Abstract
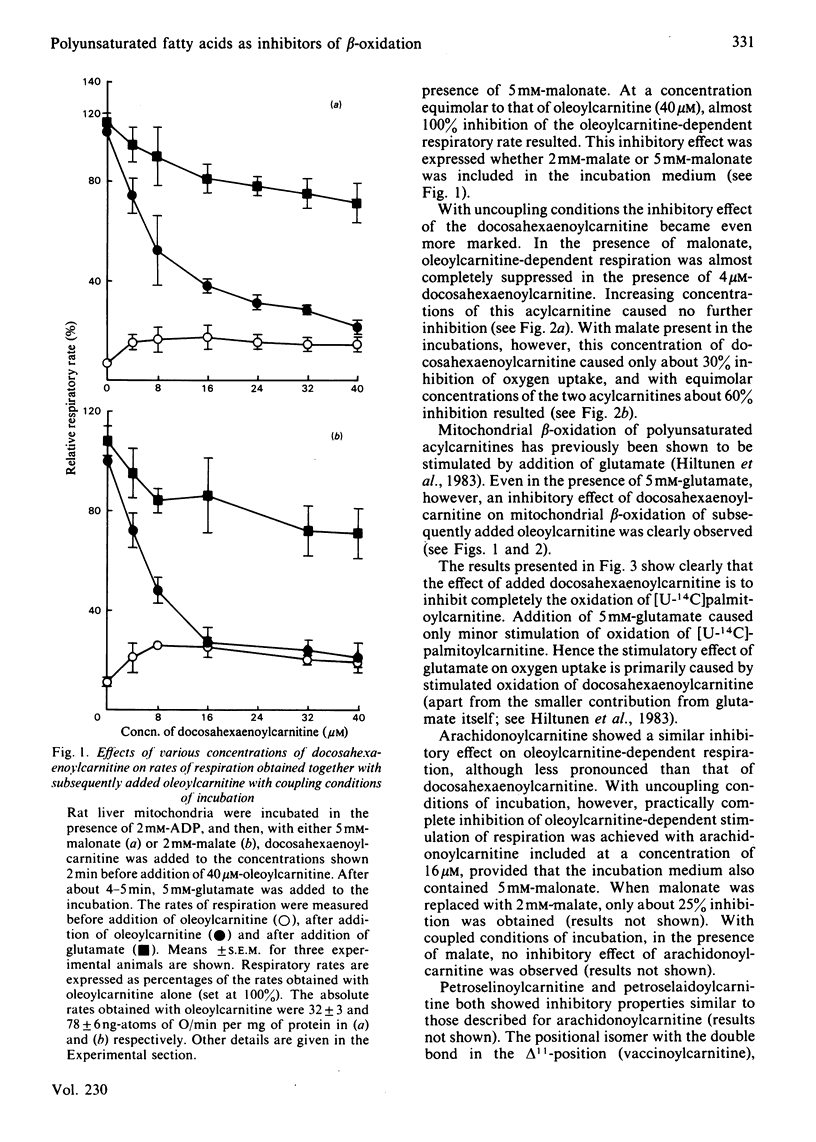
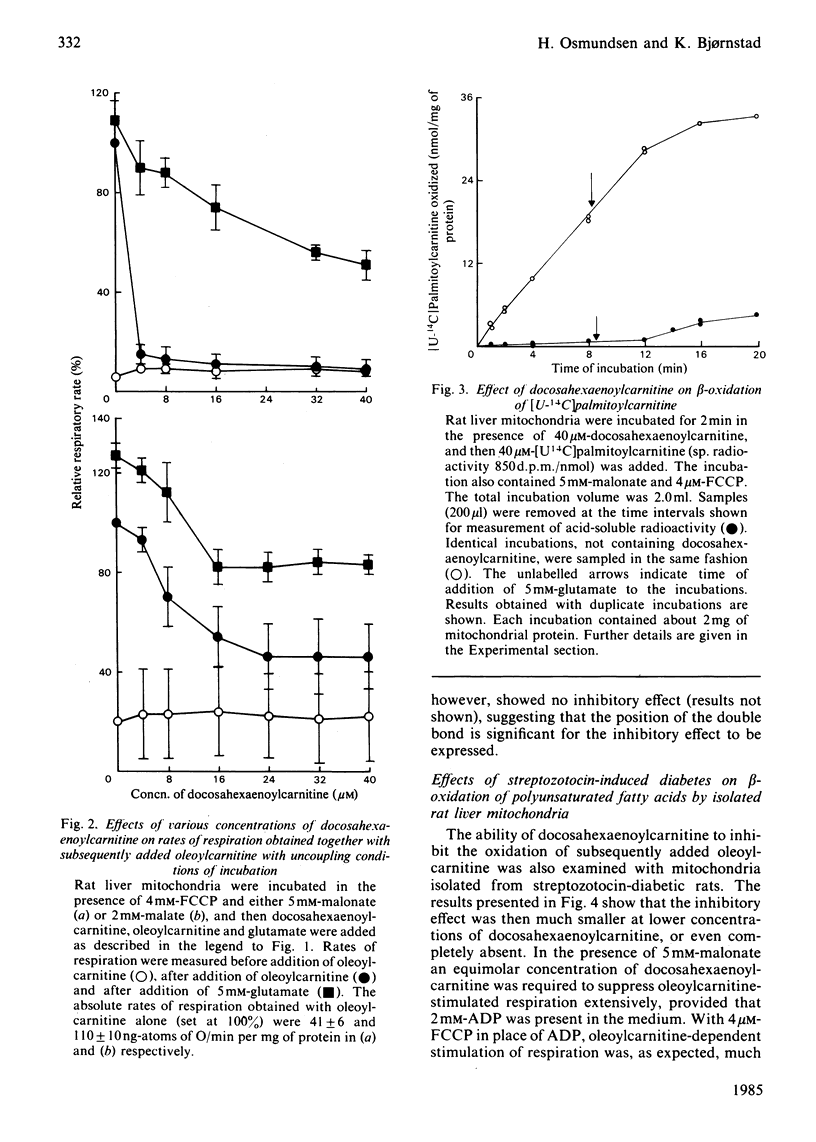
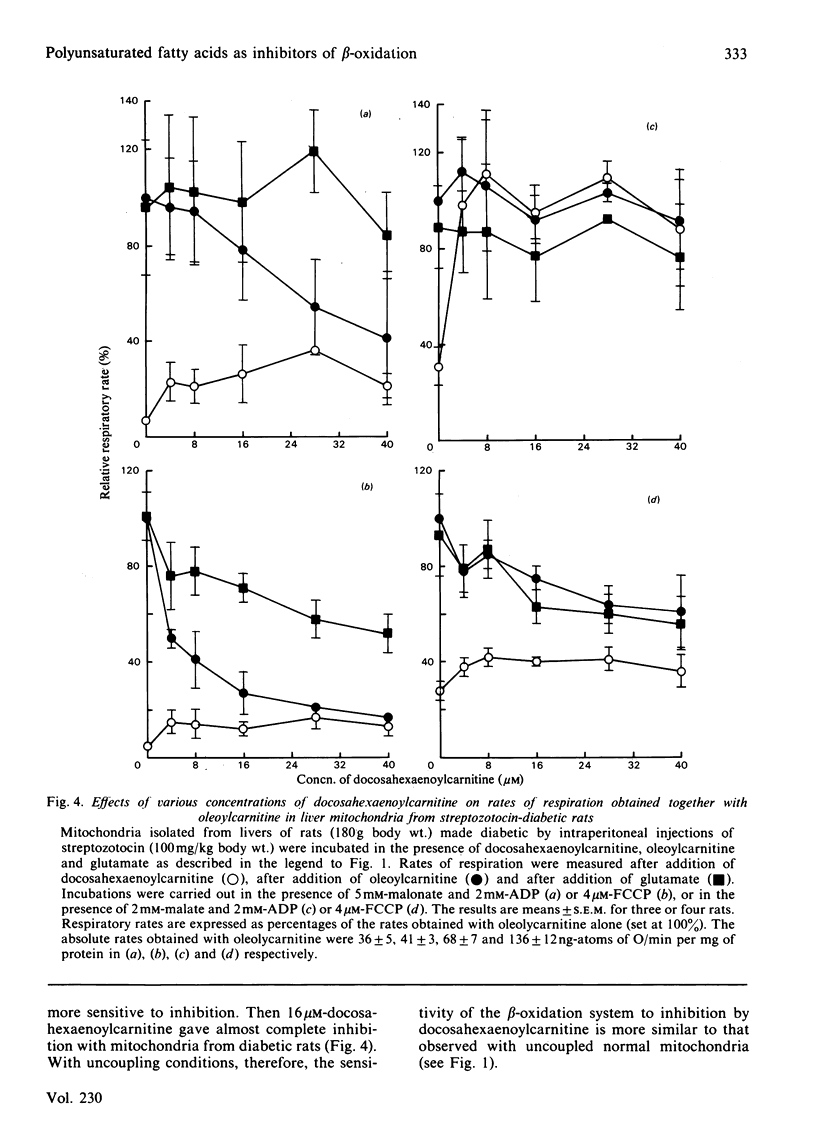
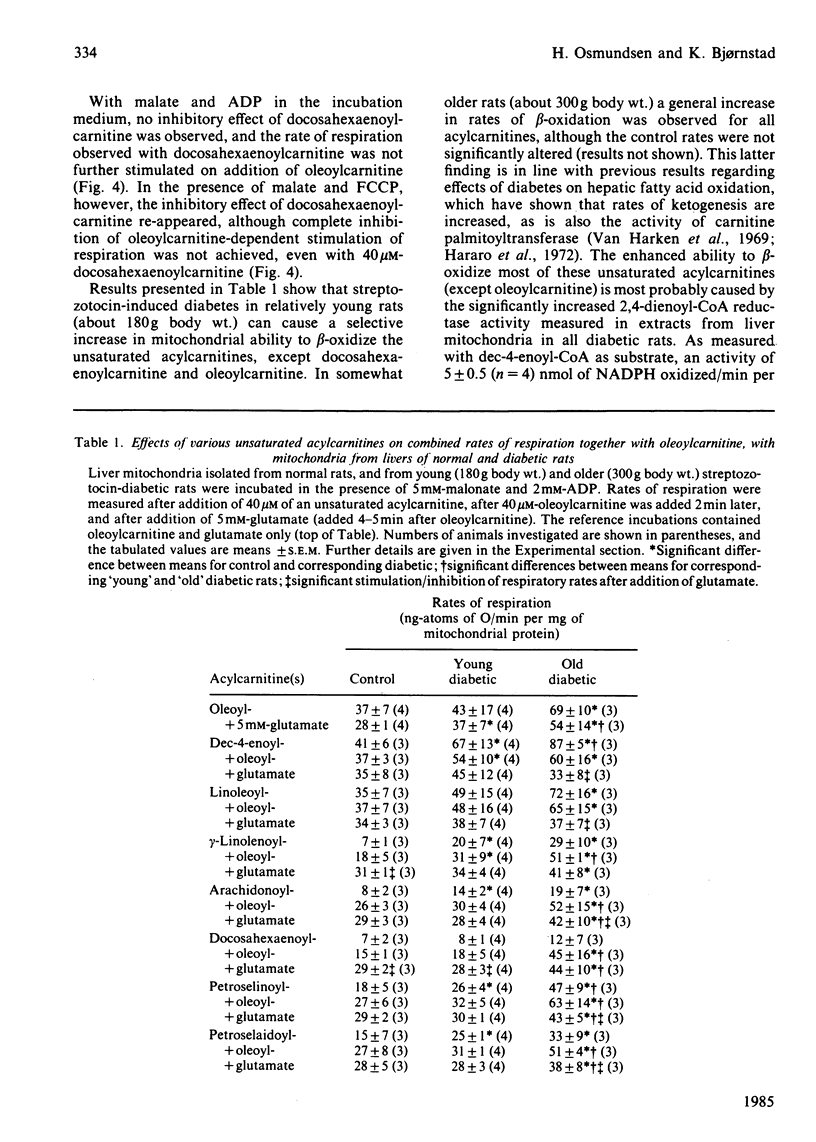
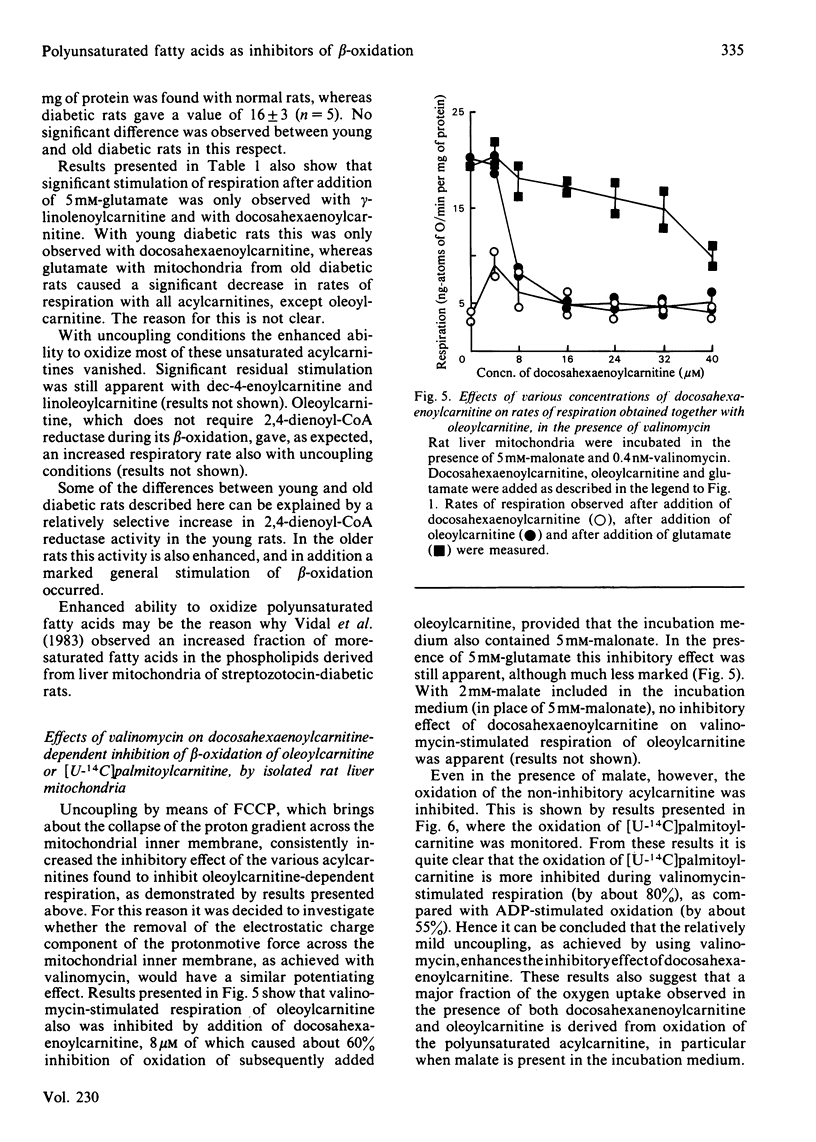
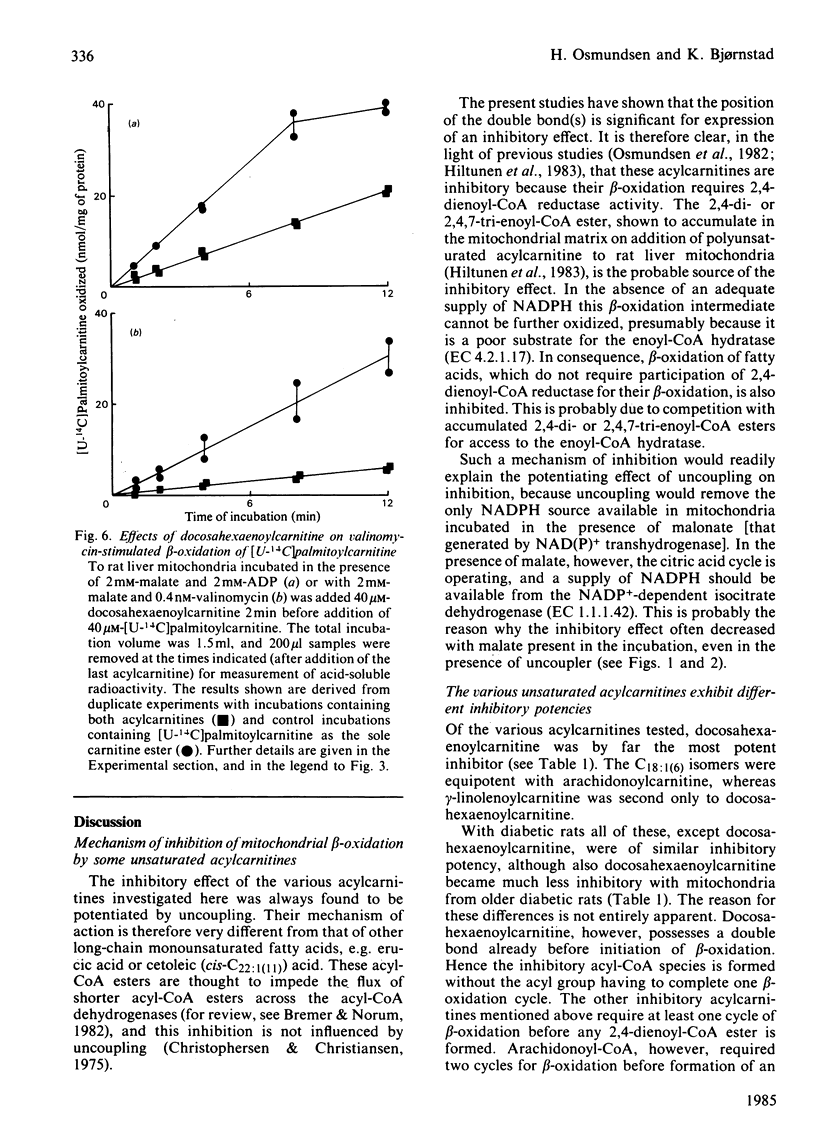
Evidence showing that some unsaturated fatty acids, and in particular docosahexaenoic acid, can be powerful inhibitors of mitochondrial beta-oxidation is presented. This inhibitory property is, however, also observed with the cis- and trans-isomers of the C18:1(16) acid. Hence it is probably the position of the double bond(s), and not the degree of unsaturation, which confers the inhibitory property. It is suggested that the inhibitory effect is caused by accumulation of 2,4-di- or 2,4,7-tri-enoyl-CoA esters in the mitochondrial matrix. This has previously been shown to occur with these fatty acids, in particular when the supply of NADPH was limiting 2,4-dienoyl-CoA reductase (EC 1.3.1.-) activity [Hiltunen, Osmundsen & Bremer (1983) Biochim. Biophys. Acta 752, 223-232]. Liver mitochondria from streptozotocin-diabetic rats showed an increased ability to beta-oxidize 2,4-dienoyl-CoA-requiring acylcarnitines. Docosahexaenoylcarnitine was also found to be less inhibitory