Abstract
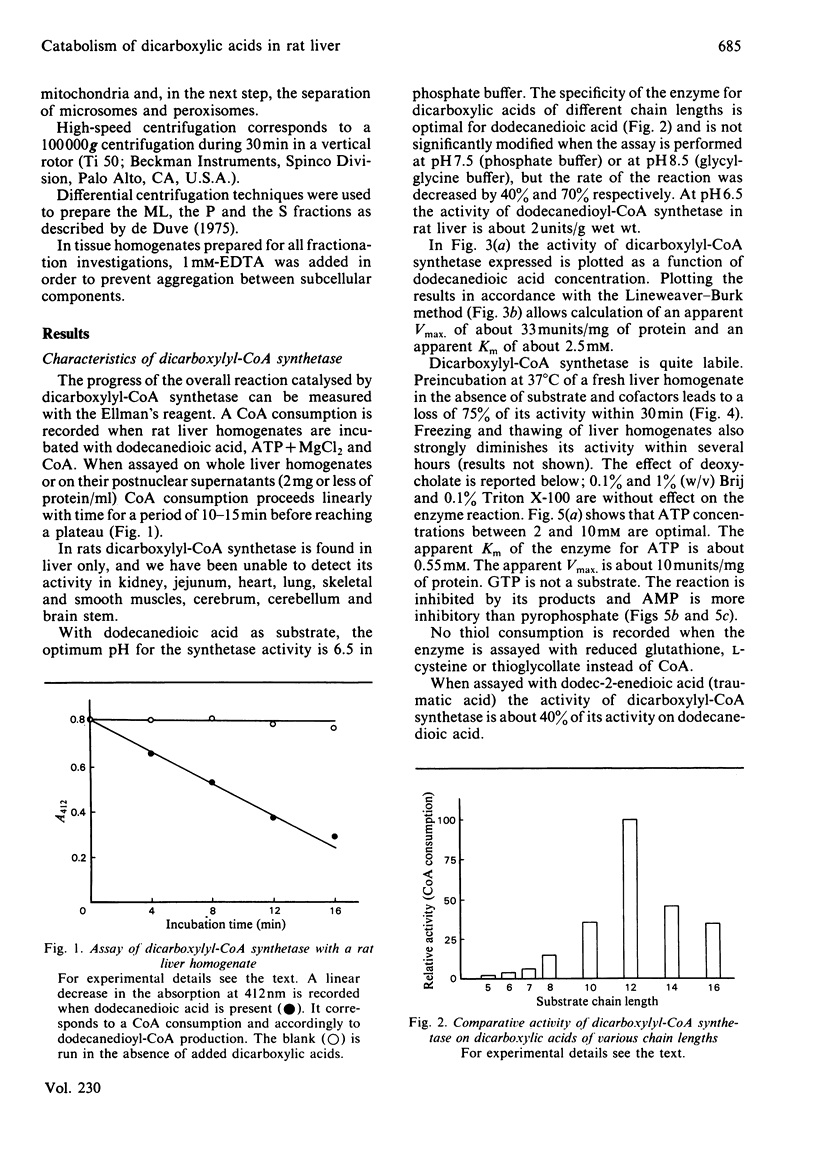
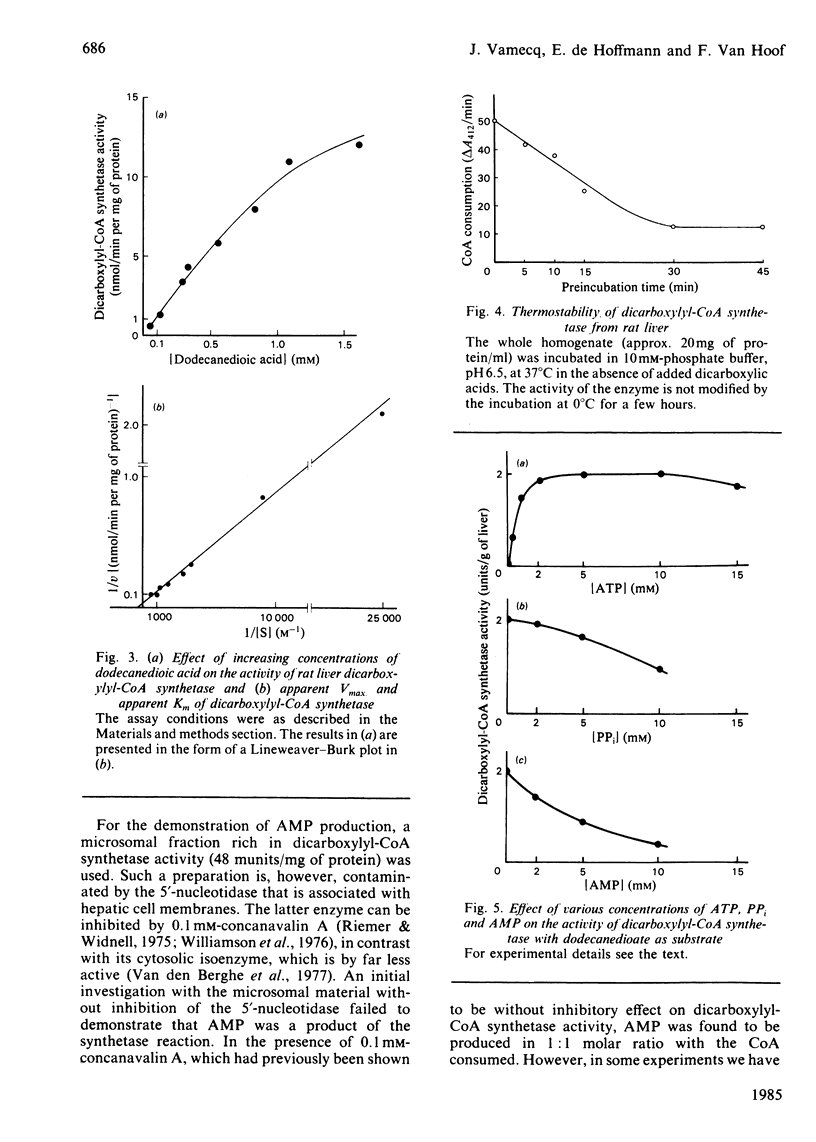
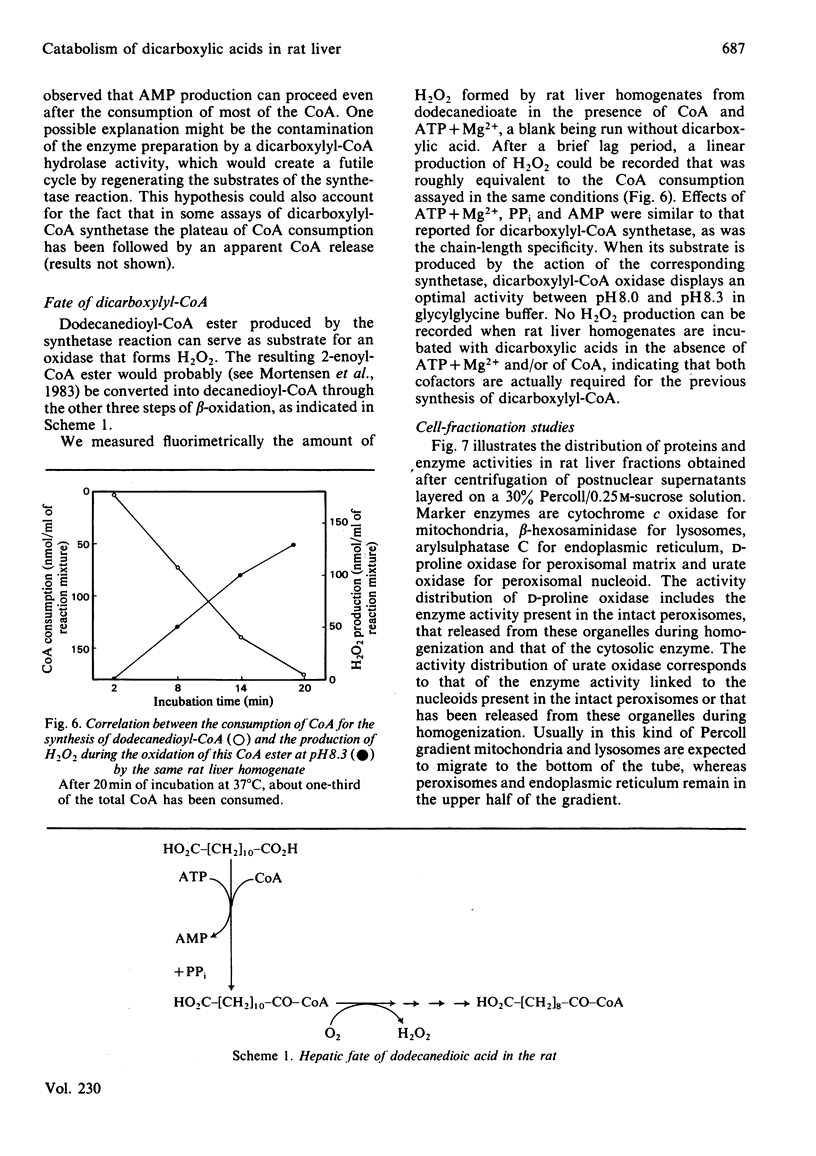
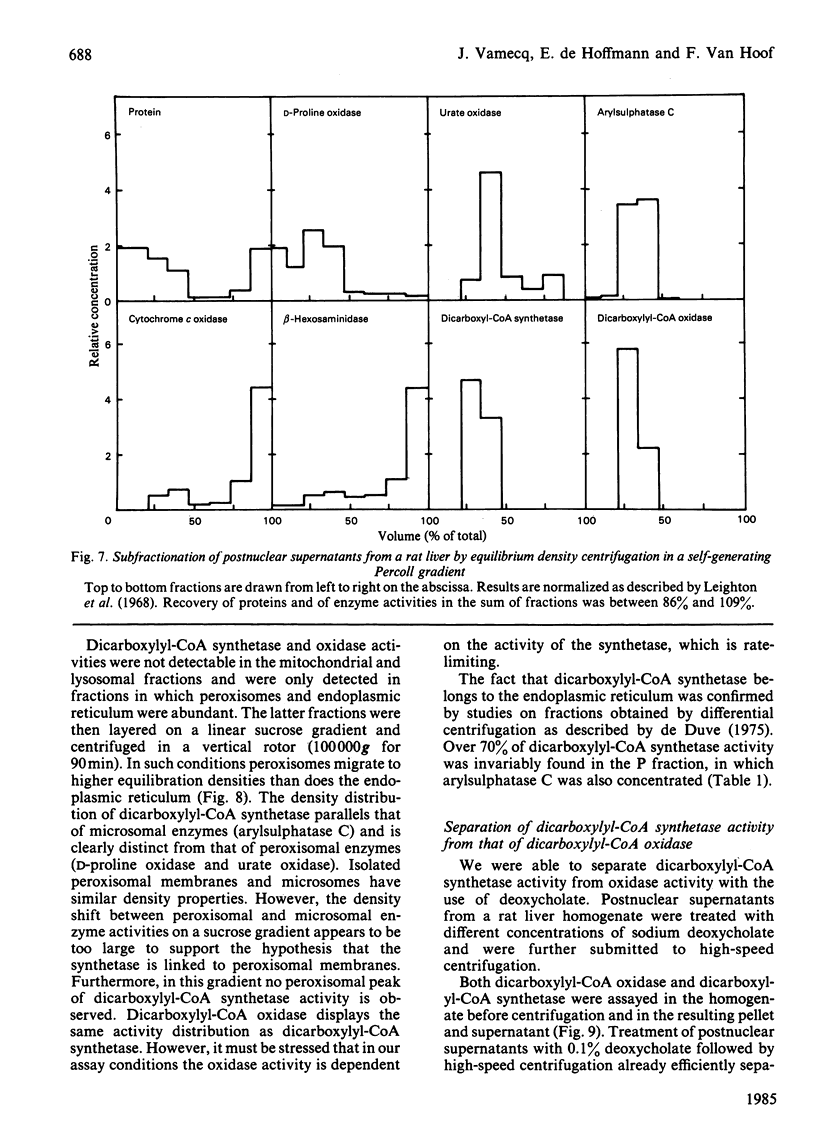
Dicarboxylic acids are products of the omega-oxidation of monocarboxylic acids. We demonstrate that in rat liver dicarboxylic acids (C5-C16) can be converted into their CoA esters by a dicarboxylyl-CoA synthetase. During this activation ATP, which cannot be replaced by GTP, is converted into AMP and PPi, both acting as feedback inhibitors of the reaction. Thermolabile at 37 degrees C, and optimally active at pH 6.5, dicarboxylyl-CoA synthetase displays the highest activity on dodecanedioic acid (2 micromol/min per g of liver). Cell-fractionation studies indicate that this enzyme belongs to the hepatic microsomal fraction. Investigations about the fate of dicarboxylyl-CoA esters disclosed the existence of an oxidase, which could be measured by monitoring the production of H2O2. In our assay conditions this H2O2 production is dependent on and closely follows the CoA consumption. It appears that the chain-length specificity of the handling of dicarboxylic acids by this catabolic pathway (activation to acyl-CoA and oxidation with H2O2 production) parallels the pattern of the degradation of exogenous dicarboxylic acids in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Akhtar R. A., Hawthorne J. N. Acetylcholine increases the breakdown of triphosphoinositide of rabbit iris muscle prelabelled with [32P] phosphate. Biochem J. 1977 Jan 15;162(1):61–73. doi: 10.1042/bj1620061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Tana J., Rose G., Shapiro B. The purification and properties of microsomal palmitoyl-coenzyme A synthetase. Biochem J. 1971 Apr;122(3):353–362. doi: 10.1042/bj1220353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., Rahman-Li Y., Sellinger O. Z., Wattiaux R., Jacques P., De Duve C. Tissue fractionation studies. 