Abstract
Objective
Obesity and associated low-level local systemic inflammation have been linked to an increased rate of developing knee osteoarthritis (OA). Aerobic exercise has been shown to protect the knee from obesity-induced joint damage. The aims of this study were to determine (1) if resistance training provides beneficial metabolic effects similar to those previously observed with aerobic training in rats consuming a high-fat/high-sucrose (HFS) diet and (2) if these metabolic effects mitigate knee OA in a diet-induced obesity model in rats.
Design
Twelve-week-old Sprague-Dawley rats were randomized into 4 groups: (1) a group fed an HFS diet subjected to aerobic exercise (HFS+Aer), (2) a group fed an HFS diet subjected to resistance exercise (HFS+Res), (3) a group fed an HFS diet with no exercise (HFS+Sed), and (4) a chow-fed sedentary control group (Chow+Sed). HFS+Sed animals were heavier and had greater body fat, higher levels of triglycerides and total cholesterol, and more joint damage than Chow+Sed animals.
Results
The HFS+Res group had higher body mass and body fat than Chow+Sed animals and higher OA scores than animals from the HFS+Aer group. Severe bone lesions were observed in the HFS+Sed and Chow+Sed animals at age 24 weeks, but not in the HFS+Res and HFS+Aer group animals.
Conclosion
In summary, aerobic training provided better protection against knee joint OA than resistance training in this rat model of HFS-diet-induced obesity. Exposing rats to exercise, either aerobic or resistance training, had a protective effect against the severe bone lesions observed in the nonexercised rats.
Keywords: obesity, high-fat/high-sucrose diet, resistance exercise, aerobic exercise, osteoarthritis, knee, metabolic disease
Introduction
Osteoarthritis (OA) is the most common type of arthritis, often affecting multiple joints. 1 It is associated with articular cartilage breakdown, thickening of the subchondral bone plate, and inflammation of the synovium. 2 The pain and stiffness associated with OA often affect mobility, and thus the ability to do simple activities of everyday life. 3 It is estimated that 1 in 4 Canadians will have knee OA by 2035. 2 Consumption of a diet that is low in fiber and high in fat and sugar has been associated with increased rates of obesity, metabolic syndrome, and a specific phenotype of OA called metabolic OA. 4 This phenotype has been associated with chronic systemic inflammation of the host. 5 Previous studies in our laboratory showed that exposing Sprague-Dawley rats to an HFS diet for 12 weeks increased body fat, blood lipid levels, systemic inflammation, and knee joint damage,6-10 and similar findings have been reported by other groups in different rat strains and species.11,12
Exercise is an effective method to control obesity and related comorbidities by reducing body fat, sytemic inflammation, 13 and chronic inflammation and improving blood circulation. 14 Specifically, aerobic and resistance exercise have been recommended as effective nonpharmacological treatments for cardiovascular and metabolic diseases, 15 and diet and exercise interventions have been effective in reducing weight and interleukin-6 levels in humans, but the detailed mechanisms linking obesity and metabolic syndrome with knee OA remain unclear. 16 Few studies have been aimed at investigating the effects of different types of exercise on knee joint damage associated with obesity in metabolic OA. It has been reported that muscle strengthening and aerobic exercise improve pain and disability associated with everyday movements, such as walking and stair climbing in patients with knee OA.17-19 For example, de Almeida et al.20,21 reported that the combination of aerobic and resistance exercise (circuit periodized) reduced pain, fat mass, and lipid profiles in patients with knee OA. Recently, Rios et al. 6 reported that prebiotic fiber supplementation, aerobic exercise, and the combination of exercise and fiber supplementation interventions prevented knee OA in Sprague-Dawley rats and resulted in decreased levels of systematic inflammation, decreased body mass and body fat percentage, reductions in leptin levels in serum and synovial fluid, and promotion of gut health through a diverse microbiome. Furthermore, Collins et al. 22 suggested that predominantly slow-twitch-fibred muscles with an inherent high capacity for aerobic metabolism, in contrast to fast-twitch-fibred muscles, are largely protected from degeneration associated with diet-induced obesity. Skeletal muscles are known to be effective endocrine organs producing and releasing myokines in response to exercise which, in turn, influence metabolism in other tissues and organs. 23
Resistance exercise intervention for 12 weeks has also been shown to be effective in reducing weight and body fat in obese women. 24 Others reported an increase in muscular strength and physical performance with resistance exercise training in sarcopenic patients with obesity.25,26 However, evidence of the effects of systematic resistance training on histologically quantified knee OA is not available. In view of the clinical importance of resistance/strength training in musculoskeletal health and rehabilitation 27 and current successes with nonpharmacological exercise intervention, 28 it is important to determine the potential benefits of resistance exercise in a well-controlled manner using preclinical models to explore the possible mechanistic pathways associated with resistance training in preserving musculoskeletal health and protecting against diet-induced metabolic knee OA.