at lower concentrations with incubation under coupled conditions. With uncoupling conditions there was little difference between mitochondria from normal and diabetic rats in these respects. This correlates with a 5-fold stimulation of 2,4-dienoyl-CoA reductase activity found in mitochondria from streptozotocin-diabetic rats.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borrebaek B., Osmundsen H., Bremer J. In vivo induction of 4-enoyl-CoA reductase by clofibrate in liver mitochondria and its effect on pent-4-enoate metabolism. Biochem Biophys Res Commun. 1980 Apr 29;93(4):1173–1180. doi: 10.1016/0006-291x(80)90613-0. [DOI] [PubMed] [Google Scholar]
- Bremer J., Norum K. R. Metabolism of very long-chain monounsaturated fatty acids (22:1) and the adaptation to their presence in the diet. J Lipid Res. 1982 Feb;23(2):243–256. [PubMed] [Google Scholar]
- Bremer J., Wojtczak A. B. Factors controlling the rate of fatty acid -oxidation in rat liver mitochondria. Biochim Biophys Acta. 1972 Dec 8;280(4):515–530. doi: 10.1016/0005-2760(72)90131-2. [DOI] [PubMed] [Google Scholar]
- Cervenka J., Osmundsen H. Synthesis of unsaturated carnitine esters with N-acyl imidazoles. J Lipid Res. 1982 Nov;23(8):1243–1246. [PubMed] [Google Scholar]
