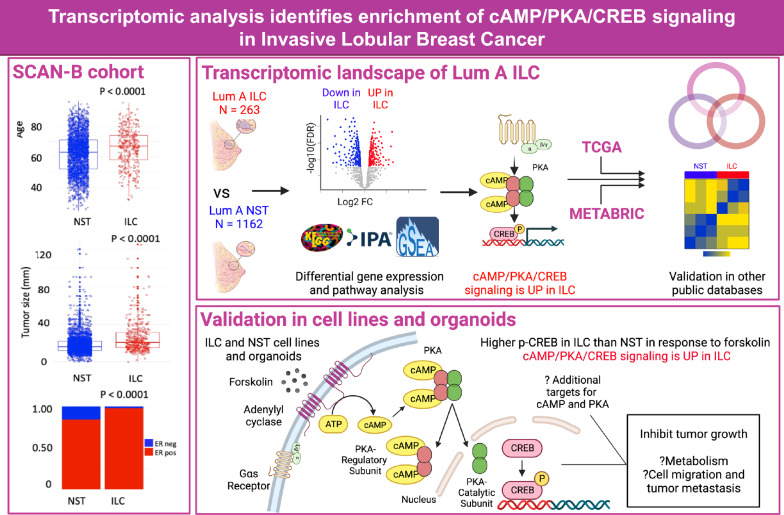
Abstract
Objective
Invasive lobular breast cancer (ILC) is the most common special type of breast cancer and has unique clinicopathological and molecular hallmarks that differentiate it from the more common invasive carcinoma—no special type (NST). Despite these differences, ILC and NST are treated as a single entity and there is a lack of ILC-targeted therapies. To fill this gap, we sought to identify novel molecular alterations in ILC that could be exploited for targeted therapies.
Methods
Differential gene expression and Geneset Enrichment and Variation analyses were performed on RNA-seq data from three large public breast cancer databases—the Sweden Cancerome Analysis Network-Breast (SCAN-B; luminal A ILC N = 263, luminal A NST N = 1162), The Cancer Genome Atlas (TCGA; luminal A ILC N = 157, luminal A NST N = 307) and Molecular Taxonomy of Breast Cancer International Consortium (METABRIC; luminal A ILC N = 65, luminal A NST N = 533). Pathways enriched in overlapping differentially expressed genes from these datasets were clustered using Jaccard similarity to identify pathways enriched in ILC. The cAMP/PKA/CREB signaling was studied in ILC, ILC-like and NST cell lines and patient-derived organoids (PDOs) using forskolin, an activator of the pathway.
Results
Clinicopathological features of patients with ILC and NST in SCAN-B were similar to prior population-based studies. There was a consistent pattern of up-regulation of cAMP/PKA/CREB related signaling in ILC compared to NST in SCAN-B, TCGA and METABRIC. Treatment with forskolin resulted in a greater increase in phospho-CREB in ILC cell lines and organoids than NST. CRISPR deletion of CDH1 in NST cell lines did not alter response of cells to forskolin as measured by phospho-CREB. Forskolin treatment caused growth inhibition in ILC and NST, with ILC cell lines being more sensitive to forskolin-mediated growth inhibition.
Conclusion
In three separate datasets, cAMP/PKA/CREB signaling was identified to be higher in ILC than NST. This in silico finding was validated in cell line and organoid models. Loss of CDH1 was not sufficient to mediate this phenotype. Future studies should investigate the mechanisms for differential cAMP/PKA/CREB signaling and the potential for therapeutic targeting in patients with ILC.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-024-01900-y.
Keywords: Invasive lobular cancer (ILC), Invasive carcinoma—No Special Type (NST), Protein Kinase A (PKA), Cyclic AMP (cAMP), CAMP Response Element Binding Protein (CREB), Forskolin
Background
Invasive lobular breast cancer (ILC) is the most common special subtype of breast cancer and comprises 10–15% of all invasive breast cancer. Compared to the no special type (NST), ILC tumors have several unique clinical and molecular features. ILC tend to present at later stages, with more lymph node involvement and more frequent metastases to the peritoneum, ovary, and gastrointestinal tract than NST [1–3]. Patients with estrogen-receptor positive (ER +) ILC have significantly worse long-term disease-specific and overall survival than ER + NST [1], attributable to late recurrences. E-Cadherin (CDH1) loss is a pathognomonic feature of ILC and results in discohesive growth of lobular cells. ILC tumors also demonstrate enrichment of PTEN loss, Akt activation, and mutations in TBX3 and FOXA1 [4]. IGF-1 (Insulin-like Growth Factor–I) and other growth factor signaling pathways are up-regulated in ILC as compared to NST and these are mediated by loss of CDH1 [4, 5]. Despite these differences, ILC is managed similar to NST based upon its intrinsic molecular subtype [6]. Treatment response to neoadjuvant chemotherapy is often poor in ILC, resulting in lower rates of complete pathological response and consequently higher rates of mastectomy [7], which is also driven by larger and often multi-focal tumors. Majority of ILCs are ER + and hence endocrine therapy is the primary adjuvant therapy in these patients [1]. Given the unique molecular alterations in ILC and differences in prognosis and treatment response between the two histologic types, we sought to investigate the molecular landscape of ILC and identify novel druggable targets.
The Sweden Cancerome Analysis Network-Breast (SCAN-B) is a large population-based database of early-stage primary breast cancer patients with RNA-sequencing and clinicopathological data [8]. SCAN-B has a large cohort of 386 ILC cases and 2602 NST cases and is ideal for hypothesis-generating bioinformatics analysis. The Cancer Genome Atlas (TCGA) Study and Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) contain gene expression data from additional ILC and NST tumor samples and these databases have previously been used for comparative studies of NST and ILC [4, 9]. In the current study, we aimed to identify molecular pathways that are consistently enriched in ILC in these three databases. As the majority of ILC are ER + luminal A [9], this analysis was limited to ER + luminal A ILC and NST samples to ensure homogeneity within the groups.
In our study, we report significant up-regulation of c(cyclic)AMP/PKA/CREB signaling in ILC compared to NST tumors. cAMP is an important second messenger that regulates several physiological and pathological processes. cAMP activates Protein Kinase A (PKA) by releasing its catalytic subunit (PKA-Cα). Activated PKA phosphorylates the transcription factor cAMP Response Element Binding protein (CREB) at Ser133 among other targets [10]. CREB is a key transcription factor that regulates the expression of several genes involved in tumorigenesis and metastasis [11]. Depending on the tumor type and context, the cAMP/PKA/CREB signaling may have a tumor suppressive or tumor promoting effect [10].
This is the first study to systematically investigate enrichment of molecular pathways in ILC using three large datasets; SCAN-B, TCGA and METABRIC. Our study identified consistent activation of the cAMP/PKA/CREB pathway across three datasets, which should be studied further with the goal of implementing precision medicine for patients with ILC.
Materials and methods
Datasets
Gene expression and clinical features for SCAN-B (N = 263 patients with luminal A ILC, N = 1162 with luminal A NST had gene expression and clinical data) were obtained from Gene expression Omnibus GEO:GSE202203 [12] and SCAN‐B MutationExplorer (http://oncogenomics.bmc.lu.se/MutationExplorer) [13] respectively. Data for TCGA (luminal A ILC N = 157, luminal A NST N = 307) and METABRIC (luminal A ILC N = 65, luminal A NST N = 533) were obtained from GEO:GSE62944 [14] and Synapse software platform (syn1688369; Sage Bionetworks, Seattle, WA, USA) respectively. PAM50 classification was provided by the authors in the SCAN-B dataset [13]. For TCGA and METABRIC, we used PAM50 assignment from our previous publication [9]. The genefu R package was used for PAM50 assignment in all three datasets. Patient and tumor characteristics were compared between ILC and NST in SCAN-B. Wilcoxon and chi-squared tests were used to compare continuous and categorical variables respectively.
Differential gene expression analysis
Differential expression analysis was performed between luminal A ILC and luminal A NST samples in SCAN-B and TCGA using the R package, DESeq2 after correcting for tumor purity estimated by the package “ESTIMATE” [15]. Genes with a false discovery rate (FDR) < 0.05 were considered significantly differentially expressed. For microarray data from METABRIC, probes with the highest interquartile range were selected for genes that matched to multiple probes. Significance Analysis of Microarrays (SAM) was used to detect the differentially expressed genes (DEGs). FDR < 0.05 was used to call DEGs. Downstream pathway analysis was performed using EnrichR (https://maayanlab.cloud/Enrichr/) and Ingenuity Pathway Analysis (IPA, QIAGEN Inc., https://digitalinsights.qiagen.com/IPA).