Therefore, the purpose of this study was to determine if a 12-week resistance exercise training program exhibits similar beneficial metabolic effects and prevents knee joint damage as observed for an aerobic training intervention in an established rat model of diet-induced metabolic/obesity knee OA. 6 ,8-10, 29 We hypothesized that the resistance exercise program is as effective in preventing knee joint damage as the aerobic exercise program in rats fed an HFS diet.
Methods
Animals and Diets
Twelve-week-old male Sprague-Dawley rats purchased from the University of Calgary Life and Environmental Sciences Animal Resource Center (LESARC) were fed either an HFS diet (20% of total weight as fat, 50% sucrose, 20% protein, and 10% fiber and micronutrients; custom Diet #102412, Dyets, Inc., Bethlehem, PA) or a chow diet (5% of total weight as fat, 47.5% carbohydrates [only 4% from sucrose], 25% protein fiber and micronutrients, and 10% moisture; Lab Diet 5001, St.Louis, Missouri, United States). Rats were divided into 4 groups: (1) a group fed an HFS diet and exposed to aerobic exercise (HFS+Aer, n = 12), (2) a group fed an HFS diet and exposed to resistance exercise training (HFS+Res, n = 7), (3) a group fed an HFS diet with no exercise intervention/sedentary (HFS+Sed, n = 12), and (4) a group fed the chow/control diet with no exercise intervention/sedentary (Chow+Sed, n = 8). To assess the degree of knee OA, the following comparisons were made: (1) To test if exercise, independent of its type, had a beneficial effect on knee OA, the knee OA scores of the exercised animals (HFS+Aer and HFS+Res) were combined (EXE, n = 19) and compared to those of nonexercised rats (HFS+Sed and Chow+Sed). (2) In order to identify if one exercise modality was more effective than the other in preventing obesity-induced metabolic knee OA, the knee OA scores of the HFS+Res group rats were compared to those of the HFS+Aer group rats. (3) To test if exercise had a beneficial effect on knee OA independent of the diet, sedentary group animals (HFS+Sed and Chow+Sed) were compared as a combined sedentary group (SED, n = 20) to the exercise group rats (EXE, n = 19).
All rats started the HFS diet and the exercise interventions at 12 weeks of age, and all interventions lasted for 12 weeks. Animals were housed at 21 °C on a 12:12 light-dark cycle and had access to diet and water ad libitum. All protocols were approved by the University of Calgary Life and Environmental Sciences Animal Care Committee. All methods were conducted following the Guide for the Care and Use of Laboratory Animals at the University of Calgary. This study is reported in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Exercise Protocols
Aerobic Exercise
The aerobic exercise intervention consisted of progressive treadmill training for 30 minutes each, 5 days per week, reaching speeds of 25 m/min. Exercise intensity and duration were progressively increased over the first 4 weeks and then maintained at 25 m/min for 30 minutes a day for the last 8 weeks. This exercise protocol meets the minimal physical activity guidelines recommended for humans 30 and has been used previously and shown to be safe for this rat model. 31
Resistance Exercise
The resistance exercise consisted of a progressive ladder-climbing exercise program performed 3 days per week.32,33 Rats were trained to climb a 1-m almost-vertical (80° incline from horizontal) ladder with a weight attached at the base of their tails. Each day of training consisted of the first climb carried out with a load of 50% of body mass, the second climb with 75%, the third climb with 90%, and the fourth climb with 100%, after which 30 g was added to each subsequent climb until failure to complete the ladder climb. After each successful ladder climb, rats were given a 2-minute rest. This procedure was repeated until the rats were unable to complete the ladder climb, and the load for the last successful ladder climb was designated as the maximum load. Animals started with an average load of 540 g and ended up with 1,200 g by week 12. Rats using this protocol completed, on average, 5 (range 4-11) climbs per day. The only stimulation necessary to encourage the rats to climb was an occasional hand prod to the base of the tail.32,34 The rats’ ability to climb the ladder with additional weights was continuously monitored by the greatest load carried each week during training. This exercise protocol was developed to simulate human progressive resistance training. 33
Body Composition
At the end of the 12-week intervention period, body composition was measured using dual-energy x-ray absorptiometry (GE Medical Systems Lunar, Madison, WI), with a software program for small animals.
Knee Joint Histology
At the end of the experimental period (12 weeks), rats were anesthetized using 5% isoflurane, then euthanized by severing the aorta and vena cava. Knee joints were harvested, the excess muscle was removed, and joints were fixed in a 10% neutral buffered formalin solution for 2-4 weeks. Knee joints were then decalcified in Cal-x-II (Fisher Scientific, Ottawa, Ontario, Canada) at room temperature for 4 weeks, with daily changes in the first 2 weeks, then changes every 2 days. The endpoint of decalcification was determined by chemical testing using a 5% ammonium oxalate solution. The joints were then dehydrated in a graded series of alcohol, cleared in xylene, and infiltrated with paraffin using a Leica TP1020 automated processor (Leica Biosystems, Nussloch, Germany). Knee Joints were then embedded in a mix of Paraffin Plus and Paraffin Xtra wax (Fisher Scientific). Serial sagittal sections of 10 µm were obtained and mounted on Superfrost Plus slides (Fisherbrand, Canada). Slides were stained with hematoxylin, fast green, and safranin-O using Leica Autostainer XL (Leica Biosystems, Nussloch, Germany). Sections were scored using a modified Mankin score for cartilage (0-14) and a modified (0-22) Osteoarthritis Research Society International histological bone score. 7 Scores for synovium thickening (0-4) and meniscus damage (0-3) were added. The total score for each knee was obtained by summing the scores from the tibia and femur in the medial and lateral compartments, respectively. Scores for synovium and menisci were also added to make up the total score. Knee joints from all groups were scored by 2 independent and blinded assessors.