17. Intracellular distribution of monoamine oxidase, aspartate aminotransferase, alanine aminotransferase, D-amino acid oxidase and catalase in rat-liver tissue. Biochem J. 1964 Jul;92(1):179–184. doi: 10.1042/bj0920179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besrat A., Polan C. E., Henderson L. M. Mammalian metabolism of glutaric acid. J Biol Chem. 1969 Mar 25;244(6):1461–1467. [PubMed] [Google Scholar]
- Björkhem I., Danielsson H. Omega- and (omega - 1)-oxidation of fatty acids by rat liver microsomes. Eur J Biochem. 1970 Dec;17(3):450–459. doi: 10.1111/j.1432-1033.1970.tb01186.x. [DOI] [PubMed] [Google Scholar]
- Björkhem I. On the mechanism of regulation of omega oxidation of fatty acids. J Biol Chem. 1976 Sep 10;251(17):5259–5266. [PubMed] [Google Scholar]
- Borg L., Lindstedt S., Steen G., Hjalmarson O. Aliphatic C 6 -C 14 dicarboxylic acids in urine from an infant with fatal congenital lactic acidosis. Clin Chim Acta. 1972 Oct;41:363–366. doi: 10.1016/0009-8981(72)90532-3. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duve C. Exploring cells with a centrifuge. Science. 1975 Jul 18;189(4198):186–194. doi: 10.1126/science.1138375. [DOI] [PubMed] [Google Scholar]
- Fell G. S. Analytical procedures for diagnosis of trace element disorders. J Inherit Metab Dis. 1983;6 (Suppl 1):5–8. doi: 10.1007/BF01811316. [DOI] [PubMed] [Google Scholar]
- Gaunt G. L., de Duve C. Subcellular distribution of D-amino acid oxidase and catalase in rat brain. J Neurochem. 1976 Apr;26(4):749–759. doi: 10.1111/j.1471-4159.1976.tb04448.x. [DOI] [PubMed] [Google Scholar]
- Gregersen N., Brandt N. J. Ketotic episodes in glutaryl-CoA dehydrogenase deficiency (glutaric aciduria). Pediatr Res. 1979 Sep;13(9):977–981. doi: 10.1203/00006450-197909000-00005. [DOI] [PubMed] [Google Scholar]