Overlapping DEGs in SCAN-B, TCGA and METABRIC
DEGs (FDR < 0.05) between luminal A ILC and luminal A NST from SCAN-B, TCGA and METABRIC were overlapped to identify genes that were consistently altered in luminal A ILC in all three datasets. Pathway analysis was performed on these genes using IPA. To identify related pathways, Jaccard similarity (Jaccard similarity for two pathways i and j is defined as the intersection of genes in i and j divided by union of genes in i and j) was calculated for the significantly enriched pathways. Pathways with a Jaccard similarity > 0.3, and at least 1 membership were clustered using hierarchical clustering and plotted in a heatmap. Pathways with a positive IPA z-score (i.e., up-regulated in ILC) in the cluster of interest were used for further analysis.
Gene set variation analysis (GSVA) and gene set enrichment analysis (GSEA) in clinical datasets and cell lines
Gene sets relevant to cAMP/PKA/CREB signaling were downloaded from the Molecular Signature Database (MSigDB) and Kyoto Encyclopedia of Genes and Genomes (KEGG): KEGG cAMP mediated signaling [16], REACTOME PKA mediated phosphorylation of CREB [17], GOBP Cellular response to cAMP [18] and GOBP regulation of cAMP mediated signaling [19]. These gene sets along with pathways of interest from Jaccard-based clustering above were used for GSVA. GSVA was performed on variance stabilized normalized expression data generated by DESeq2 for SCAN-B and TCGA, and on normalized microarray data for METABRIC using the R package GSVA (kcdf = “Gaussian”, method = “ssgsea”). In addition, GSVA was also performed for the aforementioned gene sets in normalized gene expression data from 47 paired primary and metastatic ILC and NST (N = 36 NST, N = 11 ILC; N = 11 ovarian, N = 22 brain, N = 5 gastrointestinal and N = 9 bone) breast cancer tissues previously published by our group [20] and breast cancer cell lines obtained from Marcotte et al. (GSE73526) [21]. Cell lines from GSE73526 include ILC (HCC2185, MDAMB134, MDAMB330, SUM44PE) ILC-like (MPE600, CAMA1, HCC2218, MDAMB453, OCUBM, SKBR5, ZR7530) and NST lines (BT474, BT483, EFM19, HCC1008, HCC1419, HCC1500, LY2, MCF7, MDAMB157, MDAMB175, T47D, UACC812, ZR75-1, ZR75B). Of note, ILC-like cell lines are derived from clinical NSTs but demonstrate key ILC genetic features such as CDH1 deletion [22]. The enrichment scores were compared between luminal A ILC and luminal A NST using Wilcoxon test. GSEA (GSEA Version 2.2.2. Broad Institute) was performed on normalized counts for SCAN-B (Phenotype 1 = luminal A ILC, 0 = luminal A NST; Permutation type = Phenotype; Number of permutations = 1000; no collapse).
Survival analysis in METABRIC
The effect of the expression of cAMP/PKA/CREB related genes on survival in patients with luminal A ILC was assessed in METABRIC using the R package “survival”. Luminal A ILC tumors were divided into high and low expression groups for pathways of interest based on their GSVA scores, using the 75th or 50th percentile as cut off. Disease-specific survival (DSS) was compared between patients with tumors characterized by high and low expression using Kaplan–Meier plot and log rank test.
Cell culture
Cell lines utilized in this study were obtained from ATCC (MCF7, T47D, ZR75.1, BT474, MDAMB134-VI, CAMA1, ZR7530), DSMZ (EFM19), Asterand (SUM44PE) and our recently generated cell line WCRC25 [23] as well as CDH1 knock-out (KO) cells of MCF7 and T47D [5]. The generation of BCK4 and MPE600 has previously been described [24, 25]. The BCK4 cell line was obtained through an MTA from the University of Colorado. Cell lines were maintained in 10% fetal bovine serum (FBS; Life Technologies) supplemented media (Thermo Fisher Scientific): MDAMB134 in 1:1 DMEM: L-15; MCF7, MCF7 E-cad KO, MPE600, and CAMA1 in DMEM; and T47D, T47D E-cad KO, EFM19, BT474, ZR7530 and ZR75.1 in RPMI. SUM44PE was maintained in DMEM-F12 with 2% charcoal stripped serum (CSS; Thermo Fisher Scientific) with additional supplements as previously reported [26]. Cell lines were routinely tested for Mycoplasma and authenticated by the Genetics Core of University of Arizona (Tucson, Arizona) via short tandem repeat DNA profiling. Since different cell lines are grown in different media which could impact cell signaling, we also cultured the above cell lines with overnight incubation in DMEM + 1% FBS (referred to as low serum media) prior to treatment and harvesting.
Organoid culture
Patient derived organoids (PDOs) representing both ILC and NST were generated following the Sachs methodology [27] and then kept with 1 nM β-estradiol supplementation (Sigma-Aldrich #E8875) as previously described by us [5, 28]. PDOs were monitored to achieve appropriate confluency prior to treatment with forskolin.
Immunoblotting
Cell lines or PDOs were treated with either 25 µM Forskolin (Cell Signaling technology #3828) or vehicle (DMSO) for 20 min then immunoblotting experiments were conducted using proteins extracts as reported previously [29]. Intercept PBS blocking buffer (LiCOr #927–40000) was used for membrane blocking for one hour at room temperature. Afterwards, membranes were probed with primary antibodies (Cell Signaling Technology: Phospho-CREB (p-CREB, Ser133) (87G3) Rabbit mAb #9198, CREB (48H2) Rabbit mAb #9197 and PKA-Cα Antibody #4782, Millipore Sigma: Monoclonal Anti-β-Actin #A5441 as loading control overnight at 4 °C. Membranes were then incubated for 1 h at room temperature with secondary antibodies (anti-rabbit 800CW: LiCor #925–32211; 1:10000; anti-mouse 680LT: LiCor #925–68020). This was followed by imaging on the LiCor Odyssey CLx Imaging system, with quantifications of protein bands carried out with built in software. Protein levels of CREB, p-CREB, and PKA-CA were normalized for β-actin and the ratio of their levels after and before forskolin treatment were compared between ILC and NST cell lines. In some cases, p-CREB or CREB were blotted, membranes were stripped using NewBlot Nitro Stripping Buffer (Licor, 928–40030) for 5 min at room temperature then re-probed by adding the primary antibody for the other protein. Following the addition of the appropriate secondary antibody, the membranes were then scanned with an Odyssey CLx Imaging System.
Immunofluorescence
Cells were plated at a density of 1–2 × 105 cells per well on glass coverslips (Fisher #12-545-80P) in 24 well plates. Cells were treated with either 25 µM Forskolin (Cell Signaling technology #3828) or vehicle (DMSO) for 20 min then fixed on ice in ice cold methanol for 30 min and blocked in 0.3% Triton X-100, 5% BSA, 1X DPBS for 1 h at room temperature. Primary antibody incubation was performed overnight at 4 °C: P-CREB (Ser133) (87G3) (Cell Signaling Technology #9198; 10 ug/ml), PKA-Cα (Cell Signaling Technology #4782; 1:100). Secondary antibody incubation was performed at room temperature for 1 h followed by Hoechst 33342 staining (Thermo Scientific #62249; 1:10000). Coverslips were mounted with Aqua-Poly/Mount (Polysciences #18606–20) and images were taken on a Nikon A1 confocal microscope with a 60X oil-immersion objective lens.
Growth assay and dose response analysis
Cells were plated in 80µL of media at 5000 cells per well (for NST) or 15000 cells per well (for ILC) in ULA 96-well plates (Corning #3474). Treatments were added 24 h after seeding in an additional 20uL of respective media and then treatment was repeated every other day. Forskolin (Cell Signaling technology #3828) was dissolved in DMSO with a final ≤ 0.5% DMSO concentration in treatments. Cells were collected at day 7 and measured by CellTiter-Glo (Promega #PR-G7573) following the manufacture’s protocol. Cell viability values were analyzed following blank cell deductions. Half maximal inhibitory concentration (IC50) values for viability were calculated by nonlinear regression and statistical differences evaluated using sum-of-squares Global f-test (p < 0.05).
Results
Clinicopathological features of the SCAN-B study population
Key clinicopathological features of all patients with ILC and NST in SCAN-B are detailed in Supplementary Table 1. This is compared with data from TCGA, METABRIC and a large clinical dataset from the Great Lakes Breast Cancer Consortium [1] in Supplementary Table 1. Similar to the other datasets, patients with ILC in SCAN-B were older at diagnosis, had higher ER positivity, lower HER2 positivity, lower grade and higher proportion of luminal A subtype than NST.
