Abstract
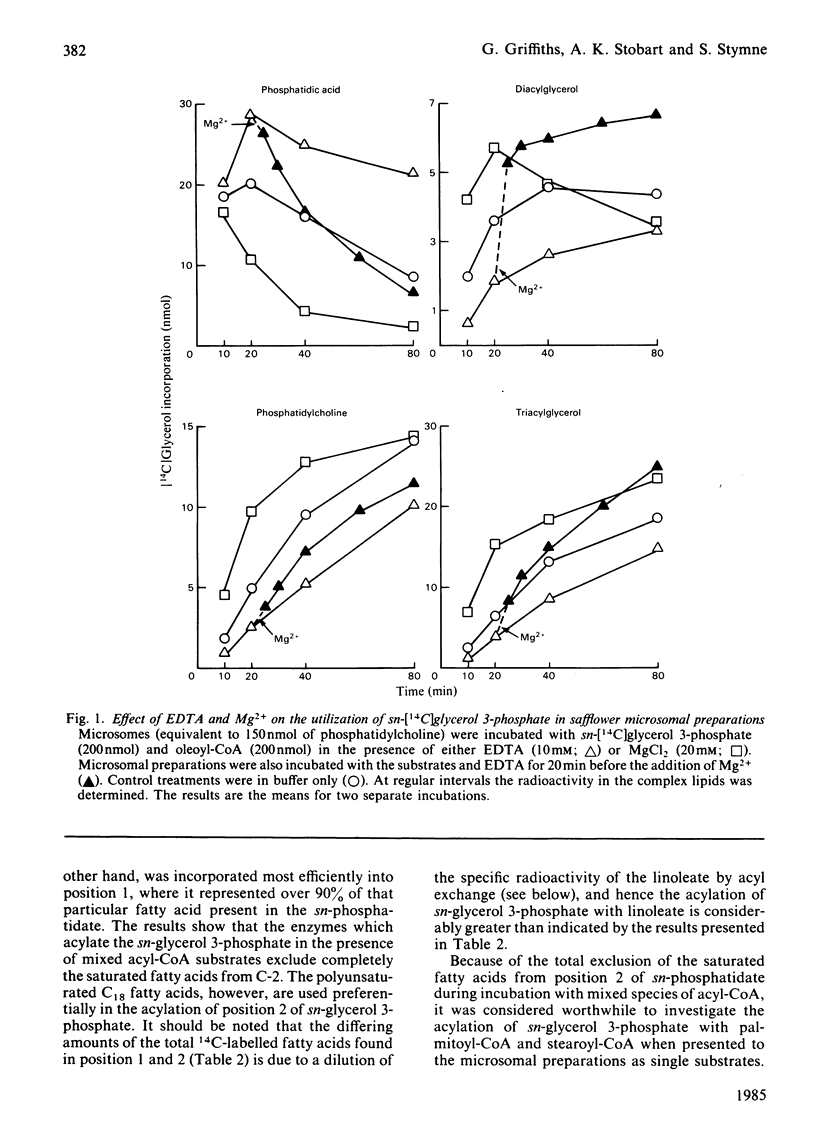
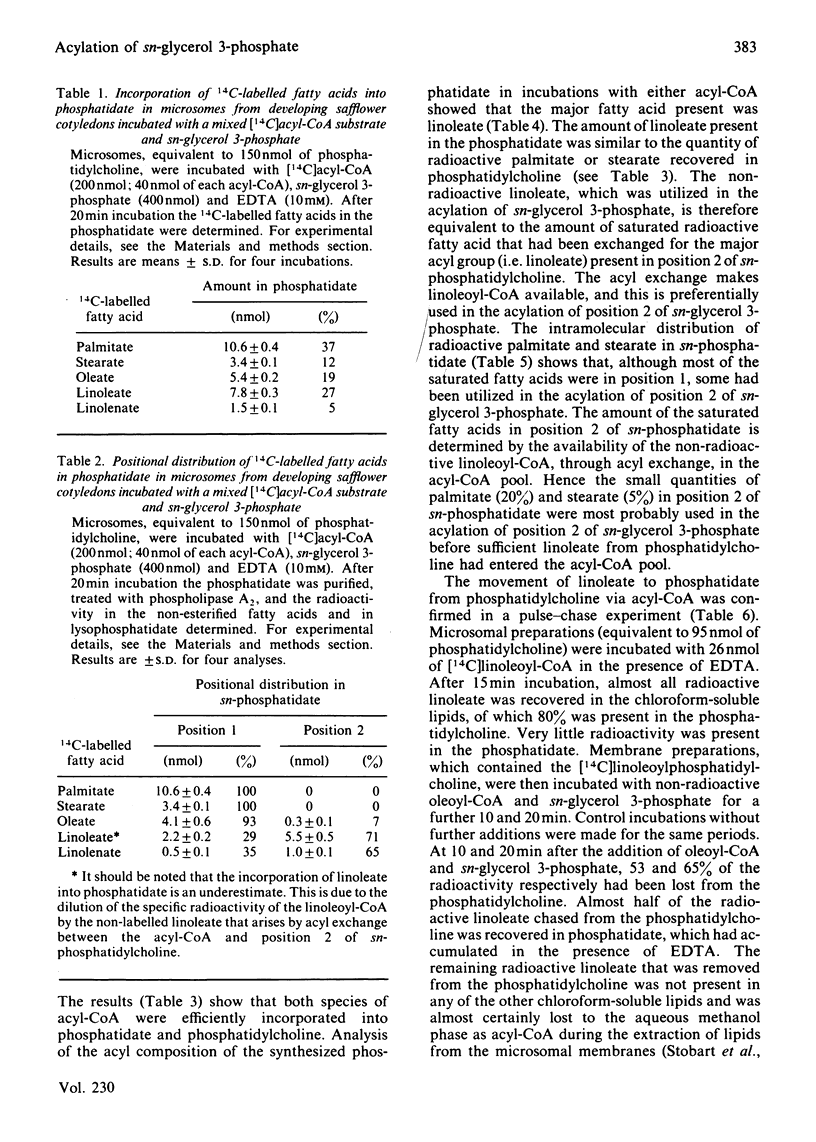
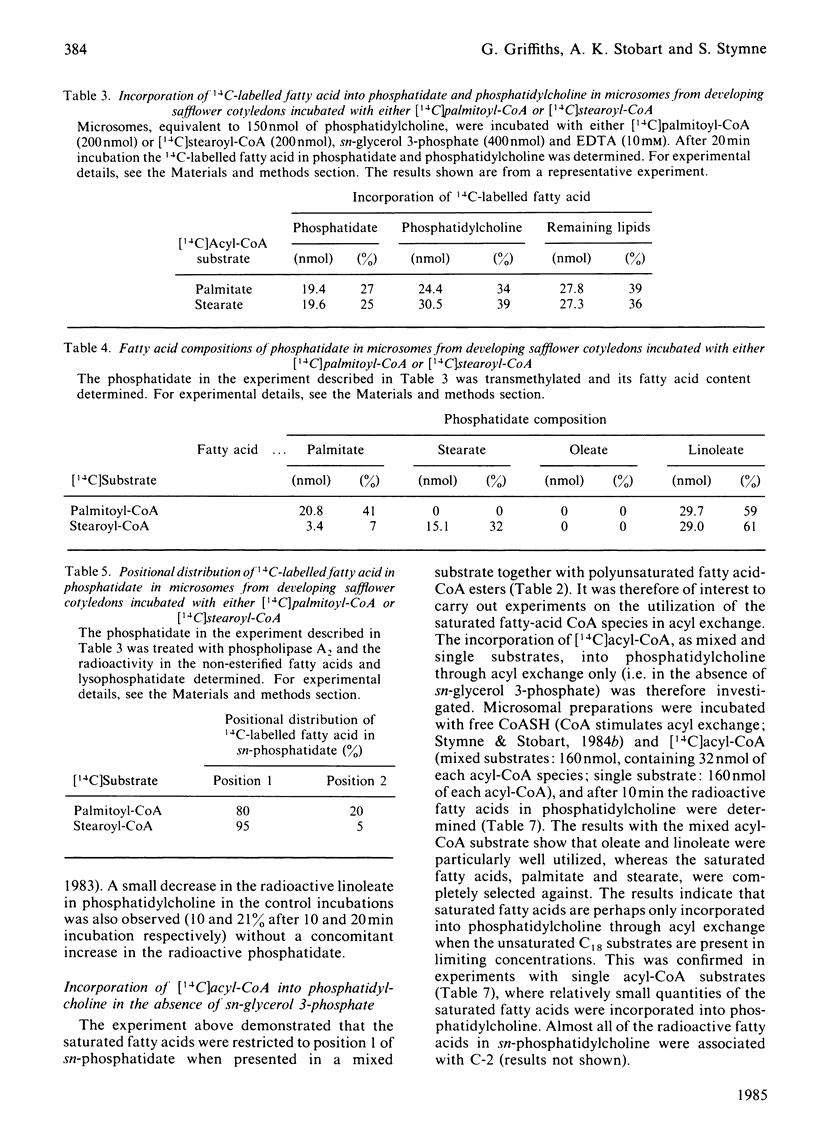
Microsomal preparations from the developing cotyledons of safflower (Carthamus tinctorius) catalysed the acylation of sn-glycerol 3-phosphate in the presence of acyl-CoA. The resulting phosphatidate was further utilized in the synthesis of diacyl- and tri-acylglycerol by the reactions of the so-called 'Kennedy pathway' [Kennedy (1961) Fed. Proc. Fed. Am. Soc. Exp. Biol. 20, 934-940]. Diacylglycerol equilibrated with the phosphatidylcholine pool when glycerol backbone, with the associated acyl groups, flowed from phosphatidate to triacylglycerol. The formation of diacylglycerol from phosphatidate through the action of a phosphatidate phosphohydrolase (phosphatidase) was substantially inhibited by EDTA and, under these conditions, phosphatidate accumulated in the microsomal membranes. The inhibition of the phosphatidase by EDTA was alleviated by Mg2+. The presence of Mg2+ in all incubation mixtures stimulated quite considerably the synthesis of triacylglycerol in vitro. Microsomal preparations incubated with acyl-CoA, sn-glycerol 3-phosphate and EDTA synthesized sufficient phosphatidate for the reliable analysis of its intramolecular fatty acid distribution. In the presence of mixed acyl-CoA substrates the sn-glycerol 3-phosphate was acylated exclusively in position 1 with the saturated fatty acids, palmitate and stearate. The polyunsaturated fatty acid linoleate was, however, utilized largely in the acylation of position 2 of sn-glycerol 3-phosphate. The affinity of the enzymes involved in the acylation of positions 1 and 2 of sn-glycerol 3-phosphate for specific species of acyl-CoA therefore governs the non-random distribution of the different acyl groups in the seed triacylglycerols. The acylation of sn-glycerol 3-phosphate in position 1 with saturated acyl components also accounts for the presence of these groups in position 1 of sn-phosphatidylcholine through the equilibration of diacylglycerol with the phosphatidylcholine pool, which occurs when phosphatidate is utilized in the synthesis of triacylglycerol. These results add further credence to our previous proposals for the regulation of the acyl quality of the triacylglycerols that accumulate in developing oil seeds [Stymne & Stobart (1984) Biochem. J. 220, 481-488; Stobart & Stymne (1985) Planta 163, 119-125].