Blood Lipid Profile
Following 16 hours of food deprivation, immediately before sacrifice, cardiac blood samples were collected. Serum samples were analyzed for lipid profile (total cholesterol, high-density lipoprotein [HDL] cholesterol, and triglycerides) using a colorimetric assay (Calgary Lab Service, Calgary, AB, Canada).
Statistical Analysis
Mann-Whitney U tests and Kruskal-Wallis with Bonferroni correction were used to compare knee joint scores, body composition, and serum lipid profiles across experimental groups. Comparisons were made using IBM SPSS statistic V26 (IBM SPSS, Armonk, NY). Significance was accepted for P < 0.05, 2-tailed test.
Results
Body Composition and Body Mass
At the end of the experimental period, animals in the HFS+Res and HFS+Sed groups were heavier and had a higher percentage of body fat than the Chow+Sed group rats (P = 0.001 and P = 0.0001, respectively) (Table 1). There was no difference in lean body mass between the four experimental groups (P > 0.05).
Table 1.
Body Mass, Body Fat Mass, and Lean Body Mass.
| Body Mass (g) | Body Fat (g) | Body Lean (g) | |
|---|---|---|---|
| Chow+Sed | 564 ± 47 | 107 ± 25 | 468 ± 30 |
| HFS+Sed | 719 ± 39 a | 203 ± 60 a | 499 ± 59 |
| HFS+Aer | 671 ± 67 | 177 ± 53 | 493 ± 32 |
| HFS+Res | 759 ± 92 a | 236 ± 75 a | 523 ± 100 |
Data displayed are means ± SD of Chow+Sed, HFS+Sed, HFS+Aer, and HFS+Res animals.
HFS = high fat/high sucrose; Chow+Sed = chow diet with no exercise; HFS+Sed = HFS diet with no exercise; HFS+Aer = fed an HFS diet and subjected to aerobic exercise; HFS+Res = fed an HFS diet and subjected to resistance exercise.
A significant difference compared to Chow+Sed animals at P < 0.05.
Knee Joint Damage
We first compared the knee damage between HFS+Sed rats, Chow+Sed rats, and the EXE group (HFS+Res and HFS+Aer, n = 19) to investigate the effect of exercise regardless of its type on joint degeneration. Four rats were excluded from the Chow+Sed group (n = 4) and 2 rats from the HFS+Sed group (n = 10) because they had severe bone lesions that have been reported previously to develop in Sprague-Dawley rats with aging. 35 Interestingly, these bone lesions were not observed in any of the EXE group animals (see the following paragraph).
OA scores were significantly greater in the HFS+Sed group rats (median = 33, range = 47) than in the Chow+Sed rats (median = 13, range = 29) (P = 0.05). There was no difference in knee OA scores between the HFS+Sed and the Chow+Sed animals compared to the EXE group animals, indicating that the knee OA score of the EXE group animals was in between those of the HFS+Sed and the Chow+Sed group animals ( Fig. 1 ).
Figure 1.
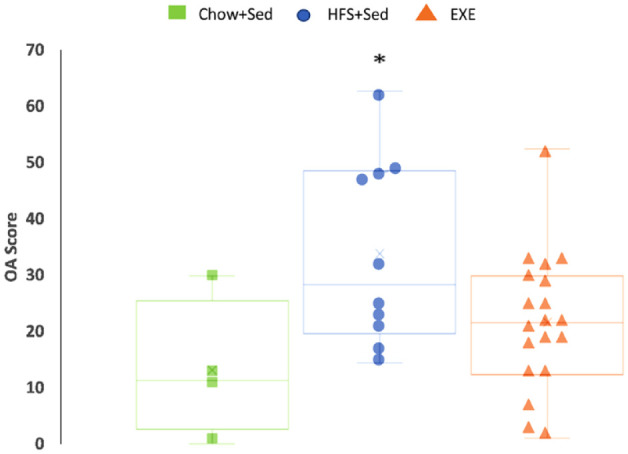
Knee joint damage represented by total knee joint OA score. Data presented as box plots showing the median and interquartile ranges and the individual values for rats fed the chow diet (Chow+Sed), animals fed the HFS diet (HFS+Sed), and animals fed the HFS diet and subjected to aerobic and resistance exercise (EXE). OA = osteoarthritis; HFS = high fat/high sucrose; Chow+Sed = chow diet with no exercise; HFS+Sed = HFS diet with no exercise intervention.
aA significant difference compared to Chow+Sed at P < 0.05.