Identifying DEGs between luminal A ILC and luminal A NST in SCAN-B
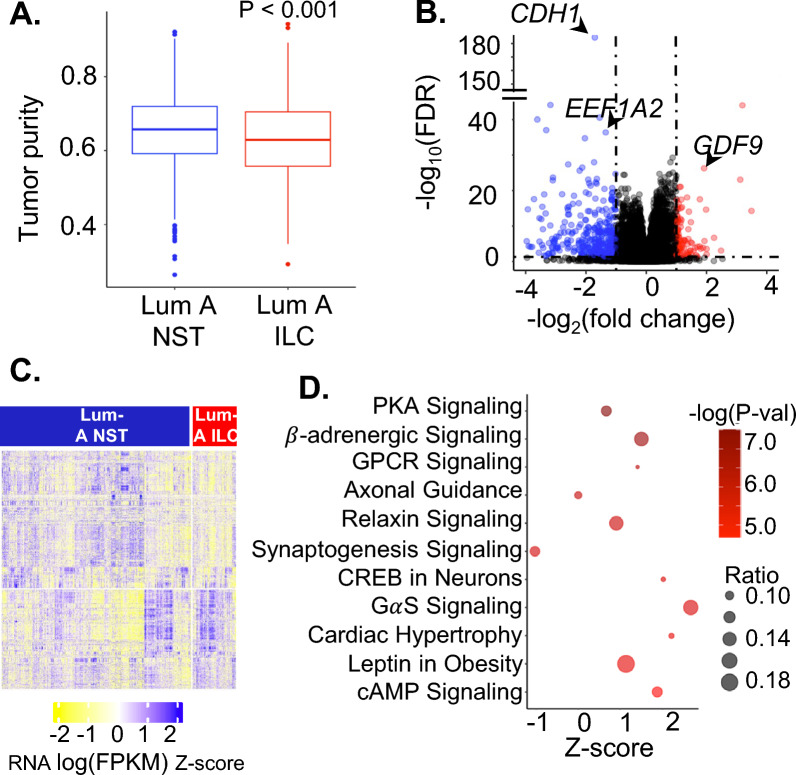
For differential gene expression analysis, we focused on the luminal A subtype. NST tumors had higher tumor purity than ILC (Fig. 1A) in SCAN-B similar to TCGA and METABRIC [9]. There were 7,585 DEGs (FDR < 0.05) between Luminal A ILC and Luminal A NST after correcting for tumor purity of which 3188 genes had higher expression in luminal A ILC (Fig. 1B, C, Supplementary Table 2). Genes with highly significant FDR that were previously reported to be altered in ILC (including CDH1) are depicted in the volcano plot [9, 30]. The enrichment analyses using IPA and EnrichR revealed that several Gαs/cAMP/PKA/CREB signaling related pathways were upregulated in Luminal A ILC compared to NST (Fig. 1D, Supplementary Fig. 1).
Fig. 1.
A Box plot depicting tumor purity of Luminal A ILC and Luminal A NST based on gene expression data from SCAN-B estimated by package ESTIMATE. B Volcano plot for differential gene expression between Luminal A ILC and Luminal A NST in SCAN-B using DESeq2; red = genes with log(FC) > 1 and FDR < 0.05 and blue—genes with log(FC) < 1 and FDR < 0.05. Data points representing CDH1, EEF1A2 and GDF9 are labeled. C Heatmap showing RNA expression (logFPKM z-scores) of differentially expressed genes in Luminal A ILC and Luminal A NST in SCAN-B. D Bubble plot depicting z score (represents overall effect on pathway in Luminal A ILC, z-score > 0 suggests upregulation and z-score < 0 suggests downregulation) on x-axis, -log(P-val) as color, and ratio (ratio of differentially expressed genes in the pathway to total number of genes in the pathway) as bubble size, for significantly enriched pathways identified by ingenuity pathway analysis of top 1000 most differentially expressed genes between Luminal A ILC and Luminal A NST (FDR < 1E-08). ILC—Invasive lobular breast cancer; NST—Invasive carcinoma—no special type; Lum A—Luminal A; SCAN-B—Sweden Cancerome Analysis Network—Breast; FDR—False Discovery Rate; KEGG—Kyoto Encyclopedia of Genes and Genomes; LogFC—Log to the base 2 of fold change; FPKM—Fragments Per Kilobase of transcript per Million mapped reads
cAMP/PKA/CREB signaling is consistently up-regulated in luminal A ILC across multiple datasets and associated with survival in patients with ILC
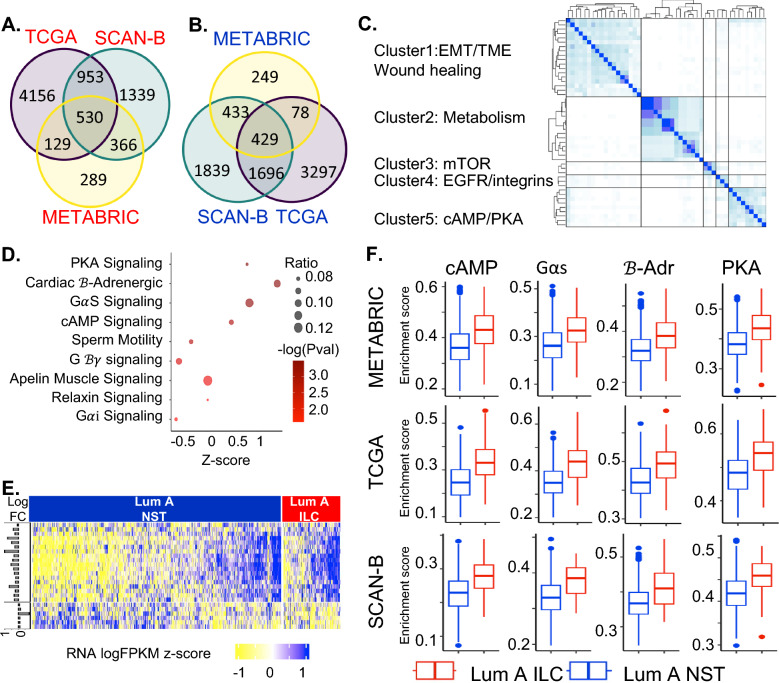
To identify molecular alterations in ILC that are consistent across multiple datasets, we overlapped the DEGs from SCAN-B, TCGA (Supplementary Table 3) and METABRIC (Supplementary Table 4). We identified 530 and 429 genes with higher and lower expressions, respectively, in Luminal A ILC vs Luminal A NST tumors in all 3 datasets (Fig. 2A, B, Supplementary Table 5). This included several ILC-related genes [4, 5, 9, 30–33]- CDH1, growth-factors (EGFR, IGF1, IGFBP6,, IGFL1, IGFL2, GDF9), genes related to lipid metabolism (FABP4, LIPE, LPL), inhibitors of differentiation (ID1 and ID2), and EEF1A2. Other known alterations [4, 34, 35] were found in at least one of the datasets: GATA3 (TCGA and SCAN-B); PTEN and PIK3CA (TCGA) and PPARG and PPARGC1A (SCAN-B).
Fig. 2.