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bjerve K. S., Daae L. N., Bremer J. The selective loss of lysophospholipids in some commonly used lipid-extraction procedures. Anal Biochem. 1974 Mar;58(1):238–245. doi: 10.1016/0003-2697(74)90463-1. [DOI] [PubMed] [Google Scholar]
- Call F. L., 2nd, Williams W. J. Phosphatidate phosphatase in human platelets. J Lab Clin Med. 1973 Oct;82(4):663–673. [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Gunstone F. D., Hamilton R. J., Padley F. B., Qureshi M. I. Glyceride studies. V. The distribution of unsaturated acyl groups in vegetable triglycerides. J Am Oil Chem Soc. 1965 Nov;42(11):965–970. doi: 10.1007/BF02632456. [DOI] [PubMed] [Google Scholar]
- Gunstone F. D., Qureshi M. I. Glyceride studies. IV. The component glycerides of ten seed oils containing linoleic acid. J Am Oil Chem Soc. 1965 Nov;42(11):961–965. doi: 10.1007/BF02632455. [DOI] [PubMed] [Google Scholar]
- Heinz E., Roughan P. G. Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 1983 Jun;72(2):273–279. doi: 10.1104/pp.72.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S., Numa S. Partial purification, properties, and subcellulsr distribution of rat liver phosphatidate phosphatase. J Biochem. 1975 Mar;77(3):501–509. doi: 10.1093/oxfordjournals.jbchem.a130751. [DOI] [PubMed] [Google Scholar]
- Hosaka K., Yamashita S. Partial purification and properties of phosphatidate phosphatase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Oct 24;796(1):102–109. [PubMed] [Google Scholar]
- Ichihara K. sn-Glycerol-3-phosphate acyltransferase in a particulate fraction from maturing safflower seeds. Arch Biochem Biophys. 1984 Aug 1;232(2):685–698. doi: 10.1016/0003-9861(84)90589-7. [DOI] [PubMed] [Google Scholar]
- KATES M. SIMPLIFIED PROCEDURES FOR HYDROLYSIS OR METHANOLYSIS OF LIPIDS. J Lipid Res. 1964 Jan;5:132–135. [PubMed] [Google Scholar]