- Christophersen B. O., Christiansen R. Z. Studies on the mechanism of the inhibitory effects of erucylcarnitine in rat heart mitochondria. Biochim Biophys Acta. 1975 Jun 23;388(3):402–412. doi: 10.1016/0005-2760(75)90099-5. [DOI] [PubMed] [Google Scholar]
- Cuebas D., Schulz H. Evidence for a modified pathway of linoleate degradation. Metabolism of 2,4-decadienoyl coenzyme A. J Biol Chem. 1982 Dec 10;257(23):14140–14144. [PubMed] [Google Scholar]
- Dommes P., Dommes V., Kunau W. H. beta-Oxidation in Candida tropicalis. Partial purification and biological function of an inducible 2,4-dienoyl coenzyme A reductase. J Biol Chem. 1983 Sep 25;258(18):10846–10852. [PubMed] [Google Scholar]
- GLOCK G. E., MCLEAN P. The intracellular distribution of pyridine nucleotides in rat liver. Exp Cell Res. 1956 Aug;11(1):234–236. doi: 10.1016/0014-4827(56)90214-2. [DOI] [PubMed] [Google Scholar]
- Harano Y., Kowal J., Yamazaki R., Lavine L., Miller M. Carnitine palmitoyltransferase activities (1 and 2) and the rate of palmitate oxidation in liver mitochondria from diabetic rats. Arch Biochem Biophys. 1972 Dec;153(2):426–437. doi: 10.1016/0003-9861(72)90360-8. [DOI] [PubMed] [Google Scholar]