A Venn diagram showing overlapping differentially expressed genes upregulated in luminal A ILC (as compared to luminal A NST) in TCGA, SCAN-B, METABRIC. B Venn diagram showing overlapping differentially expressed genes downregulated in luminal A ILC (as compared to luminal A NST) in TCGA, SCAN-B, METABRIC. C Clustering heatmap using Jaccard index for IPA pathways significantly enriched in differentially expressed genes that overlap in all 3 databases; pathways with Jaccard index > 0.3 and at least 1 membership were clustered using hierarchical clustering into clusters of related pathways. D Bubble plot depicting z score (represents overall effect on pathway in Luminal A ILC, z-score > 0 suggests upregulation and z-score < 0 suggests downregulation) in x-axis, -log(P-val) as color and ratio (ratio of differentially expressed genes in the pathway to total number of genes in the pathway) as bubble size, for pathways in Cluster 5 from the Jaccard index heatmap. The reported z-scores, p-values and ratio are based on gene expression data from SCAN-B. E Heatmap showing expression (scaled logFPKM values from SCAN-B) of genes present in the pathways in Cluster 5 of Jaccard index heatmap with a positive z-score (Protein Kinase A Signaling, Cardiac β-Adrenergic Signaling, GαS Signaling, cAMP-mediated Signaling) that are also differentially expressed between Luminal A ILC and Luminal A NST. The horizontal bars on the left depict the log fold change for the corresponding gene in luminal A ILC as compared to luminal A NST in SCAN-B. F Boxplots showing GSVA enrichment score for pathways from Cluster 5 of Jaccard index heatmap with positive z-score (cAMP = cAMP-mediated Signaling, GαS = GαS Signaling, β-Adr = Cardiac β-Adrenergic Signaling, PKA = Protein Kinase A Signaling), in Luminal A ILC (red) and Luminal A NST (blue) in SCAN-B, TCGA and METABRIC; p < 0.0001 for all comparisons. ILC—Invasive lobular breast cancer; NST—Invasive carcinoma—no special type; Lum A—Luminal A; SCAN-B—Sweden Cancerome Analysis Network—Breast; TCGA—The Cancer Genome Atlas; METABRIC—Molecular Taxonomy of Breast Cancer International Consortium; EMT—Epithelial-Mesenchymal Transition; TME—Tumor Microenvironment; mTOR—mammalian Target of Rapamycin; EGFR—Epidermal Growth Factor Receptor; IPA—Ingenuity Pathway Analysis; FPKM—Fragments Per Kilobase of transcript per Million mapped reads; PKA—Protein kinase A; cAMP—cyclic adenosine monophosohate
IPA-based pathway analysis of these 959 overlapping DEGs using logFC and FDR values from SCAN-B as input identified 115 pathways significantly enriched in the overlapping DEGs (log(P-value) > 1.5). Hierarchical clustering of these pathways based on pairwise Jaccard similarity resulted in 5 distinct clusters (Fig. 2C), including (1) Epithelial-mesenchymal transition/tumor microenvironment/wound healing, (2) Metabolism, (3) mTOR signaling, (4) EGFR and Integrins, and (5) cAMP/PKA signaling (Supplementary Table 6). Pathways from Clusters 1–4 have previously received some attention in the literature with respect to ILC biology [4, 9, 36–40] whereas cluster 5 constituted of pathways related to Gαs/cAMP/PKA/CREB signaling (Fig. 2D, E) that have not been discussed before in the context of ILC and hence we sought to focus on this discovery. Protein kinase A signaling, Cardiac β-adrenergic Signaling, Gαs Signaling, and cAMP-mediated signaling had positive z-scores suggesting up-regulation of Gαs/cAMP/PKA/CREB signaling in luminal A ILC. Table 1 describes the genes of Gαs/cAMP/PKA/CREB signaling that are significantly differentially expressed in all 3 datasets.
Table 1.
Genes relevant to cAMP/PKA/CREB signaling that are differentially expressed (FDR < 0.05) in SCAN-B, TCGA and METABRIC and their fold changes in the respective datasets
| Gene | Description | Fold change in SCAN-B | Fold chnage in TCGA | Fold change in METABRIC |
|---|---|---|---|---|
| G alpha receptors | ||||
| PTGER4 | Prostaglandin E2 receptor | 1.24 | 1.38 | 1.37 |
| ADRB2 | Beta adrenergic receptor | 1.15 | 1.32 | 1.19 |
| S1PR1 | Sphingosine-1-phosphate receptor 1 | 1.33 | 1.37 | 1.24 |
| G protein subunits | ||||
| GNAS | G protein Subunit Alpha S | 0.93 | 0.77 | 0.86 |
| GNB1 | G protein Subunit Beta 1 | 0.92 | 0.83 | 0.91 |
| GNG7 | G protein Subunit Gamma 7 | 1.19 | 1.45 | 1.15 |
| GNG11 | G protein Subunit Gamma 11 | 1.26 | 1.24 | 1.40 |
| Adenylyl cyclase | ||||
| ADCY4 | Adenylate Cyclase 4 | 1.28 | 1.68 | 1.30 |
| ADCY5 | Adenylate Cyclase 5 | 1.62 | 1.53 | 1.07 |
| Phosphodiesterases | ||||
| PDE1A | Phosphodiesterase 1A | 1.34 | 1.21 | 1.39 |
| PDE2A | Phosphodiesterase 2A | 1.47 | 1.44 | 1.11 |
| PDE4B | Phosphodiesterase 4B | 1.30 | 1.32 | 1.37 |
| PDE9A | Phosphodiesterase 9A | 1.20 | 1.34 | 1.11 |
| A-kinase Anchoring Protein | ||||
| AKAP7 | A-kinase anchoring protein 7 | 1.17 | 1.17 | 1.19 |
| AKAP8 | A-kinase anchoring protein 8 | 1.04 | 1.22 | 1.08 |
| Targets for PKA Phosphorylation | ||||
| SRC | SRC Proto-oncogene | 0.90 | 0.81 | 0.87 |
| CAMK2B | Calcium/calmodulin dependent protein kinase II | 0.57 | 0.68 | 0.75 |
| PPARG | Peroxisome proliferator-activated receptor gamma | 1.38 | 1.28 | 1.55 |
| RYR3 | Ryanodine receptor 3 | 1.76 | 1.45 | 1.09 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | 1.23 | 1.39 | 1.30 |
| LIPE | Hormone sensitive lipase | 1.79 | 1.69 | 1.24 |
| DUSP6 | Dual specificity phosphatase 6 | 1.24 | 1.22 | 1.26 |
| Other related genes | ||||
| RGS2 | Regulator of G Protein Signaling 2 | 1.16 | 1.48 | 1.25 |
| RAPGEF3 | Rap Guanine Nucleotide Exchange Factor 3 | 1.25 | 1.92 | 1.21 |
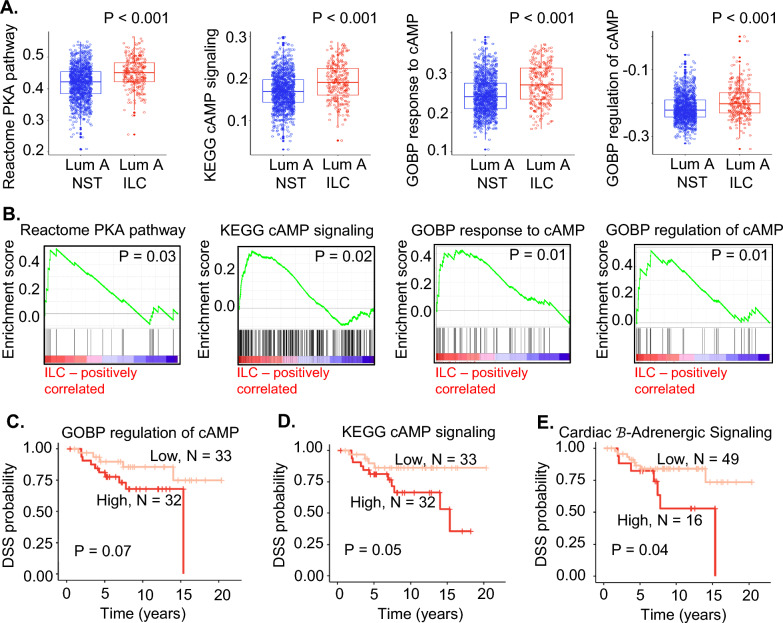
GSVA and GSEA provide alternative approaches to understanding gene expression. Indeed, pathways with a positive z-score in Jaccard cluster 5 (ie., Protein kinase A signaling, Cardiac β-adrenergic signaling, Gαs signaling, cAMP-mediated signaling) had higher GSVA scores in Luminal A ILC than NST in all 3 datasets (Fig. 2F). Additionally, GSVA was performed using genesets relevant to cAMP/PKA/CREB signaling downloaded from MSigDB and KEGG. These genesets also had higher GSVA scores in Luminal A ILC than Luminal A NST in all 3 datasets (Fig. 3A, Supplementary Table 7). Further, GSEA showed an up-regulation of these genesets in Luminal A ILC in SCAN-B (Fig. 3B).
Fig. 3.
A Overlapping box and jitter plots showing GSVA estimation scores of genesets related to cAMP/PKA/CREB signaling downloaded from MSigDB in luminal A ILC (red) vs luminal A NST (blue) from SCAN-B; Reactome PKA pathway = Reactome PKA mediated phosphorylation of CREB, KEGG cAMP Signaling = KEGG cAMP mediated signaling, GOBP response to cAMP = GOBP cellular response to cAMP, and GOBP regulation of cAMP = GOBP regulation of cAMP mediated signaling. B GSEA of genesets related to cAMP/PKA/CREB signaling downloaded from MSigDB in luminal A ILC vs luminal A NST from SCAN-B; Reactome PKA pathway = Reactome PKA mediated phosphorylation of CREB, KEGG cAMP Signaling = KEGG cAMP mediated signaling, GOBP response to cAMP = GOBP cellular response to cAMP, and GOBP regulation of cAMP = GOBP regulation of cAMP mediated signaling). C Disease-specific survival in luminal A ILC based on GSVA scores of GOBP regulation of cAMP signaling from METABRIC gene expression data; low expression (light-red) defined as < 50th percentile GSVA score, and high expression (red) defined as > / = 50th percentile GSVA score. D Disease-specific survival in luminal A ILC based on GSVA scores of KEGG cAMP mediated signaling from METABRIC gene expression data, low expression (light-red) defined as < 50th percentile GSVA score, and high expression (red) defined as > / = 50th percentile GSVA score. E Disease-specific survival in luminal A ILC based on GSVA scores of Cardiac β adrenergic signaling from METABRIC gene expression data, low expression (light-red) defined as < 75th percentile GSVA score, and high expression (red) defined as > / = 75th percentile GSVA score. GSVA—Gene Set Variation Analysis; MSigDB—Molecular Signatures Database; GSEA—Gene Set Enrichment Analysis; ILC—Invasive lobular breast cancer; NST—Invasive carcinoma—no special type; Lum A—Luminal A; SCAN-B—Sweden Cancerome Analysis Network—Breast; METABRIC- Molecular Taxonomy of Breast Cancer International Consortium; PKA—Protein kinase A, CREB—cAMP Response Element Binding protein; KEGG—Kyoto Encyclopedia of Genes and Genomes; GOBP—Gene Ontology Biological Process, DSS—Disease specific survival
In an RNA expression dataset from paired primary and metastatic breast cancer, primary ILC had higher GSVA scores for cAMP/PKA/CREB signaling pathways than primary NST (Supplementary Fig. 2A). When the GSVA scores were compared between paired primary breast tumors and metastatic lesions (ILC and NST combined), patients with brain metastases (N = 22) and ovarian metastases (N = 11) respectively had significantly higher and lower GSVA scores for some of the pathways related to cAMP/PKA/CREB signaling in the metastatic tissue than the primary breast tissue (Supplementary Fig. 2B).