- KENNEDY E. P. Biosynthesis of complex lipids. Fed Proc. 1961 Dec;20:934–940. [PubMed] [Google Scholar]
- Lamb R. G., Fallon H. J. Glycerolipid formation from sn-glycerol-3-phosphate by rat liver cell fractions. The role of phosphatidate phosphohydrolase. Biochim Biophys Acta. 1974 Apr 26;348(1):166–178. doi: 10.1016/0005-2760(74)90103-9. [DOI] [PubMed] [Google Scholar]
- Monroy G., Kelker H. C., Pullman M. E. Partial purification and properties of an acyl coenzyme A:sn-glycerol 3-phosphate acyltransferase from rat liver mitochondria. J Biol Chem. 1973 Apr 25;248(8):2845–2852. [PubMed] [Google Scholar]
- Ray T. K., Cronan J. E., Jr, Mavis R. D., Vagelos P. R. The specific acylation of glycerol 3-phosphate to monoacylglycerol 3-phosphate in Escherichia coli. Evidence for a single enzyme conferring this specificity. J Biol Chem. 1970 Dec 10;245(23):6442–6448. [PubMed] [Google Scholar]
- Siebertz H. P., Heinz E., Joyard J., Douce R. Labelling in vivo and in vitro of molecular species of lipids from chloroplast envelopes and thylakoids. Eur J Biochem. 1980;108(1):177–185. doi: 10.1111/j.1432-1033.1980.tb04710.x. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Browse J. Evidence for an oleoyl phosphatidylcholine desaturase in microsomal preparations from cotyledons of safflower (Carthamus tinctorius) seed. Biochem J. 1979 Jun 1;179(3):649–656. doi: 10.1042/bj1790649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparace S. A., Mudd J. B. Phosphatidylglycerol synthesis in spinach chloroplasts: characterization of the newly synthesized molecule. Plant Physiol. 1982 Nov;70(5):1260–1264. doi: 10.1104/pp.70.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturton R. G., Brindley D. N. Factors controlling the metabolism of phosphatidate by phosphohydrolase and phospholipase A-type activities. Effects of magnesium, calcium and amphiphilic cationic drugs. Biochim Biophys Acta. 1980 Sep 8;619(3):494–505. doi: 10.1016/0005-2760(80)90101-0. [DOI] [PubMed] [Google Scholar]