- Hiltunen J. K., Osmundsen H., Bremer J. Beta-oxidation of polyunsaturated fatty acids having double bonds at even-numbered positions in isolated rat liver mitochondria. Biochim Biophys Acta. 1983 Jul 12;752(2):223–232. doi: 10.1016/0005-2760(83)90116-9. [DOI] [PubMed] [Google Scholar]
- Holland P. C., Sherratt H. S. Biochemical effects of the hypoglycaemic compound pent-4-enoic acid and related non-hypoglycaemic fatty acids. Effects of the free acids and their carnitine esters on coenzyme A-dependent oxidations in rat liver mitochondria. Biochem J. 1973 Sep;136(1):157–171. doi: 10.1042/bj1360157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. S., Pearlstein R. D. Reversible uncoupling of oxidative phosphorylation at low oxygen tension. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5807–5811. doi: 10.1073/pnas.80.19.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunau W. H., Dommes P. Degradation of unsaturated fatty acids. Identification of intermediates in the degradation of cis-4-decenoly-CoA by extracts of beef-liver mitochondria. Eur J Biochem. 1978 Nov 15;91(2):533–544. doi: 10.1111/j.1432-1033.1978.tb12707.x. [DOI] [PubMed] [Google Scholar]
- Kunau W. H., Lauterbach F. Inhibition of linoleic acid degradation by hypoglycin A. FEBS Lett. 1978 Oct 1;94(1):120–124. doi: 10.1016/0014-5793(78)80920-x. [DOI] [PubMed] [Google Scholar]
- Osmundsen H., Bremer J. A spectrophotometric procedure for rapid and sensitive measurements of beta-oxidation. Demonstration of factors that can be rate-limiting for beta-oxidation. Biochem J. 1977 Jun 15;164(3):621–633. doi: 10.1042/bj1640621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmundsen H., Cervenka J., Bremer J. A role for 2,4-enoyl-CoA reductase in mitochondrial beta-oxidation of polyunsaturated fatty acids. Effects of treatment with clofibrate on oxidation of polyunsaturated acylcarnitines by isolated rat liver mitochondria. Biochem J. 1982 Dec 15;208(3):749–757. doi: 10.1042/bj2080749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmundsen H., Neat C. E., Norum K. R. Peroxisomal oxidation of long chain fatty acids. FEBS Lett. 1979 Mar 15;99(2):292–296. doi: 10.1016/0014-5793(79)80975-8. [DOI] [PubMed] [Google Scholar]
- Osmundsen H., Sherratt H. S. A novel mechanism for inhibition of beta-oxidation by methylenecyclopropylacetyl-CoA, a metabolite of hypoglycin. FEBS Lett. 1975 Jul 15;55(1):38–41. doi: 10.1016/0014-5793(75)80951-3. [DOI] [PubMed] [Google Scholar]