There was a trend towards worse DSS in patients with Luminal A ILC who had higher GSVA scores (> 75th or > 50th percentile) in three out of the eight cAMP/PKA/CREB signaling related pathways in METABRIC (Fig. 3C–E; Supplementary Table 8). Logrank testing showed a trend towards worse DSS based on expression of GOBP regulation of cAMP (P = 0.07, Fig. 3C) and significantly worse DSS based on expression of KEGG cAMP mediated signaling (P = 0.05, Fig. 3D) and IPA’s Cardiac β-adrenergic signaling (P = 0.04, Fig. 3E). A similar analysis did not reveal any significant association with DSS in patients with Luminal A NST (Supplementary Table 8).
cAMP/PKA/CREB signaling and forskolin mediated phosphorylation of CREB are higher in ILC cell lines
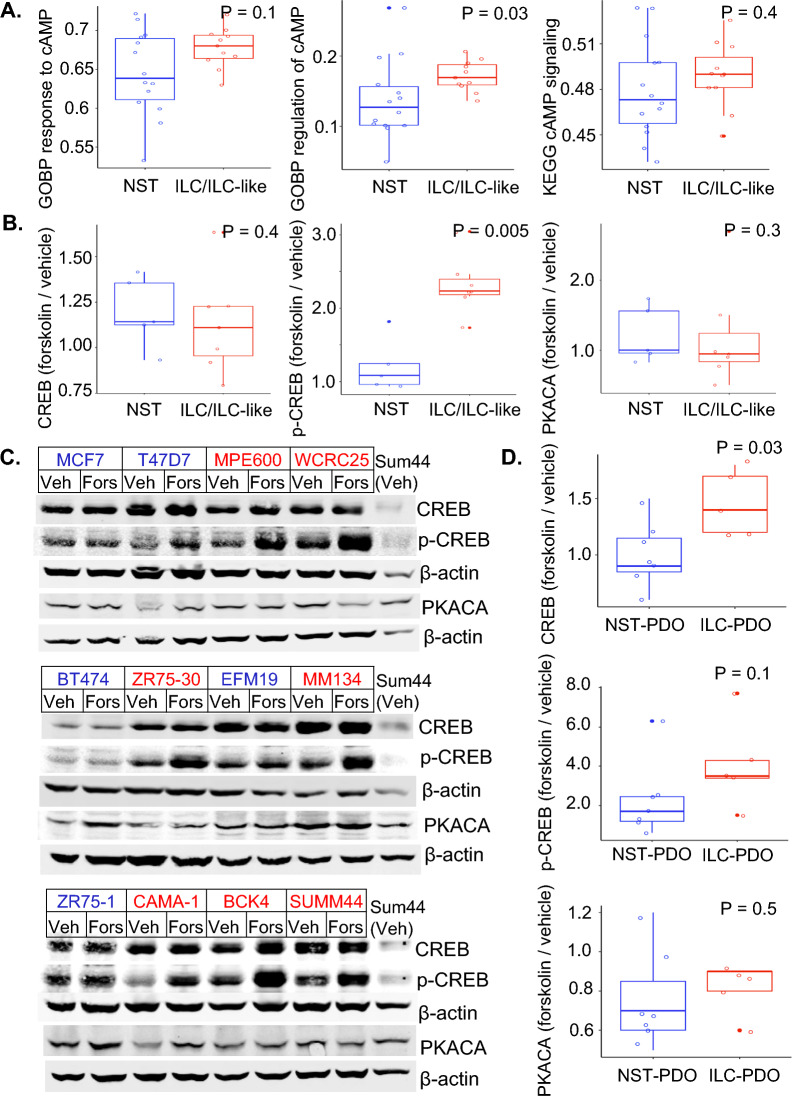
Given the bioinformatic findings showing enhanced cAMP/PKA/CREB signaling in ILC tumors, we investigated these findings using in vitro models. We first compared the expression of cAMP/PKA/CREB related pathways in ILC (HCC2185, MDAMB134, MDAMB330, SUM44PE) or ILC-like (MPE600, CAMA1, HCC2218, MDAMB453, OCUBM, SKBR5, ZR7530) vs NST (BT474, BT483, EFM19, HCC1008, HCC1419, HCC1500, LY2, MCF7, MDAMB157, MDAMB175, T47D, UACC812, ZR75-1, ZR75B) cell lines using publicly available gene expression data [21]. Of the four pathways from Cluster 5 of Jaccard clustering with positive z-score (PKA signaling; Cardiac β-adrenergic Signaling; Gαs Signaling, cAMP-mediated signaling) and the four MSigDB pathways with higher GSEA/GSVA scores in luminal A ILC than luminal A NST in SCAN-B (KEGG cAMP mediated signaling; REACTOME PKA mediated phosphorylation of CREB; GOBP cellular response to cAMP; GOBP regulation of cAMP mediated signaling), GSVA scores for GOBP cellular response to cAMP, GOBP regulation of cAMP mediated signaling and KEGG cAMP mediated signaling (Fig. 4A) were higher in ILC and ILC-like cell lines as compared to NST cell lines (statistically significant for GOBP regulation of cAMP mediated signaling, P = 0.03). The GSVA scores for the other 5 pathways tested were similar in ILC/ILC-like and NST cell lines (Supplementary Fig. 3).
Fig. 4.
A Overlapping box and jitter plots depicting GSVA estimation scores for cAMP/PKA/CREB signaling related pathways in ILC and ILC like (red; ILC—HCC2185, MDAMB134, MDAMB330, SUM44PE; ILC like—MPE600, CAMA1, HCC2218, MDAMB453, OCUBM, SKBR5, ZR7530) and NST (blue; NST—BT474, BT483, EFM19, HCC1008, HCC1419, HCC1500, LY2, MCF7, MDAMB157, MDAMB175, T47D, UACC812, ZR75-1, ZR75B) cell lines based on gene expression data from Marcotte et al. 2016; GOBP response to cAMP = GOBP cellular response to cAMP, GOBP regulation of cAMP = GOBP regulation of cAMP mediated signaling and KEGG cAMP signaling = KEGG cAMP mediated signaling. B Overlapping box and jitter plots depicting ratio of quantified levels of western blot for CREB, p-CREB and PKACA in ILC/ILC-like and NST cell lines before and after treatment with forskolin (each normalized for corresponding levels of β-actin); ILC and ILC-like cell lines (red; MPE600, WCRC25, ZR75-30, MM134, CAMA-1, BCK4, SUM44PE) and NST cell lines (blue; MCF7, T47D, BT474, EFM19, ZR75-1); Y-axis represents the ratio of levels of CREB, p-CREB, and PKACA with forskolin treatment to their baseline levels (vehicle treatment). C Western blot images for levels of CREB, p-CREB, and PKACA in ILC and ILC-like (red; MPE600, WCRC25, ZR75-30, MM134, CAMA-1, BCK4, SUM44PE) and NST (blue; MCF7, T47D, BT474, EFM19, ZR75-1) cell lines with Vehicle (Veh) and Forskolin (Fors) treatment. D Overlapping box and jitter plots depicting ratio of quantified levels of western blot for CREB, p-CREB and PKACA in patient-derived organoids (PDOs) before and after treatment with forskolin (each normalized for corresponding levels of β-actin): ILC PDOs (PDO146 PDO86 PDO53 PDO46 PDO30) and NST PDOs (PDO126 PDO56 PDO134 PDO123 PDO111 PDO102 PDO129); Y-axis represents the ratio of levels of CREB, p-CREB, and PKACA with forskolin treatment to their baseline levels (vehicle treatment). GSVA—Gene Set Variation Analysis; ILC—Invasive lobular breast cancer; NST—Invasive carcinoma—no special type; PKA—Protein kinase A; PKACA—Protein kinase A catalytic α subunit; CREB—cAMP Response Element Binding protein; p-CREB—phospho CREB, phosphorylated at Ser133; KEGG—Kyoto Encyclopedia of Genes and Genomes; GOBP—Gene Ontology Biological Process; PDO—Patient Derived Organoids
We next used an activator of cAMP/PKA/CREB pathway, forskolin, which stimulates adenylate cyclase and thereby increases intracellular cAMP levels [41] for in vitro studies. Treatment of ILC/ILC-like and NST cell lines with forskolin for 20 min increased CREB phosphorylation at Ser133 (Fig. 4B) and did not alter the expression levels of CREB or PKA-Cα compared with vehicle treatment (Fig. 4C). The forskolin induced phosphorylation of CREB was twofold higher in ILC/ILC-like cell lines as compared to NST (P = 0.005). ILC/ILC-like cell lines incubated overnight in low serum media also demonstrated enhanced CREB phosphorylation than NST (P = 0.02, Supplementary Fig. 4) indicating that ILC/ILC-like cell lines have higher cAMP/PKA/CREB signaling irrespective of the growth media. Baseline level of p-CREB was higher in ILC/ILC-like cell lines than NST in low serum media (P = 0.048, Supplementary Fig. 5).
