore A23187 or arachidonic acid, both of which liberate Ca2+ irreversibly. Inositol 1,4-bisphosphate was ineffective in releasing Ca2+ from these intracellular membranes. The results support the role of Ins(1,4,5)P3 as a specific intracellular mediator, transducing the action of excitatory agonists acting on the platelet surface into metabolic, mechanochemical and other functional events, known to occur during platelet activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anctil M., Shimomura O. Mechanism of photoinactivation and re-activation in the bioluminescence system of the ctenophore Mnemiopsis. Biochem J. 1984 Jul 1;221(1):269–272. doi: 10.1042/bj2210269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Authi K. S., Lagarde M., Crawford N. Diacylglycerol lipase activity in human platelet intracellular and surface membranes. Some kinetic properties and fatty acid specificity. FEBS Lett. 1985 Jan 21;180(1):95–101. doi: 10.1016/0014-5793(85)80239-8. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Phosphatidylinositol hydrolysis: a multifunctional transducing mechanism. Mol Cell Endocrinol. 1981 Nov;24(2):115–140. doi: 10.1016/0303-7207(81)90055-1. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Prentki M., Irvine R. F., Berridge M. J., Wollheim C. B. Inositol 1,4,5-trisphosphate mobilizes intracellular Ca2+ from permeabilized insulin-secreting cells. Biochem J. 1984 Oct 15;223(2):467–473. doi: 10.1042/bj2230467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brattin W. J., Jr, Waller R. L., Recknagel R. O. Analysis of microsomal calcium sequestration by steady state isotope exchange. Enzyme kinetics and role of membrane permeability. J Biol Chem. 1982 Sep 10;257(17):10044–10051. [PubMed] [Google Scholar]
- Burgess G. M., Irvine R. F., Berridge M. J., McKinney J. S., Putney J. W., Jr Actions of inositol phosphates on Ca2+ pools in guinea-pig hepatocytes. Biochem J. 1984 Dec 15;224(3):741–746. doi: 10.1042/bj2240741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey F., Menashi S., Crawford N. Localization of cyclo-oxygenase and thromboxane synthetase in human platelet intracellular membranes. Biochem J. 1982 Jun 15;204(3):847–851. doi: 10.1042/bj2040847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham A. C. A survey of readily available chelators for buffering calcium ion concentrations in physiological solutions. Cell Calcium. 1983 Feb;4(1):33–46. doi: 10.1016/0143-4160(83)90047-7. [DOI] [PubMed] [Google Scholar]
- Graff G., Nahas N., Nikolopoulou M., Natarajan V., Schmid H. H. Possible regulation of phospholipase C activity in human platelets by phosphatidylinositol 4',5'-bisphosphate. Arch Biochem Biophys. 1984 Jan;228(1):299–308. doi: 10.1016/0003-9861(84)90071-7. [DOI] [PubMed] [Google Scholar]
- Hack N., Carey F., Crawford N. The inhibition of platelet cyclo-oxygenase by aspirin is associated with the acetylation of a 72kDa polypeptide in the intracellular membranes. Biochem J. 1984 Oct 1;223(1):105–111. doi: 10.1042/bj2230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack N., Crawford N. Two-dimensional polyacrylamide-gel electrophoresis of the proteins and glycoproteins of purified human platelet surface and intracellular membranes. Biochem J. 1984 Aug 15;222(1):235–246. doi: 10.1042/bj2220235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Suematsu E., Hashimoto T., Hamachi T., Koga T. Release of Ca2+ from a non-mitochondrial store site in peritoneal macrophages treated with saponin by inositol 1,4,5-trisphosphate. Biochem J. 1984 Oct 1;223(1):229–236. doi: 10.1042/bj2230229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Dawson R. M. Phosphatidylinositol-4,5-bisphosphate phosphodiesterase and phosphomonoesterase activities of rat brain. Some properties and possible control mechanisms. Biochem J. 1984 Feb 15;218(1):177–185. doi: 10.1042/bj2180177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Lagarde M., Guichardant M., Menashi S., Crawford N. The phospholipid and fatty acid composition of human platelet surface and intracellular membranes isolated by high voltage free flow electrophoresis. J Biol Chem. 1982 Mar 25;257(6):3100–3104. [PubMed] [Google Scholar]
- Menashi S., Authi K. S., Carey F., Crawford N. Characterization of the calcium-sequestering process associated with human platelet intracellular membranes isolated by free-flow electrophoresis. Biochem J. 1984 Sep 1;222(2):413–417. doi: 10.1042/bj2220413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menashi S., Weintroub H., Crawford N. Characterization of human platelet surface and intracellular membranes isolated by free flow electrophoresis. J Biol Chem. 1981 Apr 25;256(8):4095–4101. [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Rourke F. A., Halenda S. P., Zavoico G. B., Feinstein M. B. Inositol 1,4,5-trisphosphate releases Ca2+ from a Ca2+-transporting membrane vesicle fraction derived from human platelets. J Biol Chem. 1985 Jan 25;260(2):956–962. [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Prentki M., Wollheim C. B., Lew P. D. Ca2+ homeostasis in permeabilized human neutrophils. Characterization of Ca2+-sequestering pools and the action of inositol 1,4,5-triphosphate. J Biol Chem. 1984 Nov 25;259(22):13777–13782. [PubMed] [Google Scholar]
- Siess W., Binder H. Thrombin induces the rapid formation of inositol bisphosphate and inositol trisphosphate in human platelets. FEBS Lett. 1985 Jan 21;180(1):107–112. doi: 10.1016/0014-5793(85)80241-6. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Vickers J. D., Kinlough-Rathbone R. L., Mustard J. F. Accumulation of the inositol phosphates in thrombin-stimulated, washed rabbit platelets in the presence of lithium. Biochem J. 1984 Dec 1;224(2):399–405. doi: 10.1042/bj2240399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., McConnell R. T., Lapetina E. G. The rapid formation of inositol phosphates in human platelets by thrombin is inhibited by prostacyclin. J Biol Chem. 1984 Nov 10;259(21):13199–13203. [PubMed] [Google Scholar]