- REYE R. D., MORGAN G., BARAL J. ENCEPHALOPATHY AND FATTY DEGENERATION OF THE VISCERA. A DISEASE ENTITY IN CHILDHOOD. Lancet. 1963 Oct 12;2(7311):749–752. doi: 10.1016/s0140-6736(63)90554-3. [DOI] [PubMed] [Google Scholar]
- Thayer W. S. Inhibition of mitochondrial fatty acid oxidation in pentenoic acid-induced fatty liver. A possible model for Reye's syndrome. Biochem Pharmacol. 1984 Apr 15;33(8):1187–1194. doi: 10.1016/0006-2952(84)90169-2. [DOI] [PubMed] [Google Scholar]
- Tischler M. E., Friedrichs D., Coll K., Williamson J. R. Pyridine nucleotide distributions and enzyme mass action ratios in hepatocytes from fed and starved rats. Arch Biochem Biophys. 1977 Nov;184(1):222–236. doi: 10.1016/0003-9861(77)90346-0. [DOI] [PubMed] [Google Scholar]
- Van Harken D. R., Dixon C. W., Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J Biol Chem. 1969 May 10;244(9):2278–2285. [PubMed] [Google Scholar]
- Vidal J. C., McIntyre J. O., Churchill P., Andrew J. A., Péhuet M., Fleischer S. Influence of diabetes on rat liver mitochondria: decreased unsaturation of phospholipid and D-beta-hydroxybutyrate dehydrogenase activity. Arch Biochem Biophys. 1983 Jul 15;224(2):643–658. doi: 10.1016/0003-9861(83)90252-7. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]