The HFS+Res group animals had significantly higher knee joint scores than the Chow+Sed group animals (P = 0.03). No difference in joint scores was found between HFS+Aer and Chow+Sed groups. This suggests that the aerobic training was more effective than the resistance training in mitigating knee joint degeneration.
HFS+Res group rats had significantly higher knee joint OA scores (median = 30, range = 20) than the HFS+Aer group rats (median = 19, range = 27; P = 0.028; Fig. 2 ).
Figure 2.
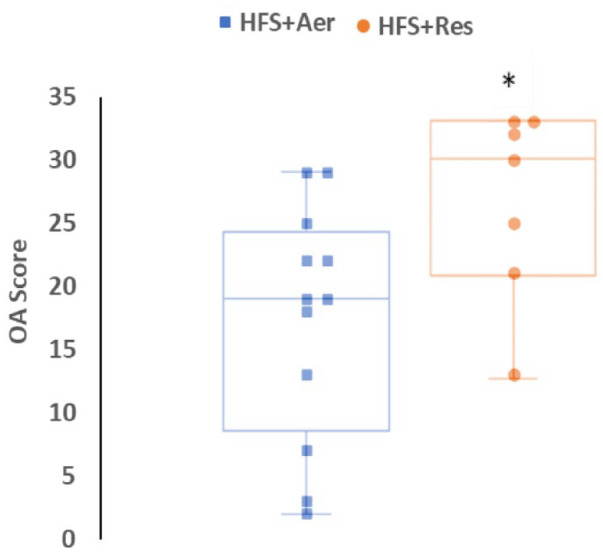
Knee joint damage assessed using total knee joint OA scores. Data presented as box plots showing the median and interquartile ranges and the individual values for animals fed an HFS diet subjected to aerobic exercise (HFS+Aer) and animals fed an HFS diet subjected to resistance exercise training (HFS+Res). OA = osteoarthritis; HFS = high fat/high sucrose; HFS+Aer = HFS diet subjected to aerobic exercise; HFS+Res = HFS diet subjected to resistance exercise.
aA significant difference at P < 0.05.
Rats exposed to the HFS diet and exercise (either aerobic or resistance; EXE) had significantly less knee joint degeneration (median = 22, range = 50) than sedentary group animals exposed to the HFS or the chow diet when including the age-related severe bone lesions that were observed frequently in the sedentary group animals (median = 39, range = 68, P = 0.022, Fig. 3 and Fig.4 ).
Figure 3.
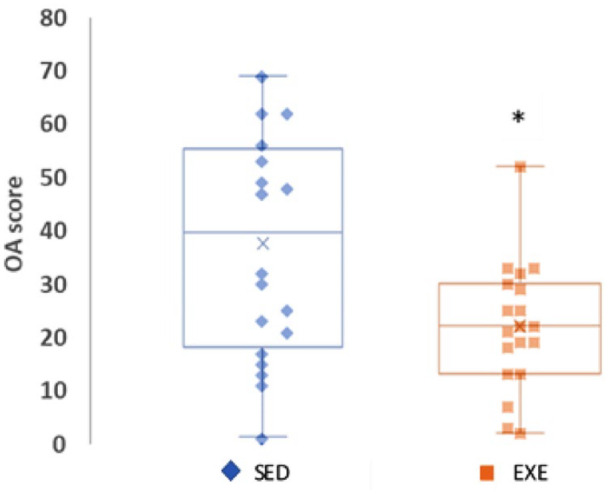
Knee joint damage assessed using total knee joint OA scores. Data are presented as box plots showing the median and interquartile ranges and the individual values for all animals in the nonexercised/sedentary groups (SED = Chow+Sed and HFS+Sed) and all animals fed an HFS diet and subjected to resistance and aerobic exercise training (EXE). OA = osteoarthritis; HFS = high fat/high sucrose; Chow+Sed = chow diet with no exercise; HFS+Sed = HFS diet with no exercise intervention.
aA significant difference at P < 0.05.
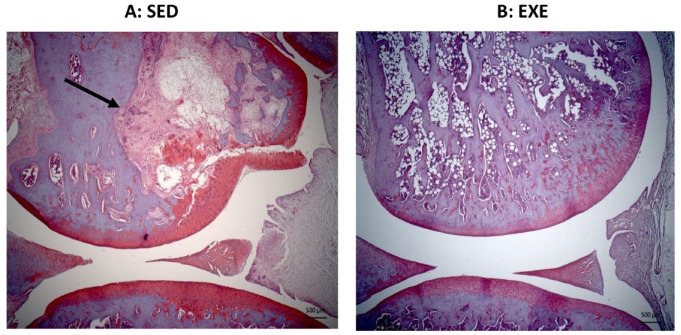
Figure 4.