- Gregersen N., Kølvraa S., Rasmussen K., Mortensen P. B., Divry P., David M., Hobolth N. General (medium-chain) acyl-CoA dehydrogenase deficiency (non-ketotic dicarboxylic aciduria): quantitative urinary excretion pattern of 23 biologically significant organic acids in three cases. Clin Chim Acta. 1983 Aug 15;132(2):181–191. doi: 10.1016/0009-8981(83)90246-2. [DOI] [PubMed] [Google Scholar]
- Gregersen N., Lauritzen R., Rasmussen K. Suberylglycine excretion in the urine from a patient with dicarboxylic aciduria. Clin Chim Acta. 1976 Aug 2;70(3):417–425. doi: 10.1016/0009-8981(76)90355-7. [DOI] [PubMed] [Google Scholar]
- Gregersen N., Wintzensen H., Christensen S. K., Christensen M. F., Brandt N. J., Rasmussen K. C6-C10-dicarboxylic aciduria: investigations of a patient with riboflavin responsive multiple acyl-CoA dehydrogenation defects. Pediatr Res. 1982 Oct;16(10):861–868. doi: 10.1203/00006450-198210000-00012. [DOI] [PubMed] [Google Scholar]
- Guilbualt G. G., Kramer D. N., Hackley E. A new substrate for fluorometric determination of oxidative enzymes. Anal Chem. 1967 Feb;39(2):271–272. doi: 10.1021/ac60246a029. [DOI] [PubMed] [Google Scholar]
- Ichiha K., Kushunose E., Kusunose M. Some properties and distribution of the omega-hydroxylation system of medium-chain fatty acids. Biochim Biophys Acta. 1969 Jun 10;176(4):704–712. [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Enzymatic synthesis of the coenzyme A derivatives of long chain fatty acids. J Biol Chem. 1953 Sep;204(1):329–343. [PubMed] [Google Scholar]
- Krisans S. K., Mortensen R. M., Lazarow P. B. Acyl-CoA synthetase in rat liver peroxisomes. Computer-assisted analysis of cell fractionation experiments. J Biol Chem. 1980 Oct 25;255(20):9599–9607. [PubMed] [Google Scholar]
- Kølvraa S., Gregersen N., Christensen E., Hobolth N. In vitro fibroblast studies in a patient with C6-C10-dicarboxylic aciduria: evidence for a defect in general acyl-CoA dehydrogenase. Clin Chim Acta. 1982 Nov 24;126(1):53–67. doi: 10.1016/0009-8981(82)90361-8. [DOI] [PubMed] [Google Scholar]