- Stymne S., Appelqvist L. A. The biosynthesis of linoleate from oleoyl-CoA via oleoyl-phosphatidylcholine in microsomes of developing safflower seeds. Eur J Biochem. 1978 Oct;90(2):223–229. doi: 10.1111/j.1432-1033.1978.tb12594.x. [DOI] [PubMed] [Google Scholar]
- Stymne S., Stobart A. K. Evidence for the reversibility of the acyl-CoA:lysophosphatidylcholine acyltransferase in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons and rat liver. Biochem J. 1984 Oct 15;223(2):305–314. doi: 10.1042/bj2230305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne S., Stobart A. K., Glad G. The role of the acyl-CoA pool in the synthesis of polyunsaturated 18-carbon fatty acids and triacylglycerol production in the microsomes of developing safflower seeds. Biochim Biophys Acta. 1983 Jul 12;752(2):198–208. doi: 10.1016/0005-2760(83)90113-3. [DOI] [PubMed] [Google Scholar]
- Stymne S., Stobart A. K. The biosynthesis of triacylglycerols in microsomal preparations of developing cotyledons of sunflower (Helianthus annuus L.). Biochem J. 1984 Jun 1;220(2):481–488. doi: 10.1042/bj2200481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez M., Nicholls D. G., Brindley D. N. [The relationship between palmitoyl-coenzyme A synthetase activity and esterification of sn-glycerol 3-phosphate in rat liver mitochondria]. Biochem J. 1973 Apr;132(4):697–706. doi: 10.1042/bj1320697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai Y., Lands W. E. Positional specificity of sn-glycerol 3-phosphate acylation during phosphatidate formation by rat liver microsomes. J Biochem. 1974 Oct;76(4):847–860. [PubMed] [Google Scholar]
- Yamashita S., Hosaka K., Numa S. Resolution and reconstitution of the phosphatidate-synthesizing system of rat-liver microsomes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3490–3492. doi: 10.1073/pnas.69.11.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S., Numa S. Partial purification and properties of glycerophosphate acyltransferase from rat liver. Formation of 1-acylglycerol 3-phosphate from sn-glycerol 3-phosphate and palmityl coenzyme A. Eur J Biochem. 1972 Dec 18;31(3):565–573. doi: 10.1111/j.1432-1033.1972.tb02566.x. [DOI] [PubMed] [Google Scholar]