Histological slides showing knee joint integrity. (A) Severe lesions commonly seen in the femur of rats in the nonexercised/sedentary groups (Sed = Chow+Sed and HFS+Sed). OA femur score = 33 (maximum = 36). (B) Exemplar joint showing healthy cartilage and bone of the rats subjected to aerobic or resistance exercise (EXE = HFS+Aer and HFS+Res). OA femur score = 2 (maximum = 36). The black arrow indicates a severe bone lesion and bone degeneration associated with a collapse of the adjacent cartilage. OA = osteoarthritis; HFS = high fat/high sucrose; HFS+Aer = HFS diet and subjected to aerobic exercise; HFS+Res = HFS diet and subjected to resistance exercise.
Serum Lipid Profile
Rats from the HFS+Sed group had significantly greater serum total cholesterol, triglyceride, and HDL levels than Chow+Sed group rats (P = 0.007, P = 0.00, and P = 0.002, respectively; Table 2 ). HFS+Aer group rats had significantly lower triglyceride levels and triglyceride to HDL cholesterol ratios than HFS+Sed group animals (P = 0.009 and P = 0.006, respectively) and higher levels of HDL cholesterol than Chow+Sed group animals (P = 0.029). HFS+Res group animals had lower levels of HDL cholesterol than the HFS+Sed group rats (P = 0.011) and higher levels of triglyceride to HDL cholesterol ratios than Chow+Sed group animals (P = 0.00). Animals from the HFS+Res group had greater triglyceride to HDL cholesterol ratios than the HFS+Aer group animals (P = 0.004). No differences were found in serum total cholesterol, triglyceride, and HDL levels between HFS+Aer and HFS+Res group animals.
Table 2.
Serum Lipid Profiles.
| Group | Chow+Sed | HFS+Sed | HFS+Aer | HFS+Res | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | |
| Total cholesterol (mmol/L) | 1.27 ± 0.29 | 1.26 (0.64) | 1.86 ± 0.2 a | 1.9 (0.4) | 1.85 ± 0.3 a | 1.78 (0.81) | 1.92 ± 0.4 | 1.82 (1.08) |
| Triglyceride (mmol/L) | 0.53 ± 0.25 | 0.52 (0.6) | 1.45 ± 0.45 a | 1.5 (1.3) | 0.81 ± 0.2 b | 0.85 (0.67) | 1.16 ± 0.4 | 1.05 (0.99) |
| HDL (mmol/L) | 1.19 ± 0.25 | 1.16 (0.1.2) | 1.7 ± 0.2 a | 1.6 (0.5) | 1.64 ± 0.2 a | 1.6 (0.47) | 1.2 ± 0.3 b | 1.1 (0.81) |
| Ratio: Tryg/HDL | 0.43 ± 0.12 | 0.45 (0.31) | 0.83 ± 0.2 | 0.78 (0.6) | 0.50 ± 0.14b,c | 0.54 (0.38) | 1.02 ± 0.4 a | 0.9 (0.93) |
Data displayed are means ± SD and median (range).
HFS = high fat/high sucrose; Chow+Sed = chow diet with no exercise; HFS+Sed = HFS diet with no exercise; HFS+Aer = fed an HFS diet and subjected to aerobic exercise; HFS+Res = fed an HFS diet and subjected to resistance exercise; HDL = high-density lipoprotein; Tryg = triglycerides.
A significant difference compared to the Chow+Sed group animals at P < 0.05.
A significant difference compared to HFS+Sed at P < 0.05.
A significant difference compared to HFS+Res at P < 0.05.
Discussion
The primary purpose of this study was to determine if resistance training has similar beneficial effects in preventing knee joint degeneration as those previously observed with aerobic training in rats consuming an HFS diet for 12 weeks. The key findings of this study were that rats consuming an HFS diet for 12 weeks without any type of exercise intervention had more severe joint OA, as assessed by a validated OA scoring system, than control animals fed a balanced chow diet. Rats in the HFS+Aer group had lower knee joint scores, indicating mild joint OA, than rats in the HFS+Res group. An interesting result was the presence of severe bone lesions at 24 weeks in both the Chow+Sed control and the HFS+Sed group rats, that is, in the nonexercised group rats regardless of diet.