- LIPMANN F., TUTTLE L. C. Lipase-catalysed condensation of fatty acids with hydroxylamine. Biochim Biophys Acta. 1950 Jan;4(1-3):301–309. doi: 10.1016/0006-3002(50)90036-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Poole B., Beaufay H., Baudhuin P., Coffey J. W., Fowler S., De Duve C. The large-scale separation of peroxisomes, mitochondria, and lysosomes from the livers of rats injected with triton WR-1339. Improved isolation procedures, automated analysis, biochemical and morphological properties of fractions. J Cell Biol. 1968 May;37(2):482–513. doi: 10.1083/jcb.37.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENON G. K., FRIEDMAN D. L., STERN J. R. Enzymic synthesis of glutaryl-coenzyme A. Biochim Biophys Acta. 1960 Nov 4;44:375–377. doi: 10.1016/0006-3002(60)91583-3. [DOI] [PubMed] [Google Scholar]
- MITZ M. A., HEINRIKSON R. L. Omega hydroxy fatty acid dehydrogenase. Biochim Biophys Acta. 1961 Jan 1;46:45–50. doi: 10.1016/0006-3002(61)90644-8. [DOI] [PubMed] [Google Scholar]
- MYERS D. K., SLATER E. C. The enzymic hydrolysis of adenosine triphosphate by liver mitochondria. I. Activities at different pH values. Biochem J. 1957 Dec;67(4):558–572. doi: 10.1042/bj0670558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaerts G. P., Van Veldhoven P., Van Broekhoven A., Vandebroek G., Debeer L. J. Evidence that peroxisomal acyl-CoA synthetase is located at the cytoplasmic side of the peroxisomal membrane. Biochem J. 1982 Apr 15;204(1):17–23. doi: 10.1042/bj2040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen P. B. C6--C10-dicarboxylic aciduria in starved, fat-fed and diabetic rats receiving decanoic acid or medium-chain triacylglycerol. An in vivo measure of the rate of beta-oxidation of fatty acids. Biochim Biophys Acta. 1981 May 22;664(2):349–355. doi: 10.1016/0005-2760(81)90057-6. [DOI] [PubMed] [Google Scholar]
