Abstract
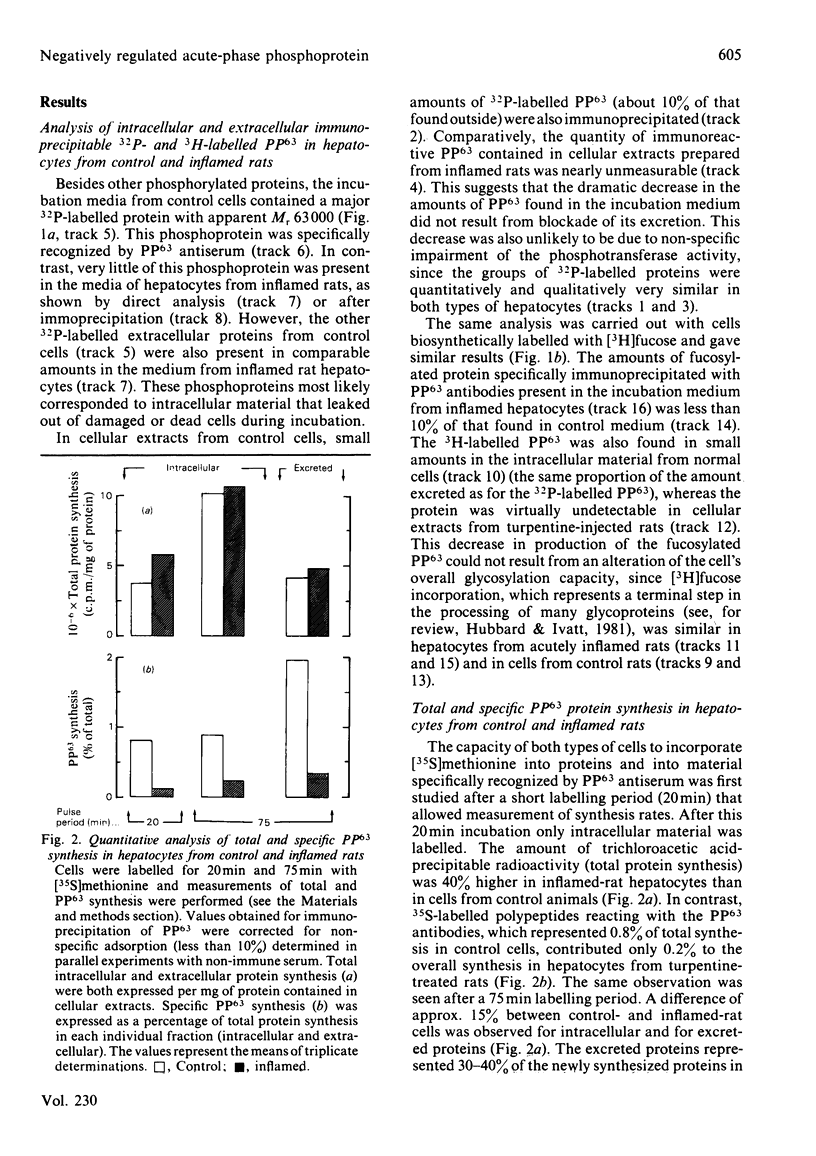
The effect of acute inflammation on the production of the major phosphorylated protein (PP63) excreted by rat hepatocytes was investigated. Both intra- and extracellular forms of the protein labelled with [32P]Pi, [3H]fucose and [35S]methionine were immunoprecipitated with monospecific polyclonal antibodies, and relative rates of PP63 synthesis were measured. The hepatocytes of acutely inflamed rats produced and excreted 85% less 32P- and 3H-labelled PP63 than did control cells. This decreased amount of PP63 did not result from an impairment in the phosphorylation or glycosylation processes or from a blockade in excretion, but rather was found to be due to extensive shut-off in biosynthesis of the protein as measured by [35S]methionine incorporation. Thus PP63 would appear to represent a new negatively regulated acute-phase protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andus T., Gross V., Tran-Thi T. A., Schreiber G., Nagashima M., Heinrich P. C. The biosynthesis of acute-phase proteins in primary cultures of rat hepatocytes. Eur J Biochem. 1983 Jul 1;133(3):561–571. doi: 10.1111/j.1432-1033.1983.tb07500.x. [DOI] [PubMed] [Google Scholar]
- Baumann H., Firestone G. L., Burgess T. L., Gross K. W., Yamamoto K. R., Held W. A. Dexamethasone regulation of alpha 1-acid glycoprotein and other acute phase reactants in rat liver and hepatoma cells. J Biol Chem. 1983 Jan 10;258(1):563–570. [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Sauder D. N., Koj A. Human keratinocytes and monocytes release factors which regulate the synthesis of major acute phase plasma proteins in hepatic cells from man, rat, and mouse. J Biol Chem. 1984 Jun 10;259(11):7331–7342. [PubMed] [Google Scholar]
- Chandra T., Kurachi K., Davie E. W., Woo S. L. Induction of alpha 1-antitrypsin mRNA and cloning of its cDNA. Biochem Biophys Res Commun. 1981 Nov 30;103(2):751–758. doi: 10.1016/0006-291x(81)90513-1. [DOI] [PubMed] [Google Scholar]
- Crane L. J., Miller D. L. Plasma protein induction by isolated hepatocytes. Mol Cell Biochem. 1983;53-54(1-2):89–109. doi: 10.1007/BF00225248. [DOI] [PubMed] [Google Scholar]