The increase in knee joint OA score in the HFS+Res animals compared to the HFS+Aer may be explained by the significantly greater amount of total body fat in HFS+Res rats than in HFS+Aer rats. Previous studies showed that when lipids accumulate in adipose tissue due to overnutrition, monocytes infiltrate the adipose tissue and differentiate into macrophages which are responsible for secreting pro-inflammatory cytokines, 36 thereby increasing the chronic low-grade inflammation observed in animals with obesity. Moreover, recent studies suggest that fat plays a critical role in the pathogenesis of cartilage damage by producing adipokines that increase systemic inflammation and are associated with cartilage degeneration and OA. 37
Aerobic exercise mitigated the increase in serum triglycerides observed in the HFS+Sed group rats. Moreover, the serum triglycerides to HDL cholesterol ratio was higher in HFS+Res animals than in HFS+Aer group animals, which may explain the higher OA scores in the HFS+Res group compared to those of the HFS+Aer group rats. This finding agrees with previous studies reporting that an increase in circulating free fatty acids is associated with a decrease in insulin resistance and metabolic syndrome. Free fatty acids also act as pro-inflammatory cytokines that increase systemic inflammation, which in turn affects knee joint health. 38 Our findings agree with previous studies where a relationship was found between alterations in lipid metabolism and joint damage. 6 Further studies are needed to investigate this relationship more carefully and directly, determining differences in synovial fluid cytokines and adipokines that may be related to the onset and progression of knee joint OA.
The detailed mechanisms by which aerobic exercise provided better protection against knee joint deterioration with cartilage breakdown than resistance exercise training are not known. However, it has been reported that oxygen supply to the adipose tissue is decreased in obese rodents compared to normal-weight ones.39,40 These hypoxic conditions have been associated with triggering pro-inflammatory responses from adipose tissues. 41 We speculate that aerobic exercise may stimulate adipose tissue angiogenesis and increase blood flow and oxygen supply to the adipose tissues, 42 thus decreasing the levels of hypoxia and associated inflammatory response. 14 Moreover, aerobic exercise has been reported to modulate serum insulin and leptin levels and to prevent increases in lipopolysaccharide (LPS) levels associated with consuming an HFS diet. 6 As a result, systemic inflammation and knee joint OA are reduced. However, more studies are needed to elucidate the exact mechanisms by which aerobic exercise reduces LPS levels associated with obesity, thereby preventing knee joint damage.
Severe bone lesions were observed in sedentary rats (Chow+Sed and HFS+Sed) at 24 weeks of age regardless of the type of diet they consumed. However, rats exposed to the aerobic and resistance exercise interventions were largely protected from bone lesions, suggesting that exercise in general, independent of aerobic or resistance type, provided a protective effect against these severe bone lesions. The exact mechanism by which exercise protects bone health in this rat model is not known. However, some studies reported increased peak bone mass with increasing muscle mass in response to exercise. Also, mechanical stress on bones, as caused, for example, by exercise, has been shown to result in increasing osteoblast activity and calcium deposition in humans. 43 For example, 17 weeks of treadmill exercise has been shown to improve bone mass and body composition in ovariectomized female Sprague-Dawley rats, 44 and Simas et al. 45 reported that ladder-climbing exercise was effective in reversing the degenerative effects of immobilization in the cortical bone in ovariectomized Wistar rats. Our study showed that aerobic and resistance exercise reduced the severe bone lesions that appear to be a genetic predisposition in male Sprague-Dawley rats, 46 regardless of the diet consumed.
Conclusion
Aerobic exercise was better than resistance exercise at controlling body mass, body fat, lipid profile, and knee joint OA in an established model of HFS diet-induced obesity in male Sprague-Dawley rats. Surprisingly, aerobic and resistance exercise interventions prevented the severe bone lesions observed in aging male Sprague-Dawley rats independent of diet exposure. Because these bone lesions developed in Chow+Sed and HFS+Sed rats, it appears that exercise protects or delays rats from these genetically driven bone lesions, which is likely caused by mechanisms different from the exercise protection provided to cartilage degeneration in typically developing knee joint OA.
Footnotes
Author Contributions: N.A. was responsible for data collection, data analysis, interpretation of data, drafting the manuscript, revising the manuscript, and approving the final submitted version of the manuscript under W.H. supervision. J.L.R., K.B., and S.M.M. were responsible for designing the exercise protocols, executing the study, and revising the manuscript. R.-A.S. contributed to data analysis, interpretation of data, and writing the method section. K.H.C. was responsible for critically revising the manuscript and approving the final submitted version. W.H. contributed to study design, interpretation of the data, critically revising the manuscript, and approving the final submission.
Acknowledgments and Funding: The authors thank Andrew Sawatsky, Timothy Leonard, and Venus Joumaa for technical contributions. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Arthritis Society, the Canadian Institutes of Health Research FDN-143341, the Canada Research Chair Program (CIHR) 950-200955, the Killam Foundation, and McCaig Institute for Bone and Joint Health project number 10010760.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: All methods were conducted following the Guide for the Care and Use of Laboratory Animals at the University of Calgary. This study is reported in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
ORCID iDs: Nada Abughazaleh  https://orcid.org/0000-0002-4213-2752
https://orcid.org/0000-0002-4213-2752
Jaqueline Lourdes Rios  https://orcid.org/0000-0002-3679-2869
https://orcid.org/0000-0002-3679-2869
Data Availability Statement: The data that support the findings of this study are available on request from the corresponding author.