- Mortensen P. B., Gregersen N. The biological origin of ketotic dicarboxylic aciduria. II. In vivo and in vitro investigations of the beta-oxidation of C8-C16-dicarboxylic acids in unstarved, starved and diabetic rats. Biochim Biophys Acta. 1982 Mar 12;710(3):477–484. doi: 10.1016/0005-2760(82)90132-1. [DOI] [PubMed] [Google Scholar]
- Mortensen P. B., Gregersen N. The biological origin of ketotic dicarboxylic aciduria. In vivo and in vitro investigations of the omega-oxidation of C6-C16-monocarboxylic acids in unstarved, starved and diabetic rats. Biochim Biophys Acta. 1981 Dec 23;666(3):394–404. doi: 10.1016/0005-2760(81)90298-8. [DOI] [PubMed] [Google Scholar]
- Mortensen P. B., Kølvraa S., Gregersen N., Rasmussen K. Cyanide-insensitive and clofibrate enhanced beta-oxidation of dodecanedioic acid in rat liver. An indication of peroxisomal beta-oxidation of N-dicarboxylic acids. Biochim Biophys Acta. 1982 Nov 12;713(2):393–397. doi: 10.1016/0005-2760(82)90258-2. [DOI] [PubMed] [Google Scholar]
- PREISS B., BLOCH K. OMEGA-OXIDATION OF LONG CHAIN FATTY ACIDS IN RAT LIVER. J Biol Chem. 1964 Jan;239:85–88. [PubMed] [Google Scholar]
- Passi S., Picardo M., Nazzaro-Porro M., Breathnach A., Confaloni A. M., Serlupi-Crescenzi G. Antimitochondrial effect of saturated medium chain length (C8-C13) dicarboxylic acids. Biochem Pharmacol. 1984 Jan 1;33(1):103–108. doi: 10.1016/0006-2952(84)90376-9. [DOI] [PubMed] [Google Scholar]
- Pettersen J. E. Formation of n-hexanedioic acid from hexadecanoic acid by an initial oxidation in ketotic rats. Clin Chim Acta. 1972 Oct;41:231–237. doi: 10.1016/0009-8981(72)90516-5. [DOI] [PubMed] [Google Scholar]
- Pettersen J. E. In vitro studies on the metabolism of hexadecanedioic acid and its mono-L-carnitine ester. Biochim Biophys Acta. 1973 Apr 13;306(1):1–14. doi: 10.1016/0005-2760(73)90201-4. [DOI] [PubMed] [Google Scholar]
- Pettersen J. E., Jellum E., Eldjarn L. The occurrence of adipic and suberic acid in urine from ketotic patients. Clin Chim Acta. 1972 Apr;38(1):17–24. doi: 10.1016/0009-8981(72)90202-1. [DOI] [PubMed] [Google Scholar]