We next studied the effect of forskolin treatment on the levels of CREB, p-CREB and PKA-Cα in NST and ILC PDOs. The histology of the PDOs was validated by presence or absence of E-cadherin (Supplementary Fig. 6). Akin to cell lines, forskolin treatment resulted in higher levels of p-CREB in ILC than NST PDOs, although this was not statistically significant (Fig. 4D, Supplementary Fig. 6). CREB levels were also higher with forskolin treatment in ILC PDOs. Together, these data suggest increased activity of cAMP/PKA/CREB signaling in ILC in vitro models.
Given that activated PKA-Cα localizes to the nucleus to phosphorylate CREB, we performed co-immunofluorescence of PKA-Cα and p-CREB on representative NST and ILC/ILC-like cell lines. Following forskolin treatment, PKA-Cα was more evident in the nucleus and the staining of nuclear p-CREB was enhanced across the tested cell lines (Supplementary Fig. 7) with no obvious differences among them.
Loss of E-cadherin in MCF7 and T47D with CRISPR-mediated deletion of CDH1 did not increase phosphorylation of CREB with Forskolin (Supplementary Fig. 8), suggesting that E-cadherin loss is not sufficient for increased cAMP/PKA/CREB signaling in ILC.
Forskolin inhibits in vitro cell growth
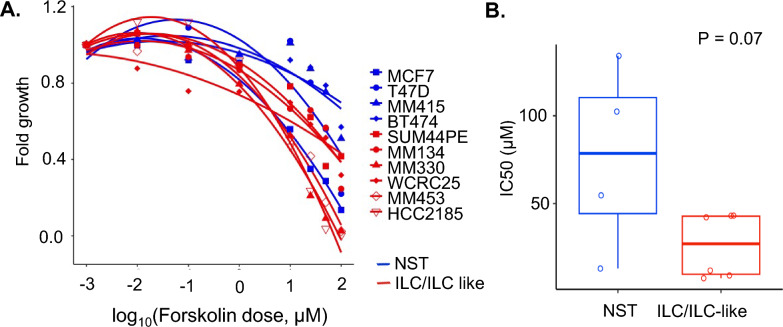
To understand the effect of increased cAMP/PKA/CREB signaling on proliferation of breast cancer cells, we performed growth assays in 6 ILC cell lines (SUM44PE, MM134, MM330, WCRC25, MM453, HCC2185) and 4 NST cell lines (MCF7, T47D, MM415, BT474) treated with forskolin. Forskolin has anti-cancer and anti-proliferative effects in multiple cancer types [42] and augments the cytotoxicity of chemotherapy agents [43]. Increasing forskolin doses inhibited cell growth in all breast cancer cell lines (Fig. 5A), with a lower IC50 in the ILC cell lines (median IC50 27 µM) compared to the NST cell lines (median IC50 78.5 µM, P = 0.07, Fig. 5B).
Fig. 5.
A Dose response curves representing growth inhibition in ILC (red: SUM44PE, MM134, MM330, WCRC25, MM453, HCC2185) and NST (blue: MCF7, T47D, MM415, BT474) cell lines in response to treatment with forskolin: Y axis represents fold change in growth after 7 days of treatment with forskolin and X axis represents log of dose of forskolin in µM B Overlapping box and jitter plot depicting IC50 (half maximal inhibitory concentration) for forskolin in ILC (red: SUM44PE, MM134, MM330, WCRC25, MM453, HCC2185) and NST (blue: MCF7, T47D, MM415, BT474) cell lines; C ILC—Invasive lobular breast cancer; NST—Invasive carcinoma—no special type; PKACA—Protein kinase A catalytic α subunit; CREB—cAMP Response Element Binding protein; p-CREB—phospho CREB, phosphorylated at Ser133; Ecad KO—E-cadherin Knock Out; IC50—Half maximal inhibitory concentration
Discussion
Differential gene expression analysis in SCAN-B identified a higher expression of pathways related to cAMP/PKA/CREB signaling in Luminal A ILC compared to Luminal A NST, which was validated in TCGA and METABRIC, as well as in vitro models. In patients with ILC (but not NST) DSS was worse with activation of cAMP and β-adrenergic signaling in the tumors. Forskolin treatment caused higher levels of p-CREB suggesting greater cAMP/PKA/CREB signaling in ILC than NST cell lines and organoids. Finally, forskolin inhibited cell growth in both ILC and NST cell lines, with a trend for increased sensitivity in ILC.
We report an up-regulation of cAMP/PKA/CREB related pathways in ILC. Multiple genes involved in cAMP/PKA/CREB signaling (G-protein coupled receptors, G-protein subunits, adenylyl cyclases, phosphodiesterases, and A-kinase anchoring proteins) were differentially expressed between ILC and NST in all datasets, but the genes coding for PKA subunits and CREB were not consistently differentially expressed. The regulation of cAMP/PKA/CREB signaling involves a complex interplay of gene/protein expression, post-translational modifications, cAMP spatial compartmentalization as well as negative feedback mechanisms [10] making it challenging to interpret the contribution of individual gene expression on overall activity of the pathway. In breast cancer, cAMP/PKA/CREB signaling regulates metabolism [44, 45] but its role has not previously been discussed in the context of ILC. ILC has a distinct metabolic phenotype among breast cancer, characterized by relative metabolic quiescence and limited glucose uptake [46]. cAMP/PKA/CREB signaling has been implicated in upregulation of PPARG, the master regulator of metabolism and its coactivator [47, 48], both of these genes were upregulated in ILC in the SCAN-B dataset and could potentially mediate some of the metabolic hallmarks of ILC.
Forskolin activates adenylyl cyclase and increases intracellular cAMP, and treatment with forskolin resulted in increased phosphorylation of CREB in ILC cell lines than NST. CREB-phosphorylation in response to forskolin is primarily mediated by the cAMP/PKA/CREB signaling [49]. Loss of CDH1 was not sufficient to increase the activity of cAMP/PKA/CREB signaling in ILC, and additional studies are necessary to understand the underlying mechanism. EPAC, the Exchange Protein directly Activated by cAMP, encoded for by RAPGEF3, was also over-expressed in ILC than NST in all three databases. EPAC controls biological functions downstream of cAMP by activating Rap1 and consequently the ERK1/2 and PI3K/AKT/mTOR signaling [50]. Higher levels of EPAC1 and its effector AKAP9 has been reported in breast cancer as compared to normal, and play a role in cell migration and apoptosis [51].
Forskolin inhibited growth in both ILC and NST with lower IC50s in ILC than NST suggesting a greater response to the inhibitory effects of forskolin in ILC than NST. Anti-proliferative effects of forskolin through cAMP/PKA mediated inhibition of ERK (Extracellular signal-Regulated Kinase) 1/2 have been previously reported in breast cancer cell lines [43]. The ratio of subtypes of PKA determines the overall effect of this pathway on proliferation- Type II PKA is anti-proliferative, while type I PKA promotes proliferation [10]. One mechanism by which PKA inhibits proliferation is by phosphorylating Raf-1 and consequently inactivating ERK [52]. cAMP/PKA/CREB signaling promotes apoptosis through mitochondrial mediated events [53]. Forskolin-mediated PKA activation results in mesenchymal to epithelial transition and loss of tumor initiating ability in aggressive breast cancers [54]. cAMP may also regulate proliferation through EPAC, which activates ERK and mTOR signaling [55]. Specific factors in the microenvironment of ILC and NST that tip the balance towards growth inhibition need further investigation. Drugs that modulate intracellular cAMP are approved in other diseases (phosphodiesterase inhibitors) and are in investigational stage for cancer therapy (cAMP analog: taclodesine) [10] and should be evaluated further for the treatment of ILC.