References
- 1. Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15-25. [DOI] [PubMed] [Google Scholar]
- 2. Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol. 2013;25(1):114-8. [DOI] [PubMed] [Google Scholar]
- 3. Teeple E, Jay GD, Elsaid KA, Fleming BC. Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J. 2013;15(2):438-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Courties A, Berenbaum F, Sellam J. The phenotypic approach to osteoarthritis: a look at metabolic syndrome-associated osteoarthritis. Joint Bone Spine. 2019;86(6):725-30. [DOI] [PubMed] [Google Scholar]
- 5. Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38-50. [DOI] [PubMed] [Google Scholar]
- 6. Rios JL, Bomhof MR, Reimer RA, Hart DA, Collins KH, Herzog W. Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci Rep. 2019;9(1):3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collins KH, Paul HA, Reimer RA, Seerattan RA, Hart DA, Herzog W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: studies in a rat model. Osteoarthr Cartil. 2015;23(11):1989-98. [DOI] [PubMed] [Google Scholar]
- 8. Collins KH, Reimer RA, Seerattan RA, Leonard TR, Herzog W. Using diet-induced obesity to understand a metabolic subtype of osteoarthritis in rats. Osteoarthr Cartil. 2015;23(6):957-65. [DOI] [PubMed] [Google Scholar]
- 9. Collins KH, Hart DA, Seerattan RA, Reimer RA, Herzog W. High-fat/high-sucrose diet-induced obesity results in joint-specific development of osteoarthritis-like degeneration in a rat model. Bone Joint Res. 2018;7(4):274-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collins KH, Hart DA, Reimer RA, Seerattan RA, Herzog W. Response to diet-induced obesity produces time-dependent induction and progression of metabolic osteoarthritis in rat knees. J Orthop Res. 2016;34(6):1010-8. [DOI] [PubMed] [Google Scholar]
- 11. Warmink K, Rios JL, van Valkengoed DR, Korthagen NM, Weinans H. Sprague Dawley rats show more severe bone loss, osteophytosis and inflammation compared to Wistar Han rats in a high-fat, high-sucrose diet model of joint damage. Int J Mol Sci. 2022;23(7):3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aboudeya HM, Shaker SA, Salama M. Effect of short-term high fat diet on resistin levels and expression of autophagy-related genes in the cartilage of male rats. Sci Rep. 2022;12(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Willis LH, Slentz CA, Bateman LA, Shields AT, Piner LW, Bales CW, et al. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol. 2012;113(12):1831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. You T, Arsenis NC, Disanzo BL, Lamonte MJ. Effects of exercise training on chronic inflammation in obesity: current evidence and potential mechanisms. Sports Med. 2013;43(4):243-56. [DOI] [PubMed] [Google Scholar]
- 15. Conti FF, Brito JDO, Bernardes N, Dias DDS, Malfitano C, Morris M, et al. Positive effect of combined exercise training in a model of metabolic syndrome and menopause: autonomic, inflammatory, and oxidative stress evaluations. Am J Physiol. 2015;309(12):R1532-9. [DOI] [PubMed] [Google Scholar]
- 16. Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nejati P, Farzinmehr A, Moradi-Lakeh M. The effect of exercise therapy on knee osteoarthritis: a randomized clinical trial. Med J Islam Repub Iran. 2015;29:1-9. [PMC free article] [PubMed] [Google Scholar]
- 18. Hurley M, Dickson K, Hallett R, Grant R, Hauari H, Walsh N, et al. Exercise interventions and patient beliefs for people with hip, knee or hip and knee osteoarthritis: a mixed methods review. Cochrane Database Syst Rev. 2018;4:CD010842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bennell KL, Nelligan RK, Kimp AJ, Schwartz S, Kasza J, Wrigley TV, et al. What type of exercise is most effective for people with knee osteoarthritis and co-morbid obesity? The TARGET randomized controlled trial. Osteoarthr Cartil. 2020;28(6):755-65. [DOI] [PubMed] [Google Scholar]
- 20. de Almeida AC, Aily JB, Pedroso MG, Gonçalves GH, de Carvalho Felinto J, Ferrari RJ, et al. A periodized training attenuates thigh intermuscular fat and improves muscle quality in patients with knee osteoarthritis: results from a randomized controlled trial. Clin Rheumatol. 2020;39(4):1265-75. [DOI] [PubMed] [Google Scholar]
- 21. de Almeida AC, Aily JB, Pedroso MG, Gonçalves GH, Pastre CM, Mattiello SM. Reductions of cardiovascular and metabolic risk factors after a 14-week periodized training model in patients with knee osteoarthritis: a randomized controlled trial. Clin Rheumatol. 2021;40(1):303-14. [DOI] [PubMed] [Google Scholar]
- 22. Collins KH, Hart DA, Smith IC, Issler AM, Reimer RA, Seerattan RA, et al. Acute and chronic changes in rat soleus muscle after high-fat high-sucrose diet. Physiol Rep. 2017;5(10):e13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379-406. [DOI] [PubMed] [Google Scholar]
- 24. Kang S, Park KM, Sung KY, Yuan Y, Lim ST. Effect of resistance exercise on the lipolysis pathway in obese pre- and postmenopausal women. J Pers Med. 2021;11(9):874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang SW, Ku JW, Lin LF, Liao CD, Chou LC, Liou TH. Body composition influenced by progressive elastic band resistance exercise of sarcopenic obesity elderly women: a pilot randomized controlled trial. Eur J Phys Rehabil Med. 2017;53(4):556-63. [DOI] [PubMed] [Google Scholar]
- 26. Chen HT, Chung YC, Chen YJ, Ho SY, Wu HJ. Effects of different types of exercise on body composition, muscle strength, and IGF-1 in the elderly with sarcopenic obesity. J Am Geriatr Soc. 2017;65(4):827-32. [DOI] [PubMed] [Google Scholar]
- 27. Ciolac EG, Rodrigues-da-Silva JM. Resistance training as a tool for preventing and treating musculoskeletal disorders. Sports Med. 2016;46(9):1239-48. [DOI] [PubMed] [Google Scholar]
- 28. Skou ST, Roos EM. Good life with osteoarthritis in Denmark (GLA:DTM): evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Musculoskelet Disord. 2017;18(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rios J, Hart DA, Reimer RA, Herzog W. Effects of prebiotics and exercise on the progression of osteoarthritis in a rat-model of metabolic disturbance. Osteoarthr Cartil. 2020;28:S203. [Google Scholar]
- 30. Canadian Society for Exercise Physiology. Guidelines [cited 2021 Apr 26]. Available from: https://csepguidelines.ca/.