- Riemer B. L., Widnell C. C. The demonstration of a specific 5'-nucleotidase activity in rat tissues. Arch Biochem Biophys. 1975 Nov;171(1):343–347. doi: 10.1016/0003-9861(75)90041-7. [DOI] [PubMed] [Google Scholar]
- Robbins K. C. In vitro enzymic omega oxidation of medium-chain fatty acids in mammalian tissue. Arch Biochem Biophys. 1968 Mar 11;123(3):531–538. doi: 10.1016/0003-9861(68)90174-4. [DOI] [PubMed] [Google Scholar]
- Roberfroid M. B., Malaveille C., Hautefeuille A., Brun G., Vo T. K., Bartsch H. Interrelationships in mice of antipyrine half-life, hepatic monooxygenase activities and liver S9-mediated mutagenicity of aflatoxin B1, benzo[alpha]pyrene 7,8-dihydrodiol, 2-acetylaminofluorene and N-nitrosomorpholine. Chem Biol Interact. 1983 Nov;47(2):175–194. doi: 10.1016/0009-2797(83)90156-4. [DOI] [PubMed] [Google Scholar]
- Vamecq J., Van Hoof F. Implication of a peroxisomal enzyme in the catabolism of glutaryl-CoA. Biochem J. 1984 Jul 1;221(1):203–211. doi: 10.1042/bj2210203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamecq J., de Hoffmann E., Van Hoof F. Mitochondrial and peroxisomal metabolism of glutaryl-CoA. Eur J Biochem. 1985 Feb 1;146(3):663–669. doi: 10.1111/j.1432-1033.1985.tb08702.x. [DOI] [PubMed] [Google Scholar]
- Van Hoof F., Hers H. G. The abnormalities of lysosomal enzymes in mucopolysacc- haridoses. Eur J Biochem. 1968 Dec;7(1):34–44. doi: 10.1111/j.1432-1033.1968.tb19570.x. [DOI] [PubMed] [Google Scholar]
- WAKABAYASHI K., SHIMAZONO N. Studies on omega-oxidation of fatty acids in vitro. I. Overall reaction and intermediate. Biochim Biophys Acta. 1963 Apr 23;70:132–142. doi: 10.1016/0006-3002(63)90733-9. [DOI] [PubMed] [Google Scholar]
- Williamson F. A., Morré D. J., Shen-Miller J. Inhibition of 5'-nucleotidase by concanavalin A: evidence for localization on the outer surface of the plasma membrane. Cell Tissue Res. 1976 Aug 10;170(4):477–484. doi: 10.1007/BF00361705. [DOI] [PubMed] [Google Scholar]