A strength of our study is the use of three large datasets: SCAN-B, TCGA and METABRIC, as well as validation in cell lines and organoids. Clustering based on Jaccard similarity helped us group redundant pathways into clusters and focus on molecular alterations previously not reported in ILC. There are some limitations of the study for example complexities related to lower tumor purity in ILC than NST. The DEGs were filtered for significance (FDR < 0.05) but not for fold change, with the rationale that identification of differences in related genes would be more important than the magnitude of difference. We focused on gene expression analysis, but the activity of cAMP/PKA/CREB signaling is also affected by post-translational modifications and spatial compartmentalization of cAMP, which we did not address here. We used forskolin to investigate cAMP/PKA/CREB pathway as prior studies have shown it mediates CREB phosphorylation primarily via cAMP and PKA signaling [49]. However, intracellular cAMP can also activate AKT/mTOR and ERK pathways through EPAC1, which in turn can phosphorylate CREB, thus other specific inhibitors should be utilized. And finally, we limited our study to the effect of cAMP/PKA/CREB signaling on growth, but it can affect several other cellular processes like metabolism, cell adhesion and migration which should be addressed in subsequent studies.
Conclusion
In conclusion, in silico analysis identified upregulation of cAMP/PKA/CREB signaling in ILC compared to NST, which was confirmed in in vitro experiments in cell lines and organoids. Loss of CDH1 alone may not be sufficient to mediate the higher cAMP/PKA/CREB signaling in ILC. Future studies should focus on the mechanisms for differential cAMP/PKA/CREB signaling in ILC and NST, its effects on cell division, migration and metabolism and investigate the potential for therapeutic targeting of this pathway especially in patients with ILC.
Supplementary Information
Acknowledgements
This project used the UPMC Hillman Cancer Center and Tissue and Research Pathology/Pitt Biospecimen Core shared resource which is supported in part by award P30CA047904. Steffi Oesterreich and Adrian Lee are also funded by the Breast Cancer Research Foundation and are Komen Scholars.
Abbreviations
- ILC
Invasive Lobular Breast Cancer
- NST
Invasive Breast Cancer–No Special Type
- ER
Estrogen Receptor
- HER2
Human Epidermal Growth Factor Receptor 2
- IGF-1
Insulin like Growth Factor-1
- SCAN-B
Sweden Cancerome Analysis Network-Breast
- TCGA
The Cancer Genome Atlas
- METABRIC
Molecular Taxonomy of Breast Cancer International Consortium
- cAMP
Cyclic Adenosine Mono Phosphate
- PKA
Protein Kinase A
- PKA-Cα
Protein Kinase A Catalytic alpha subunit
- CREB
CAMP Response Element Binding protein
- EPAC
Exchange Protein directly Activated by cAMP
- FDR
False Discovery Rate
- LogFC
Log base 2 of fold change
- SAM
Significance of Analysis of Microarrays
- DEGs
Differentially Expressed Genes
- IPA
Ingenuity Pathway Analysis
- GSVA
Gene set Variation Analysis
- GSEA
Gene set Enrichment Analysis
- MSigDB
Molecular Signature Database
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- GOBP
Gene Ontology Biological Process
- DSS
Disease-Specific Survival
- PDO
Patient Derived Organoid
- IC50
Half maximal inhibitory concentration
- ERK1/2
Extracellular signal-Regulated Kinase 1/2
Author contributions
Conceptualization: SO, AVL Data analysis and interpretation: SPN, OSS, AW Specimen acquisition: PFM, DDB Wet lab experiments: AW, JC, DDB Writing—original draft: SPN, AW Writing—review and editing: SO, AVL, OSS, DDB, PFM.
Funding
This work was in part supported by BCRF (SO, AVL).
Data availability
Data files obtained from prior studies (from our group as well as other groups) have been referenced in the main text of the manuscript Data files generated in the current study are included within the article and supplementary data files.
Declarations
Ethics approval and consent to participate
Not applicable (no human subjects or animal experiments involved).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Steffi Oesterreich and Adrian V. Lee these have contributed equally to this work as co-senior authors.
Contributor Information
Steffi Oesterreich, Email: oesterreichs@upmc.edu.
Adrian V. Lee, Email: leeav@upmc.edu
References
- 1.Oesterreich S, Nasrazadani A, Zou J, et al. Clinicopathological features and outcomes comparing patients with invasive ductal and lobular breast cancer. JNCI J National Cancer Inst. 2022;114(11):1511–22. 10.1093/jnci/djac157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathew A, Rajagopal PS, Villgran V, et al. Distinct pattern of metastases in patients with invasive lobular carcinoma of the breast. Geburtshilfe Frauenheilkd. 2017;77(6):660–6. 10.1055/s-0043-109374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue M, Nakagomi H, Nakada H, et al. Specific sites of metastases in invasive lobular carcinoma: a retrospective cohort study of metastatic breast cancer. Breast Cancer. 2017;24(5):667–72. 10.1007/s12282-017-0753-4. [DOI] [PubMed] [Google Scholar]
- 4.Ciriello G, Gatza ML, Beck AH, et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163(2):506–19. 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elangovan A, Hooda J, Savariau L, et al. Loss of E-cadherin induces IGF1R activation and reveals a targetable pathway in invasive lobular breast carcinoma. Mol Cancer Res. 2022;20(9):1405–19. 10.1158/1541-7786.MCR-22-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barroso-Sousa R, Metzger-Filho O. Differences between invasive lobular and invasive ductal carcinoma of the breast: results and therapeutic implications. Ther Adv Med Oncol. 2016;8(4):261–6. 10.1177/1758834016644156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boughey JC, Wagner J, Garrett BJ, et al. Neoadjuvant chemotherapy in invasive lobular carcinoma may not improve rates of breast conservation. Ann Surg Oncol. 2009;16(6):1606–11. 10.1245/s10434-009-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saal LH, Vallon-Christersson J, Häkkinen J, et al. The Sweden Cancerome Analysis Network - Breast (SCAN-B) Initiative: a large-scale multicenter infrastructure towards implementation of breast cancer genomic analyses in the clinical routine. Genome Med. 2015;7(1):20. 10.1186/s13073-015-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du T, Zhu L, Levine KM, et al. Invasive lobular and ductal breast carcinoma differ in immune response, protein translation efficiency and metabolism. Sci Rep. 2018;8(1):7205. 10.1038/s41598-018-25357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Kong Q, Wang J, Jiang Y, Hua H. Complex roles of cAMP–PKA–CREB signaling in cancer. Exp Hematol Oncol. 2020;9(1):32. 10.1186/s40164-020-00191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao X, Li BX, Mitton B, Ikeda A, Sakamoto KM. Targeting CREB for cancer therapy: friend or foe. Curr Cancer Drug Targets. 2010;10(4):384–91. 10.2174/156800910791208535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalal H, Dahlgren M, Gladchuk S, Brueffer C, Gruvberger-Saal SK, Saal LH. Clinical associations of ESR2 (estrogen receptor beta) expression across thousands of primary breast tumors. Sci Rep. 2022;12(1):4696. 10.1038/s41598-022-08210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brueffer C, Gladchuk S, Winter C, et al. The mutational landscape of the SCAN-B real-world primary breast cancer transcriptome. EMBO Mol Med. 2020;12(10): e12118. 10.15252/emmm.202012118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman M, Jackson LK, Johnson WE, Li DY, Bild AH, Piccolo SR. Alternative preprocessing of RNA-Sequencing data in The Cancer Genome Atlas leads to improved analysis results. Bioinformatics. 2015;31(22):3666–72. 10.1093/bioinformatics/btv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshihara K, Shahmoradgoli M, Martínez E, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4(1):2612. 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.KEGG PATHWAY Database (https://www.genome.jp/pathway/hsa04024)
- 17.GSEA-MSigDB (https://www.gsea-msigdb.org/gsea/msigdb/cards/REACTOME_PKA_MEDIATED_PHOSPHORYLATION_OF_CREB)
- 18.GSEA-MSigDB (https://www.gsea-msigdb.org/gsea/msigdb/cards/GOBP_CELLULAR_RESPONSE_TO_CAMP)
- 19.GSEA-MSigDB (https://www.gsea-msigdb.org/gsea/msigdb/cards/GOBP_REGULATION_OF_CAMP_MEDIATED_SIGNALING)