- 31. Rios JL, Boldt KR, Mather JW, Seerattan RA, Hart DA, Herzog W. Quantifying the effects of different treadmill training speeds and durations on the health of rat knee joints. Sport Med Open. 2018;4(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boldt KR, Joumaa V, Turnbull J, Fedak PWM, Herzog W. Mechanical and structural remodeling of cardiac muscle following aerobic and resistance exercise training in rats. Med Sci Sport Exerc. 2021;53:1583–94. [DOI] [PubMed] [Google Scholar]
- 33. Hornberger TA, Jr, Farrar RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004;29(1):16-31. [DOI] [PubMed] [Google Scholar]
- 34. Duncan ND, Williams DA, Lynch GS. Adaptations in rat skeletal muscle following long-term resistance exercise training. Eur J Appl Physiol Occup Physiol. 1998;77(4):372-8. [DOI] [PubMed] [Google Scholar]
- 35. Kato M, Onodera T. Spontaneous osteochondrosis in rats. Lab Anim. 1984;18:179-87. [DOI] [PubMed] [Google Scholar]
- 36. Castoldi A, De Souza CN, Saraiva Câmara NO, Moraes-Vieira PM. The macrophage switch in obesity development. Front Immunol. 2016;6:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Collins KH, Lenz KL, Pollitt EN, Ferguson D, Hutson I, Springer LE, et al. Adipose tissue is a critical regulator of osteoarthritis. Proc Natl Acad Sci. 2021;118(1):e2021096118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frohnert BI, Jacobs DR, Jr, Steinberger J, Moran A, Steffen LM, Sinaiko AR. Relation between serum free fatty acids and adiposity, insulin resistance, and cardiovascular risk factors from adolescence to adulthood. Diabetes. 2013;62(9):3163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Woo CY, Jang JE, Lee SE, Koh EH, Lee KU. Mitochondrial dysfunction in adipocytes as a primary cause of adipose tissue inflammation. Diabetes Metab J. 2019;43:247-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goossens GH, Blaak EE. Adipose tissue oxygen tension: implications for chronic metabolic and inflammatory diseases. Curr Opin Clin Nutr Metab Care. 2012;15(6):539-46. [DOI] [PubMed] [Google Scholar]
- 41. Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56(4):901-11. [DOI] [PubMed] [Google Scholar]
- 42. Disanzo BL, You T. Effects of exercise training on indicators of adipose tissue angiogenesis and hypoxia in obese rats. Metabolism. 2014;63(4):452-5. [DOI] [PubMed] [Google Scholar]
- 43. Daly RM, Duckham RL, Gianoudis J. Evidence for an interaction between exercise and nutrition for improving bone and muscle health. Curr Osteoporos Rep. 2014;12(2):219-26. [DOI] [PubMed] [Google Scholar]
- 44. Gao L, Li Y, Yang YJ, Zhang DY. The effect of moderate-intensity treadmill exercise on bone mass and the transcription of peripheral blood mononuclear cells in ovariectomized rats. Front Physiol. 2021;12:729910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simas JMM, Brancalhão RMC, De Ribeiro LFC, Bertolini GRF. Influence of ladder climbing exercise on bone of rats induced to osteoporosis and immobilization. Arch Med Del Deport. 2016;33(3):176-82. [Google Scholar]
- 46. Kato M, Onodera T, Femoral M. Early changes of osteochondrosis in medial femoral condyles from rats. Vet Pathol. 1987;24:80-6. [DOI] [PubMed] [Google Scholar]