- Gross V., Andus T., Tran-Thi T. A., Bauer J., Decker K., Heinrich P. C. Induction of acute phase proteins by dexamethasone in rat hepatocyte primary cultures. Exp Cell Res. 1984 Mar;151(1):46–54. doi: 10.1016/0014-4827(84)90354-9. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Iynedjian P. B., Auberger P., Guigoz Y., Le Cam A. Pretranslational regulation of tyrosine aminotransferase and phosphoenolpyruvate carboxykinase (GTP) synthesis by glucagon and dexamethasone in adult rat hepatocytes. Biochem J. 1985 Jan 1;225(1):77–84. doi: 10.1042/bj2250077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. C., Ashton F. E., Friesen A. D., Chou B. Studies on acute phase proteins of rat serum. II. Determination of the contents of 1 -acid glycoprotein, 2-macroglobulin, and albumin in serum from rats suffering from induced inflammation. Can J Biochem. 1972 Aug;50(8):871–880. doi: 10.1139/o72-122. [DOI] [PubMed] [Google Scholar]
- Koj A. Comparison of synthesis and secretion of plasma albumin, fibrinogen and alpha 2-macroglobulin by slices of Morris hepatomas and rat liver. Br J Exp Pathol. 1980 Jun;61(3):332–338. [PMC free article] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Cam A., Guillouzo A., Freychet P. Ultrastructual and biochemical studies of isolated adult rat hepatocytes prepared under hypoxic conditions. Cryopreservation of hepatocytes. Exp Cell Res. 1976 Mar 15;98(2):382–395. doi: 10.1016/0014-4827(76)90448-1. [DOI] [PubMed] [Google Scholar]
- Northemann W., Andus T., Gross V., Nagashima M., Schreiber G., Heinrich P. C. Messenger RNA activities of four acute phase proteins during inflammation. FEBS Lett. 1983 Sep 19;161(2):319–322. doi: 10.1016/0014-5793(83)81033-3. [DOI] [PubMed] [Google Scholar]
- Salavert A., Iynedjian P. B. Regulation of phosphoenolpyruvate carboxykinase (GTP) synthesis in rat liver cells. Rapid induction of specific mRNA by glucagon or cyclic AMP and permissive effect of dexamethasone. J Biol Chem. 1982 Nov 25;257(22):13404–13412. [PubMed] [Google Scholar]
- Schreiber G., Howlett G., Nagashima M., Millership A., Martin H., Urban J., Kotler L. The acute phase response of plasma protein synthesis during experimental inflammation. J Biol Chem. 1982 Sep 10;257(17):10271–10277. [PubMed] [Google Scholar]