- 20.Levine KM, Priedigkeit N, Basudan A, et al. FGFR4 overexpression and hotspot mutations in metastatic ER+ breast cancer are enriched in the lobular subtype. NPJ Breast Cancer. 2019;5:19. 10.1038/s41523-019-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcotte R, Sayad A, Brown KR, et al. Functional genomic landscape of human breast cancer drivers, vulnerabilities, and resistance. Cell. 2016;164(1–2):293–309. 10.1016/j.cell.2015.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sflomos G, Schipper K, Koorman T, et al. Atlas of lobular breast cancer models: challenges and strategic directions. Cancers (Basel). 2021;13(21):5396. 10.3390/cancers13215396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elangovan A, Bossart EA, Basudan A, et al. Abstract P5–12–03: Wcrc-25: a novel luminal invasive lobular carcinoma cell line model. Cancer Res. 2022. 10.1158/1538-7445.SABCS21-P5-12-03. [Google Scholar]
- 24.Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–27. 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jambal P, Badtke MM, Harrell JC, et al. Estrogen switches pure mucinous breast cancer to invasive lobular carcinoma with mucinous features. Breast Cancer Res Treat. 2013;137(2):431–48. 10.1007/s10549-012-2377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du T, Sikora MJ, Levine KM, et al. Key regulators of lipid metabolism drive endocrine resistance in invasive lobular breast cancer. Breast Cancer Res. 2018;20(1):106. 10.1186/s13058-018-1041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachs N, de Ligt J, Kopper O, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172(1–2):373-386.e10. 10.1016/j.cell.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Ding K, Chen F, Priedigkeit N, et al. Single cell heterogeneity and evolution of breast cancer bone metastasis and organoids reveals therapeutic targets for precision medicine. Ann Oncol. 2022;33(10):1085–8. 10.1016/j.annonc.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagle AM, Levine KM, Tasdemir N, et al. Loss of E-cadherin enhances IGF1-IGF1R pathway activation and sensitizes breast cancers to Anti-IGF1R/InsR inhibitors. Clin Cancer Res. 2018;24(20):5165–77. 10.1158/1078-0432.CCR-18-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomlinson VA, Newbery HJ, Wray NR, et al. Translation elongation factor eEF1A2 is a potential oncoprotein that is overexpressed in two-thirds of breast tumours. BMC Cancer. 2005;5:113. 10.1186/1471-2407-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cha YJ, Kim HM, Koo JS. Expression of lipid metabolism-related proteins differs between invasive lobular carcinoma and invasive ductal carcinoma. Int J Mol Sci. 2017;18(1):232. 10.3390/ijms18010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rätze MAK, Koorman T, Sijnesael T, et al. Loss of E-cadherin leads to Id2-dependent inhibition of cell cycle progression in metastatic lobular breast cancer. Oncogene. 2022;41(21):2932–44. 10.1038/s41388-022-02314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tasdemir N, Ding K, Savariau L, et al. Proteomic and transcriptomic profiling identifies mediators of anchorage-independent growth and roles of inhibitor of differentiation proteins in invasive lobular carcinoma. Sci Rep. 2020;10(1):11487. 10.1038/s41598-020-68141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abduljabbar R, Al-Kaabi MM, Negm OH, et al. Prognostic and biological significance of peroxisome proliferator-activated receptor-gamma in luminal breast cancer. Breast Cancer Res Treat. 2015;150(3):511–22. 10.1007/s10549-015-3348-9. [DOI] [PubMed] [Google Scholar]
- 35.Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. The localisation and reduction of nuclear staining of PPARgamma and PGC-1 in human breast cancer. Oncol Rep. 2004;12(2):483–8. [PubMed] [Google Scholar]
- 36.Sflomos G, Battista L, Aouad P, et al. Intraductal xenografts show lobular carcinoma cells rely on their own extracellular matrix and LOXL1. EMBO Mol Med. 2021;13(3): e13180. 10.15252/emmm.202013180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nyante SJ, Wang T, Tan X, Ozdowski EF, Lawton TJ. Quantitative expression of MMPs 2, 9, 14, and collagen IV in LCIS and paired normal breast tissue. Sci Rep. 2019;9(1):13432. 10.1038/s41598-019-48602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao H, Langerød A, Ji Y, et al. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell. 2004;15(6):2523–36. 10.1091/mbc.e03-11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weigelt B, Geyer FC, Natrajan R, et al. The molecular underpinning of lobular histological growth pattern: a genome-wide transcriptomic analysis of invasive lobular carcinomas and grade- and molecular subtype-matched invasive ductal carcinomas of no special type. J Pathol. 2010;220(1):45–57. 10.1002/path.2629. [DOI] [PubMed] [Google Scholar]
- 40.Perrone G, Altomare V, Zagami M, et al. Caveolin-1 expression in human breast lobular cancer progression. Mod Pathol. 2009;22(1):71–8. 10.1038/modpathol.2008.154. [DOI] [PubMed] [Google Scholar]
- 41.Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981;78(6):3363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sapio L, Gallo M, Illiano M, et al. The natural cAMP elevating compound forskolin in cancer therapy: is it time? J Cell Physiol. 2017;232(5):922–7. 10.1002/jcp.25650. [DOI] [PubMed] [Google Scholar]
- 43.Illiano M, Sapio L, Salzillo A, et al. Forskolin improves sensitivity to doxorubicin of triple negative breast cancer cells via Protein Kinase A-mediated ERK1/2 inhibition. Biochem Pharmacol. 2018;152:104–13. 10.1016/j.bcp.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 44.Sola-Penna M, Paixão LP, Branco JR, et al. Serotonin activates glycolysis and mitochondria biogenesis in human breast cancer cells through activation of the Jak1/STAT3/ERK1/2 and adenylate cyclase/PKA, respectively. Br J Cancer. 2020;122(2):194–208. 10.1038/s41416-019-0640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu T, Yang G, Hou Y, et al. Cytoplasmic GPER translocation in cancer-associated fibroblasts mediates cAMP/PKA/CREB/glycolytic axis to confer tumor cells with multidrug resistance. Oncogene. 2017;36(15):2131–45. 10.1038/onc.2016.370. [DOI] [PubMed] [Google Scholar]
- 46.Hogan MP, Goldman DA, Dashevsky B, et al. Comparison of 18F-FDG PET/CT for systemic staging of newly diagnosed invasive lobular carcinoma versus invasive ductal carcinoma. J Nucl Med. 2015;56(11):1674–80. 10.2967/jnumed.115.161455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim S, Ha JM, Yun SJ, et al. Transcriptional activation of peroxisome proliferator-activated receptor-gamma requires activation of both protein kinase A and Akt during adipocyte differentiation. Biochem Biophys Res Commun. 2010;399(1):55–9. 10.1016/j.bbrc.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 48.Singh S, Simpson RL, Bennett RG. Relaxin activates peroxisome proliferator-activated receptor γ (PPARγ) through a pathway involving PPARγ coactivator 1α (PGC1α). J Biol Chem. 2015;290(2):950–9. 10.1074/jbc.M114.589325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Namkoong S, Kim CK, Cho YL, et al. Forskolin increases angiogenesis through the coordinated cross-talk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signaling. Cell Signal. 2009;21(6):906–15. 10.1016/j.cellsig.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 50.Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai). 2008;40(7):651–62. 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar N, Gupta S, Dabral S, Singh S, Sehrawat S. Role of exchange protein directly activated by cAMP (EPAC1) in breast cancer cell migration and apoptosis. Mol Cell Biochem. 2017;430(1):115–25. 10.1007/s11010-017-2959-3. [DOI] [PubMed] [Google Scholar]
- 52.Dhillon AS, Pollock C, Steen H, Shaw PE, Mischak H, Kolch W. Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol Cell Biol. 2002;22(10):3237–46. 10.1128/MCB.22.10.3237-3246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Zambon AC, Vranizan K, Pothula K, Conklin BR, Insel PA. Gene expression signatures of cAMP/protein kinase A (PKA)-promoted, mitochondrial-dependent apoptosis. Comparative analysis of wild-type and cAMP-deathless S49 lymphoma cells. J Biol Chem. 2008;283(7):4304–13. 10.1074/jbc.M708673200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pattabiraman DR, Bierie B, Kober KI, et al. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science. 2016. 10.1126/science.aad3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Misra UK, Pizzo SV. Epac1-induced cellular proliferation in prostate cancer cells is mediated by B-Raf/ERK and mTOR signaling cascades. J Cell Biochem. 2009;108(4):998–1011. 10.1002/jcb.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data files obtained from prior studies (from our group as well as other groups) have been referenced in the main text of the manuscript Data files generated in the current study are included within the article and supplementary data files.